
Journal of Inorganic Materials ›› 2026, Vol. 41 ›› Issue (2): 150-158.DOI: 10.15541/jim20250101
• REVIEW • Previous Articles Next Articles
LIU Zhanyi1,2,3( ), LI Mian2,3(
), LI Mian2,3( ), OUYANG Xiaoping4, CHAI Zhifang2,3, HUANG Qing2,3(
), OUYANG Xiaoping4, CHAI Zhifang2,3, HUANG Qing2,3( )
)
Received:2025-03-08
Revised:2025-04-16
Published:2025-05-09
Online:2025-05-09
Contact:
LI Mian, professor. E-mail: limian@nimte.ac.cn;About author:LIU Zhanyi (2001-), male, Master candidate. E-mail: liuzhanyi@nimte.ac.cn
Supported by:CLC Number:
LIU Zhanyi, LI Mian, OUYANG Xiaoping, CHAI Zhifang, HUANG Qing. Recent Progress on Removal of Sr/Cs from Molten Salt in Dry Reprocessing[J]. Journal of Inorganic Materials, 2026, 41(2): 150-158.
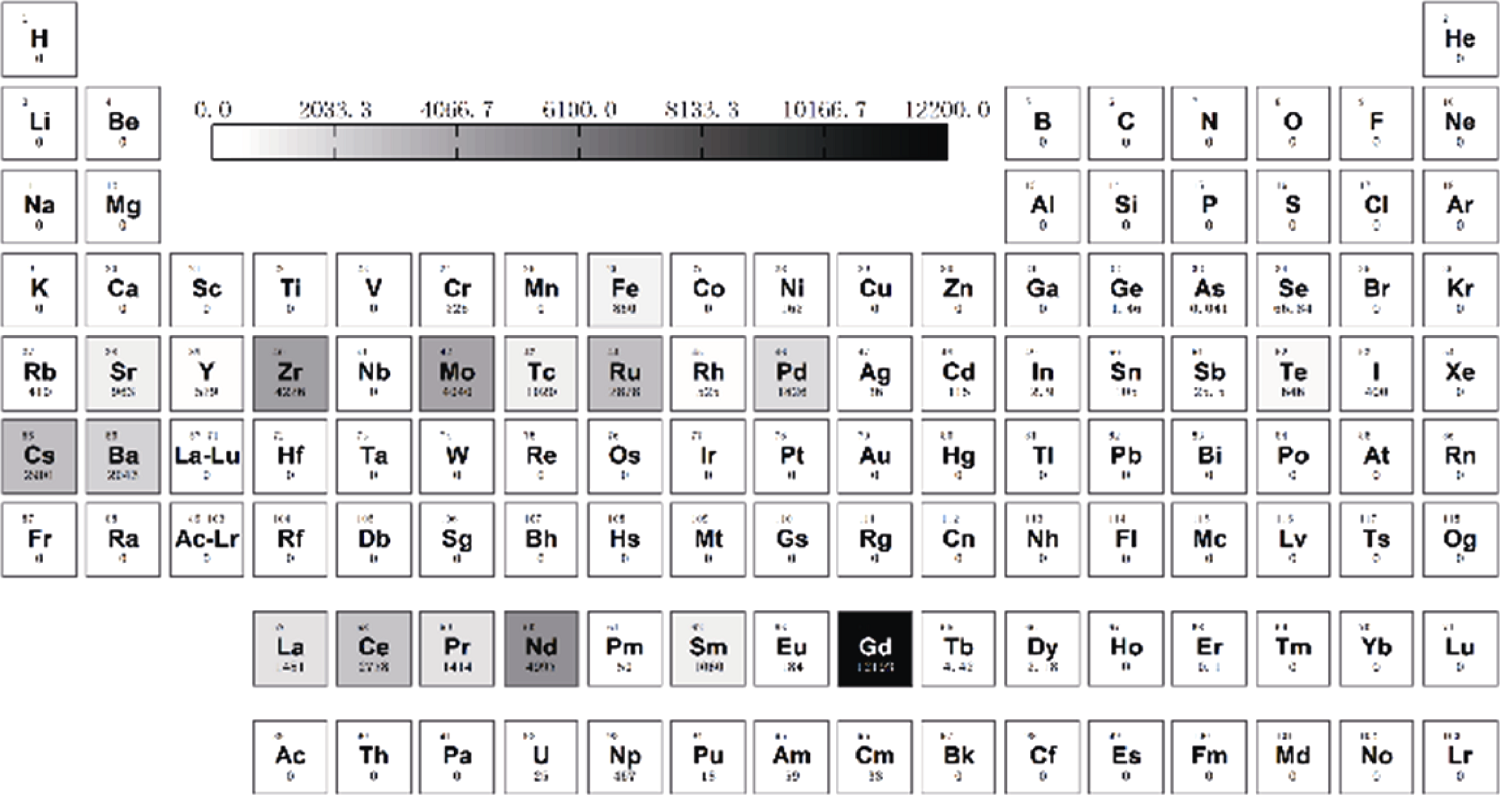
Fig. 2 Wastes arising from typical light water reactor fuel irradiated to 40000 MW·d/t[11] Elements present shadowed in grey. Numbers represent amount in milligram per kilogram of uranium
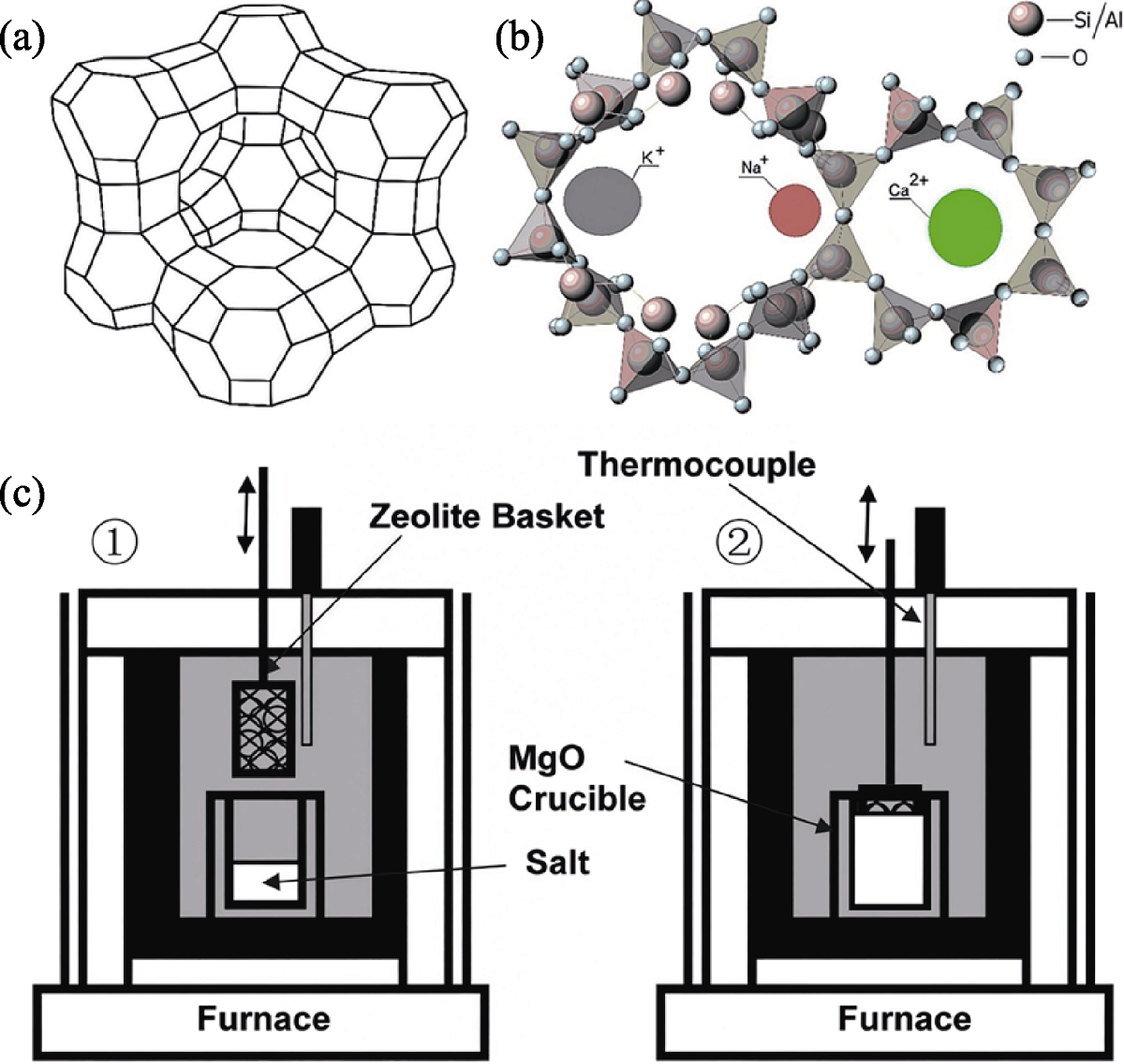
Fig. 10 Schematic diagrams of zeolite structure and zeolite ion exchange device[52,56] (a, b) Schematic structure of zeolite[52]; (c) System for molten salt-zeolite ion exchange tests[56]
| Method | Working salt | Nuclide | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|---|
| Physical method | Cold finger separation | LiCl | Sr, Cs | No impurities introduced | Difficult to scale application | [ |
