
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (8): 901-910.DOI: 10.15541/jim20250002
• REVIEW • Previous Articles Next Articles
MA Jingge1,2( ), WU Chengtie1,2(
), WU Chengtie1,2( )
)
Received:2025-01-02
Revised:2025-02-08
Published:2025-08-20
Online:2025-02-13
Contact:
WU Chengtie, professor. E-mail: chengtiewu@mail.sic.ac.cnAbout author:MA Jingge (1995-), female, PhD. E-mail: 237122364@qq.com
Supported by:CLC Number:
MA Jingge, WU Chengtie. Application of Inorganic Bioceramics in Promoting Hair Follicle Regeneration and Hair Growth[J]. Journal of Inorganic Materials, 2025, 40(8): 901-910.
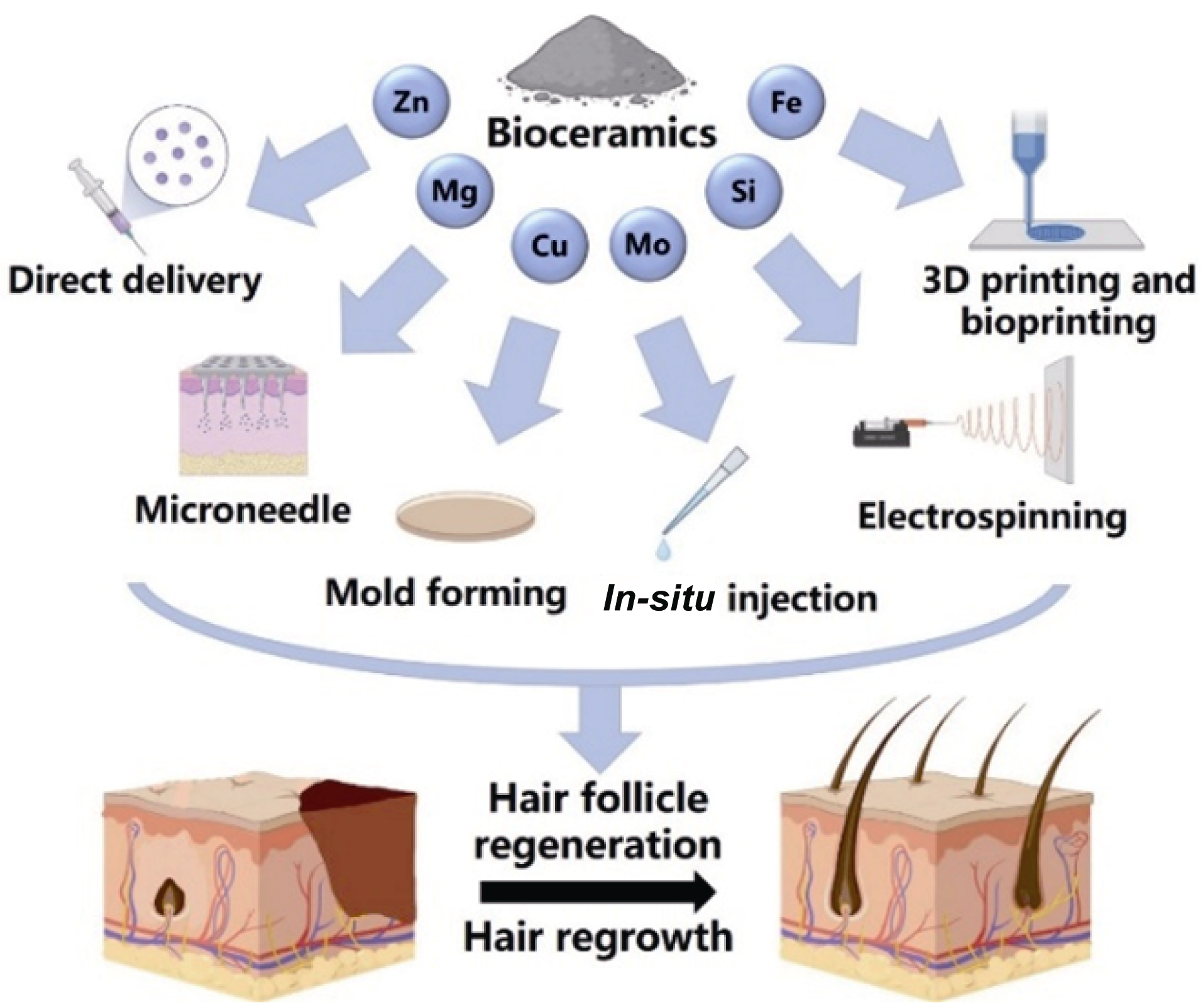
Fig. 1 Schematic diagram of the application of bioceramics containing elements such as zinc (Zn), magnesium (Mg), copper (Cu), molybdenum (Mo), silicon (Si), and iron (Fe) in hair follicle reconstruction and hair regeneration through direct delivery, microneedles, mold forming, in-situ injection, electrospinning, 3D printing, and bioprinting
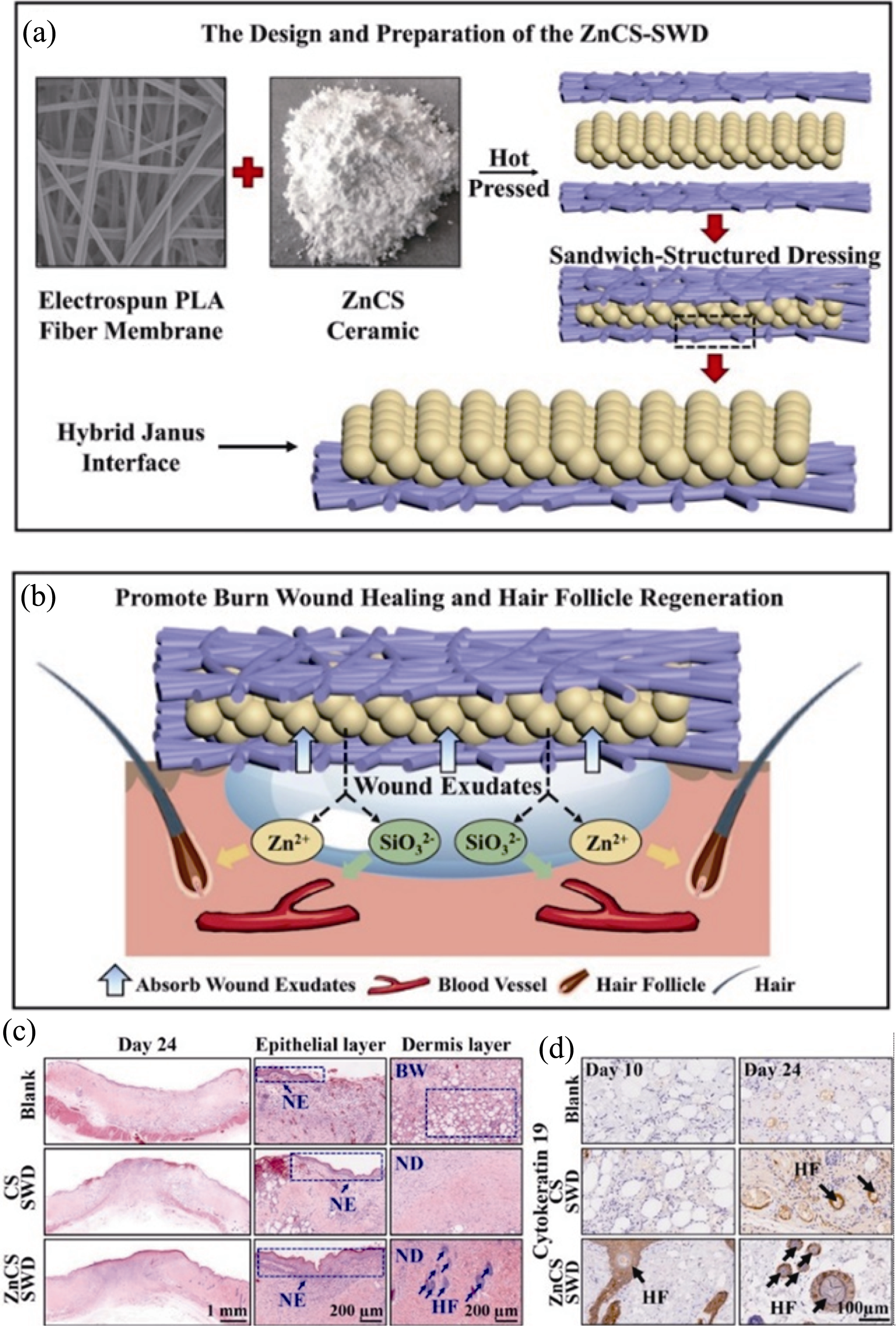