| Zone-refining process | LiCl | Sr, Cs | No impurities introduced, high removal rate, easy accessibility | Long processing time | [ | |
| LiCl-KCl | [ | |||||
| Chemical method | Precipitation | LiCl-KCl | Sr, Cs | Short processing time, low cost | Low removal rate of Cs, introduction of impurities | [ |
| Electrolysis | LiCl-KCl | Sr | High efficiency | Low removal rate, high corrosivity to equipment | [ | |
| NaCl-KCl | [ | |||||
| Ion exchange | LiCl-KCl/ NaCl-KCl | Sr, Cs | Good selectivity, high removal efficiency | Introduction of new impurities | [ | |
Table 1 Available methods for removing Sr and Cs from molten salts and their advantages and disadvantages
| Method | Working salt | Nuclide | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|---|
| Physical method | Cold finger separation | LiCl | Sr, Cs | No impurities introduced | Difficult to scale application | [ |
| Zone-refining process | LiCl | Sr, Cs | No impurities introduced, high removal rate, easy accessibility | Long processing time | [ | |
| LiCl-KCl | [ | |||||
| Chemical method | Precipitation | LiCl-KCl | Sr, Cs | Short processing time, low cost | Low removal rate of Cs, introduction of impurities | [ |
| Electrolysis | LiCl-KCl | Sr | High efficiency | Low removal rate, high corrosivity to equipment | [ | |
| NaCl-KCl | [ | |||||
| Ion exchange | LiCl-KCl/ NaCl-KCl | Sr, Cs | Good selectivity, high removal efficiency | Introduction of new impurities | [ | |
| [1] | 丁煊涛, 焦立峰, 郭峃峄, 等. 核电规模化储能集成技术进展. 热力发电, 2025, 54: 12. |
| [2] | 王宏渊. 我国快堆闭式核燃料循环体系的现状及展望. 能源工程, 2013(5): 8. |
| [3] | 张东辉, 王松平, 代智文. 我国快堆的创新与发展. 核科学与工程, 2024, 44: 980. |
| [4] |
林如山, 何辉, 唐洪彬, 等. 我国乏燃料干法后处理技术研究现状与发展. 原子能科学技术, 2020, 54: 115.
DOI |
| [5] |
YIN T Q, XUE Y, YAN Y D, et al. Recovery and separation of rare earth elements by molten salt electrolysis. International Journal of Minerals, Metallurgy and Materials, 2021, 28(6): 899.
DOI |
| [6] | MIRZA M, ABDULAZIZ R, MASKELL W C, et al. Electrochemical processing in molten salts-a nuclear perspective. Energy & Environmental Science, 2023, 16(3): 952. |
| [7] |
WILLIAMSON M A, WILLIT J. Pyroprocessing flowsheets for recycling used nuclear fuel. Nuclear Engineering and Technology, 2011, 43: 329.
DOI URL |
| [8] |
YIN T, LIU Y, JIANG S, et al. Kinetic properties and electrochemical separation of uranium on liquid bismuth electrode in LiCl-KCl melt. Journal of the Electrochemical Society, 2021, 168(3): 032503.
DOI |
| [9] | 伍思达, 林如山, 张磊, 等. 干法后处理废盐中活泼裂片元素的净化工艺研究进展. 无机盐工业, 2022, 54: 81. |
| [10] |
IIZUKA M, UOZUMI K, OGATA T, et al. Development of an innovative electrorefiner for high uranium recovery rate from metal fast reactor fuels. Journal of Nuclear Science and Technology, 2009, 46(7): 699.