Fig. 2 Sandwich-structured wound dressing containing ZnCS bioceramics for deep burn wound repair[37] (a) Schematic diagram of the sandwich structure of the composite dressing by hot compression molding of hydrophilic ZnCS bioceramics and hydrophobic PLA; (b) Promotion of angiogenesis and hair follicle regeneration by Zn and Si bioactive ions released from the composite dressing; (c, d) Hematoxylin-eosin staining (c) and hair follicle marker protein CK19 staining (d) showing new skin tissue in deep burn wounds after 24 days of treatment, with arrows in (c, d) indicating new hair follicles ZnCS bioceramics PLA: polylactic acid; SWD: sandwich- structured wound dressing; CS: calcium silicate without Zn
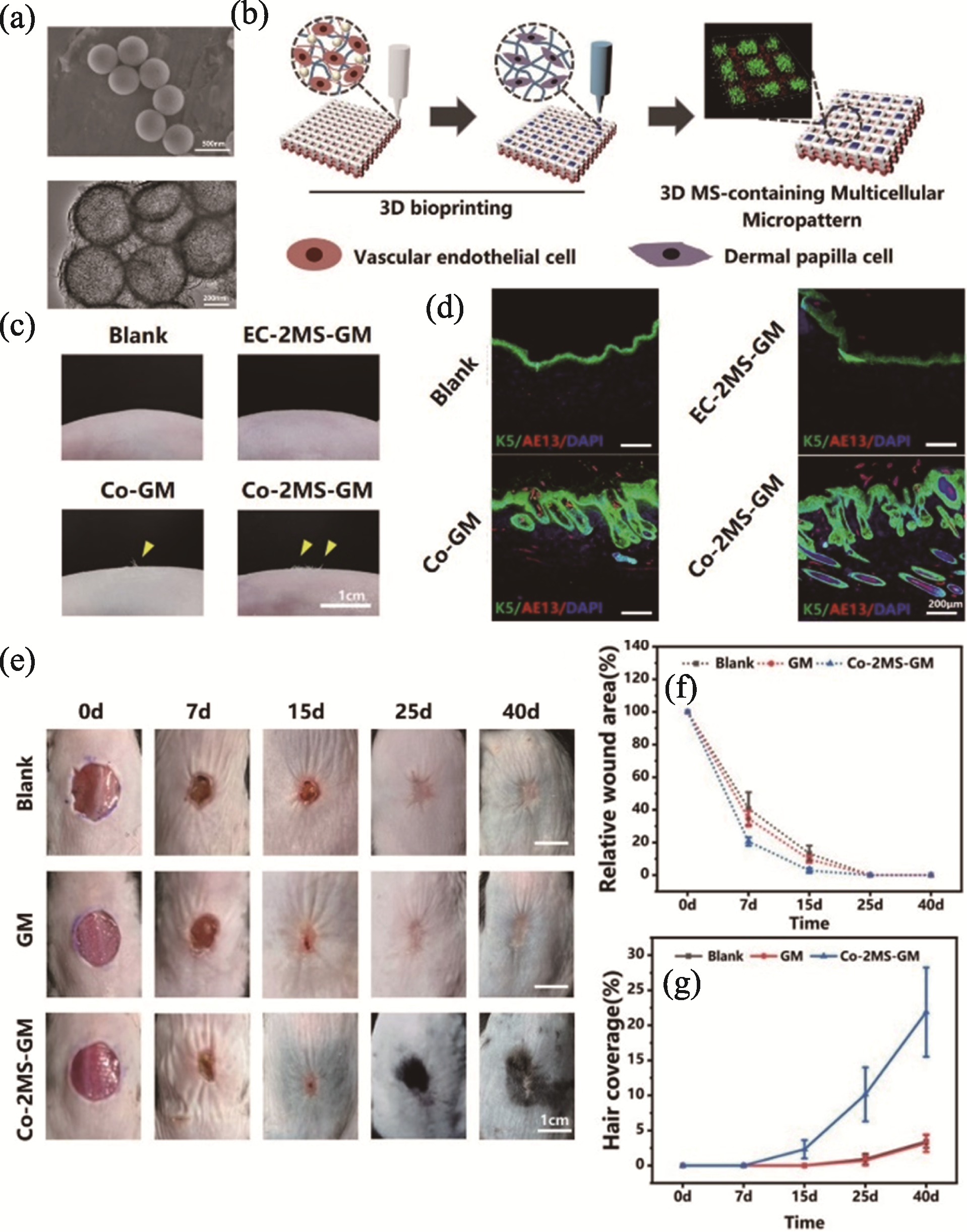
Fig. 3 3D bioprinted micropatterned multicellular scaffolds containing MS for blood vessel and hair follicle regeneration[45] (a) SEM and TEM images of MS nanospheres; (b) Schematic diagram of bioprinted micropatterned multicellular scaffolds containing MS among which distribution forms of vascular endothelial cells and dermal papilla cells simulate vascular network and punctate hair follicles in dermal tissue, respectively; (c) Hair growth in the skin of nude mice after 30 days following transplantation