DOI URL |
| [11] |
VOLKOVICH V A, GRIFFITHS T R, THIED R C. Treatment of molten salt wastes by phosphate precipitation: removal of fission product elements after pyrochemical reprocessing of spent nuclear fuels in chloride melts. Journal of Nuclear Materials, 2003, 323(1): 49.
DOI URL |
| [12] | SIMPSON M F. Projected salt waste production from a commercial pyroprocessing facility. Science and Technology of Nuclear Installations, 2013, 2013(1): 945858. |
| [13] |
CHOI E Y, WON C Y, KANG D S, et al. Production of uranium metal via electrolytic reduction of uranium oxide in molten LiCl and salt distillation. Journal of Radioanalytical and Nuclear Chemistry, 2015, 304(2): 535.
DOI URL |
| [14] |
KIM S W, JEON M K, CHOI E Y. Electrolytic behavior of SrCl2 and BaCl2 in LiCl molten salt during oxide reduction in pyroprocessing. Journal of Radioanalytical and Nuclear Chemistry, 2019, 321(1): 361.
DOI |
| [15] |
CHO Y Z, PARK G H, LEE H S, et al. Concentration of cesium and strontium elements involved in a LiCl waste salt by a melt crystallization process. Nuclear Technology, 2010, 171(3): 325.
DOI URL |
| [16] |
VANCE E R, DAVIS J, OLUFSON K, et al. Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel. Journal of Nuclear Materials, 2012, 420(1/2/3): 396.
DOI URL |
| [17] |
WANG D D, LIU Y L, JIANG S L, et al. Separation of uranium from lanthanides (La, Ce, Nd) and purification of waste salt via aluminum electrodes with different structures in LiCl-KCl eutectic. Separation and Purification Technology, 2025, 353: 128328.
DOI URL |
| [18] |
WANG D D, LIU Y L, YANG D W, et al. Separation of uranium from lanthanides (La, Sm) with sacrificial Li anode in LiCl-KCl eutectic salt. Separation and Purification Technology, 2022, 292: 121025.
DOI URL |
| [19] |
YANG D W, JIANG S L, LIU Y L, et al. Electrochemical extraction kinetics of Nd on reactive electrodes. Separation and Purification Technology, 2022, 281: 119853.
DOI URL |
| [20] |
YANG M C, ZHONG Y K, WANG D D, et al. Rapid and efficient extraction of cerium by forming Al-Ce alloys in LiCl-KCl molten salts. Separation and Purification Technology, 2024, 341: 126868.
DOI URL |
| [21] |
FIGUEIREDO B R, CARDOSO S P, PORTUGAL I, et al. Inorganic ion exchangers for cesium removal from radioactive wastewater. Separation and Purification Reviews, 2018, 47(4): 306.
DOI URL |
| [22] |
VINCENT T, VINCENT C, BARRE Y, et al. Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: synthesis and characterization. Journal of Materials Chemistry A, 2014, 2(26): 10007.
DOI URL |
| [23] |
CHEN S, HU J, HAN S, et al. A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade. Separation and Purification Technology, 2020, 251: 117340.
DOI URL |
| [24] |
LIZAGA I, GASPAR L, QUIJANO L, et al. NDVI, 137Cs and nutrients for tracking soil and vegetation development on glacial landforms in the Lake Paron Catchment (Cordillera Blanca, Peru). Science of the Total Environment, 2019, 651: 250.
DOI URL |
| [25] |
MENENDEZ-DUARTE R, FERNANDEZ S, SOTO J. The application of 137Cs to post-fire erosion in north-west Spain. Geoderma, 2009, 150(1/2): 54.
DOI URL |
| [26] | KIM G Y, JANG J, PAEK S, et al. Electrochemical removal of rare earth element in LiCl-KCl molten salt. Science and Technology of Nuclear Installations, 2020, 2020: 2392489. |
| [27] |
JANG J, LEE M, KIM G Y, et al. Cesium and strontium recovery from LiCl-KCl eutectic salt using electrolysis with liquid cathode. Nuclear Engineering and Technology, 2022, 54(10): 3957.
DOI URL |
| [28] | NIGL T P, LICHTENSTEIN T, KONG Y, et al. Electrochemical separation of alkaline-earth elements from molten salts using liquid metal electrodes. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14818. |
| [29] |
CHEN X, ZHANG Y, QU J, et al. Integrating preparation of borides and separation of alkaline- and rare-earth ions through an electrochemical alloying approach in molten salts. Separation and Purification Technology, 2022, 285: 120391.
DOI URL |
| [30] |
YUAN Y, ZHANG Y, CHEN X, et al. Electrochemical purification of waste salt from pyro-processing of spent nuclear fuels. Separation and Purification Technology, 2023, 326: 124805.
DOI URL |
| [31] |
VERSEY J R, PHONGIKAROON S, SIMPSON M F. Separation of CsCl from LiCl-CsCl molten salt by cold finger melt crystallization. Nuclear Engineering and Technology, 2014, 46(3): 395.
DOI URL |
| [32] |
CHOI J H, CHO Y Z, LEE T K, et al. Inclusion behavior of Cs, Sr, and Ba impurities in LiCl crystal formed by layer-melt crystallization: combined first-principles calculation and experimental study. Journal of Crystal Growth, 2013, 371: 84.
DOI URL |
| [33] |
LEE B, KIM G Y, CHOI J H, et al. Reactive-crystallization method for purification of LiCl-KCl eutectic salt waste. Journal of Radioanalytical and Nuclear Chemistry, 2024, 333(12): 6331.
DOI |
| [34] |
LEE H S, OH G H, LEE Y S, et al. Concentrations of CsCl and SrCl2 from a simulated LiCl Salt waste generated by pyroprocessing by using Czochralski method. Journal of Nuclear Science and Technology, 2009, 46(4): 392.
DOI URL |
| [35] |
SHIM M, CHOI H G, CHOI J H, et al. Separation of Cs and Sr from LiCl-KCl eutectic salt via a zone-refining process for pyroprocessing waste salt minimization. Journal of Nuclear Materials, 2017, 491: 9.
DOI URL |
| [36] |
CHO Y Z, LEE T K, CHOI J H, et al. Eutectic (LiCl-KCl) waste salt treatment by sequential separation process. Nuclear Engineering and Technology, 2013, 45(5): 675.
DOI URL |
| [37] |
RODRIGUEZ-LAGUNA M D R, TOLMAN K R, KROPP M T, et al. Separation of fission products from high-level waste salt systems by partial crystallization: CsCl-NaCl-LiCl-KCl study. Separation and Purification Technology, 2024, 332: 125602.
DOI URL |
| [38] |
WILLIAMS A N, PHONGIKAROON S, SIMPSON M F. Separation of CsCl from a ternary CsCl-LiCl-KCl salt via a melt crystallization technique for pyroprocessing waste minimization. Chemical Engineering Science, 2013, 89: 258.
DOI URL |
| [39] |
CHOI H G, SHIM M, LEE J H, et al. Numerical analysis of impurity separation from waste salt by investigating the change of concentration at the interface during zone refining process. Journal of Crystal Growth, 2017, 474: 69.
DOI URL |
| [40] |
DIVAKARAN S, JOSEPH J, MANOHARAN M, et al. CsCl enrichment during solidification of molten LiCl-KCl-CsCl salt mixture. Nuclear Engineering and Technology, 2024, 56(11): 4716.
DOI URL |
| [41] | 付海英, 耿俊霞, 杨洋, 等. 乏燃料干法后处理中的熔盐减压蒸馏技术. 核技术, 2018, 41: 5. |
| [42] |
EUN H C, YANG H C, CHO Y Z, et al. Vacuum distillation of a mixture of LiCl-KCl eutectic salts and RE oxidative precipitates and a dechlorination and oxidation of RE oxychlorides. Journal of Hazardous Materials, 2008, 160(2): 634.
DOI PMID |
| [43] |
EUN H C, CHOI J H, KIM N Y, et al. A reactive distillation process for the treatment of LiCl-KCl eutectic waste salt containing rare earth chlorides. Journal of Nuclear Materials, 2016, 480: 69.
DOI URL |
| [44] |
EUN H C, CHOI J H, KIM N Y, et al. A study of separation and solidification of group II nuclides in waste salt delivered from the pyrochemical process of used nuclear fuel. Journal of Nuclear Materials, 2017, 491: 149.