of micropatterned multicellular scaffolds; (d) Immunofluorescence staining images of hair follicle-related markers K5 and AE13 in the newborn skin tissue of nude mice at 30 days; (e) Hair regeneration of AGA mice on 0, 7, 15, 25, and 40 days after scaffold transplantation; (f, g) Relative wound area (f) and hair coverage statistics (g) of AGA mice MS: magnesium silicate; AGA: androgenetic alopecia; Blank: no treatment; EC-2MS-GM: treated by MS-containing scaffold encapsulated with endothelial cells; Co-GM: treated by micropatterned co-cultured scaffold; Co-2MS-GM: treated by micropatterned co-cultured scaffold incorporated with MS
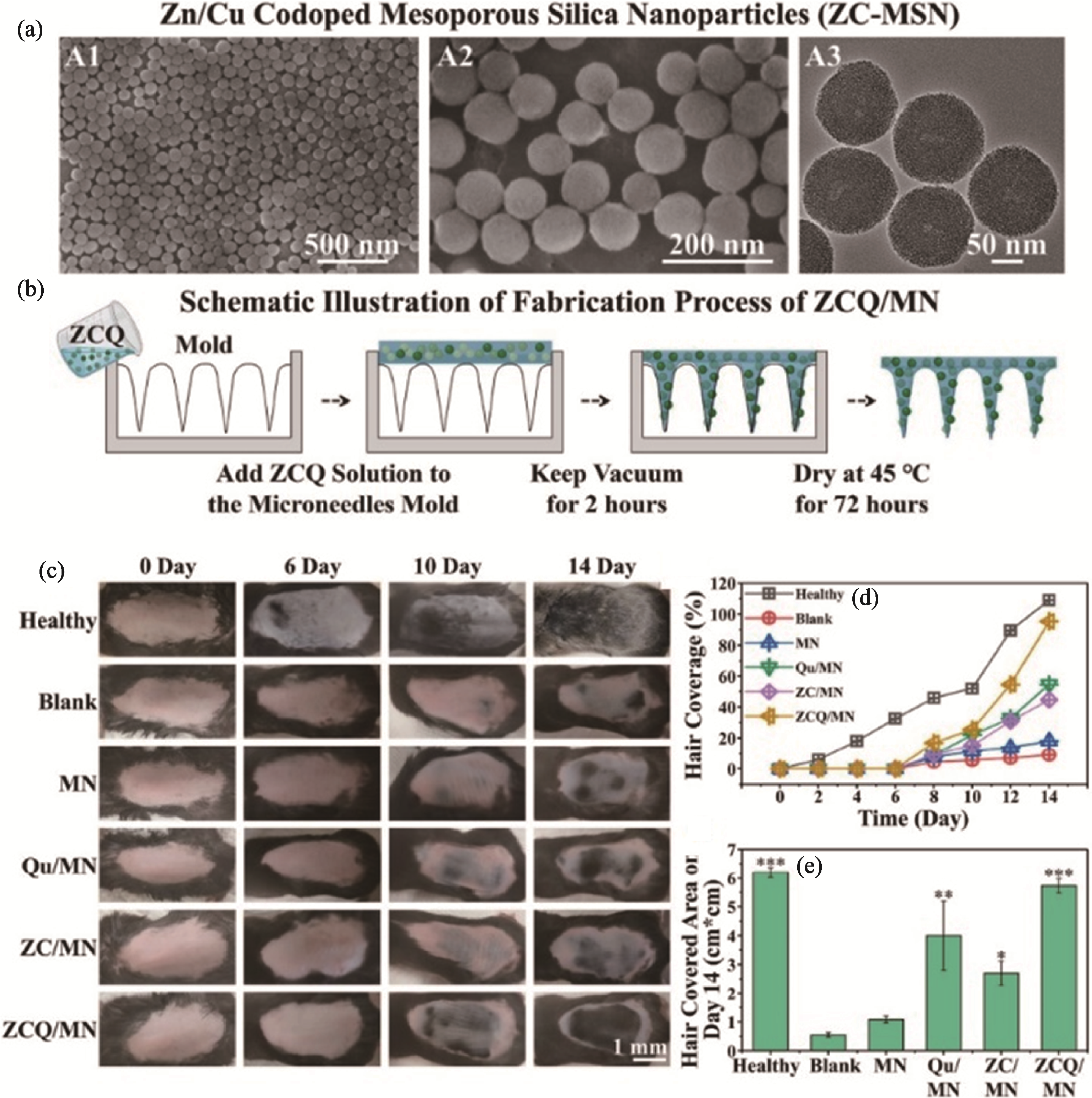
Fig. 4 ZCQ/MN microneedle patch for AGA treatment[51] (a) SEM images of Cu/Zn dual-doped mesoporous silica nanoparticles; (b) Schematic diagram of the preparation process of ZCQ/MN microneedle patch; (c) Gross photos of mice on 0, 6, 10, and 14 days after different microneedle treatments; (d) Statistics of hair coverage rate on the murine skin during 14 days; (e) Hair-covered area on the back of mice in each group after 14 days MN: pure microneedle; Qu/MN: quercetin-loaded microneedle; ZC/MN: microneedle loaded with copper/zinc dual-doped mesoporous silica;ZCQ/MN: microneedle containing copper/zinc dual-doped mesoporous silica nanoparticles loaded with quercetin
| [1] | GURTNER G C, WERNER S, BARRANDON Y, et al. Wound repair and regeneration. Nature, 2008, 453(7193): 314. |
| [2] | MARTIN P. Wound healing-aiming for perfect skin regeneration. Science, 1997, 276(5309): 75. |
| [3] | ITO M, COTSARELIS G. Is the hair follicle necessary for normal wound healing. Journal of Investigative Dermatology, 2008, 128(5): 1059. |
| [4] | TAYLOR G, LEHRER M S, JENSEN P J, et al. Involvement of follicular stem cells in forming not only the follicle but also the rpidermis. Cell, 2000, 102(4): 451. |
| [5] | JAHODA C A B, REYNOLDS A J. Hair follicle dermal sheath cells: unsung participants in wound healing. The Lancet, 2001, 358(9291): 1445. |
| [6] |
GAY D, KWON O, ZHANG Z, et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nature Medicine, 2013, 19(7): 916.
DOI PMID |
| [7] |
PRICE V H.Treatment of hair loss. New England Journal of Medicine, 1999, 341(13): 964.
DOI PMID |
| [8] |
LI M, MARUBAYASHI A, NAKAYA Y, et al. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: possible involvement of sulfonylurea receptor 2B as a target of minoxidil. Journal of Investigative Dermatology, 2001, 117(6): 1594.
PMID |
| [9] |
LACHGAR S, CHARVERON M, GALL Y, et al. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. British Journal of Dermatology, 1998, 138(3): 407.
DOI PMID |
| [10] |
STEINER J F. Clinical pharmacokinetics and pharmacodynamics of finasteride. Clinical Pharmacokinetics, 1996, 30(1): 16.
PMID |
| [11] | YUE Z, YANG F, ZHANG J, et al. Regulation and dysregulation of hair regeneration: aiming for clinical application. Cell Regeneration, 2022, 11(1): 22. |
| [12] | YUAN A R, BIAN Q, GAO J Q. Current advances in stem cell-based therapies for hair regeneration. European Journal of Pharmacology, 2020, 881: 173197. |
| [13] | GENTILE P, GARCOVICH S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: Wnt pathway, growth- factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells, 2019, 8(5): 466. |
| [14] |
MA H, FENG C, CHANG J, et al. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomaterialia, 2018, 79: 37.