DOI URL |
| [45] |
WESTPHAV B R, MARSDEN K C, PRICE J C, et al. On the development of a distillation process for the electrometallurgical treatment of irradiated spent nuclear fuel. Nuclear Engineering and Technology, 2008, 40(3): 163.
DOI URL |
| [46] |
CHO Y Z, PARK G H, YANG H C, et al. Minimization of eutectic salt waste from pyroprocessing by oxidative precipitation of lanthanides. Journal of Nuclear Science and Technology, 2009, 46(10): 1004.
DOI URL |
| [47] |
CHO Y Z, LEE T K, EUN H C, et al. Purification of used eutectic (LiCl-KCl) salt electrolyte from pyroprocessing. Journal of Nuclear Materials, 2013, 437(1/2/3): 47.
DOI URL |
| [48] |
GRIFFITHS T R, VOLKOVICH V A, YAKIMOV S M, et al. Reprocessing spent nuclear fuel using molten carbonates and subsequent precipitation of rare earth fission products using phosphate. Journal of Alloys and Compounds, 2006, 418(1): 116.
DOI URL |
| [49] |
HAN W, ZHANG Y, LIU R, et al. Purification of spent electrolyte by sequential precipitation method and its on-line monitoring. Ionics, 2021, 27(11): 4829.
DOI |
| [50] |
HAN W, ZHANG Y, LIU R, et al. Removal of RE3+, Cs+, Sr2+, Ba2+ from molten salt electrolyte by precipitation and solidification of glass- ceramics. Journal of Non-Crystalline Solids, 2023, 606: 122208.
DOI URL |
| [51] |
UOZUMI K, IIZUKA M, OMORI T. Removal of rare-earth fission products from molten chloride salt used in pyroprocessing by precipitation for consolidation into glass-bonded sodalite waste form. Journal of Nuclear Materials, 2021, 547: 152784.
DOI URL |
| [52] |
LONIN A Y, LEVENETS V V, OMELNIK O P, et al. Removal of a mixture of Cs, Sr and Co cations from an aqueous solution using composite sorbents based on natural and synthetic zeolites. Journal of Radioanalytical and Nuclear Chemistry, 2022, 331(12): 5517.
DOI |
| [53] |
HAO W, YAN N, XIE M, et al. Origin of the exceptional selectivity of NaA zeolite for the radioactive isotope 90Sr2+. Inorganic Chemistry Frontiers, 2022, 9(23): 6258.
DOI URL |
| [54] |
YANG H M, PARK C W, KIM I, et al. Sulfur-modified chabazite as a low-cost ion exchanger for the highly selective and simultaneous removal of cesium and strontium. Applied Surface Science, 2021, 536: 147776.
DOI URL |
| [55] | LEXA D, JOHNSON I. Occlusion and ion exchange in the molten (lithium chloride-potassium chloride-alkali metal chloride) salt plus zeolite 4A system with alkali metal chlorides of sodium, rubidium, and cesium. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, 2001, 32(3): 429. |
| [56] |
SACHDEV P, SIMPSON M F, FRANK S M, et al. Selective separation of Cs and Sr from LiCl-based salt for electrochemical processing of oxide spent nuclear fuel. Separation Science and Technology, 2008, 43(9/10): 2709.
DOI URL |
| [57] |
PARK H S, KIM I T, CHO Y J, et al. Removal behavior of Cs from molten salt by using zeolitic materials. Journal of Radioanalytical and Nuclear Chemistry, 2010, 283(2): 267.
DOI URL |
| [58] |
SHALTRY M, PHONGIKAROON S, SIMPSON M F. Ion exchange kinetics of fission products between molten salt and zeolite-A. Microporous and Mesoporous Materials, 2012, 152: 185.
DOI URL |
| [59] |
YOO T S, FRANK S M, SIMPSON M F, et al. Salt-zeolite ion-exchange equilibrium studies for a complete set of fission products in molten LiCl-KCl. Nuclear Technology, 2010, 171(3): 306.
DOI URL |
| [60] |
SIMPSON M F, GOUGAR M L D. Two-site equilibrium model for ion exchange between monovalent cations and zeolite-A in a molten salt. Industrial & Engineering Chemistry Research, 2003, 42(18): 4208.
DOI URL |
| [61] |
PHONGIKAROON S, SIMPSON M F. Equilibrium model for ion exchange between multivalent cations and zeolite-A in a molten salt. AICHE Journal, 2006, 52(5): 1736.
DOI URL |
| [62] |
TANG J H, JIN J C, LI W A, et al. Highly selective cesium(I) capture under acidic conditions by a layered sulfide. Nature Communications, 2022, 13: 658.
DOI |
| [63] |
QIU K, ZHANG Y, LI S, et al. Water-stable S-functionalized Ti3C2 MXene for high-performance Sr and Cs adsorption. Surfaces and Interfaces, 2024, 53: 105072.
DOI URL |
| [1] | SUN Lian, ZHANG Leilei, XUE Zexu, WU Kun, CHEN Ye, LI Zhiyuan, WANG Lukai, WANG Zungang. Research Progress on Zero-dimensional Metal Halide Scintillators towards Radiation Detection Applications [J]. Journal of Inorganic Materials, 2026, 41(2): 159-176. |
| [2] | REN Xianpei, LI Chao, HU Qiwei, XIANG Hui, PENG Yuehong. Research Progress on Mott-Schottky Hydrogen Evolution Catalysts Based on Metal/Transition Metal Compounds [J]. Journal of Inorganic Materials, 2026, 41(2): 137-149. |
| [3] | FAN Yuzhu, WANG Yuan, WANG Linyan, XIANG Meiling, YAN Yuting, LI Benhui, LI Min, WEN Zhidong, WANG Haichao, CHEN Yongfu, QIU Huidong, ZHAO Bo, ZHOU Chengyu. Graphene Oxide-based Adsorbents for Pb(II) Removing in Water: Progresses on Synthesis, Performance and Mechanism [J]. Journal of Inorganic Materials, 2026, 41(1): 12-26. |
| [4] | XU Jintao, GAO Pan, HE Weiyi, JIANG Shengnan, PAN Xiuhong, TANG Meibo, CHEN Kun, LIU Xuechao. Recent Progress on Preparation of 3C-SiC Single Crystal [J]. Journal of Inorganic Materials, 2026, 41(1): 1-11. |
| [5] | YU Shengyang, SU Haijun, JIANG Hao, YU Minghui, YAO Jiatong, YANG Peixin. A Review of Pore Defects in Ultra-high Temperature Oxide Ceramics by Laser Additive Manufacturing: Formation and Suppression [J]. Journal of Inorganic Materials, 2025, 40(9): 944-956. |
| [6] | LIU Jiangping, GUAN Xin, TANG Zhenjie, ZHU Wenjie, LUO Yongming. Research Progress on Catalytic Oxidation of Nitrogen-containing Volatile Organic Compounds [J]. Journal of Inorganic Materials, 2025, 40(9): 933-943. |
| [7] | XIAO Xiaolin, WANG Yuxiang, GU Peiyang, ZHU Zhenrong, SUN Yong. Advances in Regulation of Damaged Skin Regeneration by Two-dimensional Inorganic Materials [J]. Journal of Inorganic Materials, 2025, 40(8): 860-870. |
| [8] | MA Jingge, WU Chengtie. Application of Inorganic Bioceramics in Promoting Hair Follicle Regeneration and Hair Growth [J]. Journal of Inorganic Materials, 2025, 40(8): 901-910. |
| [9] | ZHANG Hongjian, ZHAO Ziyi, WU Chengtie. Inorganic Biomaterials on Regulating Neural Cell Function and Innervated Tissue Regeneration: A Review [J]. Journal of Inorganic Materials, 2025, 40(8): 849-859. |
| [10] | AI Minhui, LEI Bo. Micro-nanoscale Bioactive Glass: Functionalized Design and Angiogenic Skin Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 921-932. |
| [11] | WANG Yutong, CHANG Jiang, XU He, WU Chengtie. Advances in Silicate Bioceramic/Bioglass for Wound Healing: Effects, Mechanisms and Application Ways [J]. Journal of Inorganic Materials, 2025, 40(8): 911-920. |
| [12] | MA Wenping, HAN Yahui, WU Chengtie, LÜ Hongxu. Application of Inorganic Bioactive Materials in Organoid Research [J]. Journal of Inorganic Materials, 2025, 40(8): 888-900. |
| [13] | LUO Xiaomin, QIAO Zhilong, LIU Ying, YANG Chen, CHANG Jiang. Inorganic Bioactive Materials Regulating Myocardial Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 871-887. |
| [14] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [15] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||