DOI PMID |
| [15] | KARGOZAR S, HAMZEHLOU S, BAINO F. Can bioactive glasses be useful to accelerate the healing of epithelial tissues. Materials Science and Engineering: C, 2019, 97: 1009. |
| [16] | KUMAR R, MOHANTY S. Hydroxyapatite: a versatile bioceramic for tissue engineering application. Journal of Inorganic and Organometallic Polymers and Materials, 2022, 32(12): 4461. |
| [17] | SCHATKOSKI V M, MONTANHEIRO T L D, DE MENEZES B R C,et al. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceramics International, 2021, 47(3): 2999. |
| [18] |
HOPPE A, GÜLDAL N S, BOCCACCINI A R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials, 2011, 32(11): 2757.
DOI PMID |
| [19] | BROKESH A M, GAHARWAR A K. Inorganic biomaterials for regenerative medicine. ACS Applied Materials & Interfaces, 2020, 12(5): 5319. |
| [20] | MAZZONI E, IAQUINTA M R, LANZILLOTTI C, et al. Bioactive materials for soft tissue repair. Frontiers in Bioengineering and Biotechnology, 2021, 9: 613787. |
| [21] |
KOSTER M I, ROOP D R. Mechanisms regulating epithelial stratification. Annual Review of Cell and Developmental Biology, 2007, 23: 93.
PMID |
| [22] | MATHES S H, RUFFNER H, GRAF-HAUSNER U. The use of skin models in drug development. Advanced Drug Delivery Reviews, 2014, 69/70: 81. |
| [23] |
RODRIGUES M, KOSARIC N, BONHAM C A, et al. Wound healing: a cellular perspective. Physiological Reviews, 2019, 99(1): 665.
DOI PMID |
| [24] |
ROUSSELLE P, BRAYE F, DAYAN G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Advanced Drug Delivery Reviews, 2019, 146: 344.
DOI PMID |
| [25] | SCHLAKE T. Determination of hair structure and shape. Seminars in Cell & Developmental Biology, 2007, 18(2): 267. |
| [26] | DUVERGER O, MORASSO M I. To grow or not to grow: hair morphogenesis and human genetic hair disorders. Seminars in Cell & Developmental Biology, 2014, 25/26: 22. |
| [27] |
HIGGINS C A, WESTGATE G E, JAHODA C A B. From telogen to exogen: mechanisms underlying formation and subsequent loss of the hair club fiber. Journal of Investigative Dermatology, 2009, 129(9): 2100.
DOI PMID |
| [28] |
KULESSA H, TURK G, HOGAN B L M. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO Journal, 2000, 19(24): 6664.
PMID |
| [29] | ROMPOLAS P, DESCHENE E R, ZITO G, et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature, 2012, 487(7408): 496. |
| [30] |
GU Y, BIAN Q, ZHOU Y, et al. Hair follicle-targeting drug delivery strategies for the management of hair follicle-associated disorders. Asian Journal of Pharmaceutical Sciences, 2022, 17(3): 333.
DOI PMID |
| [31] | PLONKA P M, HANDJISKI B, POPIK M, et al. Zinc as an ambivalent but potent modulator of murine hair growth in vivo - preliminary observations. Experimental Dermatology, 2005, 14(11): 844. |
| [32] | PLONKA P M, HANDJISKI B, MICHALCZYK D, et al. Oral zinc sulphate causes murine hair hypopigmentation and is a potent inhibitor of eumelanogenesis in vivo. British Journal of Dermatology, 2006, 155(1): 39. |
| [33] | HAN B, FANG W H, ZHAO S, et al. Zinc sulfide nanoparticles improve skin regeneration. Nanomedicine: Nanotechnology, Biology and Medicine, 2020, 29: 102263. |
| [34] | NOZARI M, GHOLIZADEH M, OGHANI F Z, et al. Studies on novel chitosan/alginate and chitosan/bentonite flexible films incorporated with ZnO nano particles for accelerating dermal burn healing: in vivo and in vitro evaluation. International Journal of Biological Macromolecules, 2021, 184: 235. |
| [35] | KHALID A, KHAN R, UL-ISLAM M, et al. Bacterial cellulose- zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydrate Polymers, 2017, 164: 214. |
| [36] |
ZHANG Y, CHANG M L, BAO F, et al. Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale, 2019, 11(13): 6315.
DOI PMID |
| [37] |
ZHANG Z, LI W, LIU Y, et al. Design of a biofluid-absorbing bioactive sandwich-structured Zn-Si bioceramic composite wound dressing for hair follicle regeneration and skin burn wound healing. Bioactive Materials, 2021, 6(7): 1910.
DOI PMID |
| [38] | YU J, XU Y Z, ZHANG Z W B, et al. Strontium zinc silicate bioceramic composite electrospun fiber membrane for hair follicle regeneration in burn wounds. Composites Part B: Engineering, 2023, 266: 110953. |
| [39] |
ELIN R J. Magnesium: the fifth but forgotten electrolyte. American Journal of Clinical Pathology, 1994, 102(5): 616.
PMID |
| [40] | VOLPE S L. Magnesium in disease prevention and overall health. Advances in Nutrition, 2013, 4(3): 378S. |
| [41] |
ZANDI N, DOLATYAR B, LOTFI R, et al. Biomimetic nanoengineered scaffold for enhanced full-thickness cutaneous wound healing. Acta Biomaterialia, 2021, 124: 191.
DOI PMID |
| [42] | LIN X, LI Y, LUO W, et al. Leucine-activated nanohybrid biofilm for skin regeneration via improving cell affinity and neovascularization capacity. Journal of Materials Chemistry B, 2020, 8(35): 7966. |
| [43] | XU S, ZHANG Y, DAI B, et al. Green-prepared magnesium silicate sprays enhance the repair of burn-skin wound and appendages regeneration in rats and minipigs. Advanced Functional Materials, 2024, 34(9): 2307439. |
| [44] | HIGGINS C A, CHEN J C, CERISE J E, et al. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proceedings of the National Academy of Sciences, 2013, 110(49): 19679. |
| [45] |
MA J, QIN C, WU J, et al. 3D multicellular micropatterning biomaterials for hair regeneration and vascularization. Materials Horizons, 2023, 10(9): 3773.
DOI PMID |
| [46] |
YUAN A, XIA F, BIAN Q, et al. Ceria nanozyme-integrated microneedles reshape the perifollicular microenvironment for androgenetic alopecia treatment. ACS Nano, 2021, 15(8): 13759.
DOI PMID |
| [47] | MARTIN F, LINDEN T, KATSCHINSKI D M, et al. Copper- dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood, 2005, 105(12): 4613. |
| [48] | WANG P, PENG L, LIN J, et al. Enzyme hybrid virus-like hollow mesoporous CuO adhesive hydrogel spray through glucose- activated cascade reaction to efficiently promote diabetic wound healing. Chemical Engineering Journal, 2021, 415: 128901. |
| [49] | ZHANG Z W B, CHANG D, ZENG Z, et al. CuCS/Cur composite wound dressings promote neuralized skin regeneration by rebuilding the nerve cell "factory" in deep skin burns. Materials Today Bio, 2024, 26: 101075. |
| [50] | ZHANG Z W B, DAI Q X, ZHANG Y, et al. Design of a multifunctional biomaterial inspired by ancient Chinese medicine for hair regeneration in burned skin. ACS Applied Materials & Interfaces, 2020, 12(11): 12489. |
| [51] | ZHANG Z, LI W, CHANG D, et al. A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual- doped mesoporous silica nanocomposite microneedle patch. Bioactive Materials, 2023, 24: 81. |
| [52] | MA B, DANG W T, YANG Z B, et al. MoS2 Nanoclusters-based biomaterials for disease-impaired wound therapy. Applied Materials Today, 2020, 20: 100735. |
| [53] | WU J, MA J, ZHUANG H, et al. 3D bioprinting of calcium molybdate nanoparticles-containing immunomodulatory bioinks for hair regrowth. Nano Today, 2023, 51: 101917. |
| [54] | MA J, WU J, ZHANG H, et al. 3D Printing of diatomite incorporated composite scaffolds for skin repair of deep burn wounds. International Journal of Bioprinting, 2022, 8(3): 163. |
| [55] |
ABOLGHASEMZADE S, POURMADADI M, RASHEDI H, et al. PVA based nanofiber containing CQDs modified with silica NPs and silk fibroin accelerates wound healing in a rat model. Journal of Materials Chemistry B, 2021, 9(3): 658.
DOI PMID |
| [56] | ZHANG Z, ZHANG Y, LI W, et al. Curcumin/Fe-SiO2 nano composites with multi-synergistic effects for scar inhibition and hair follicle regeneration during burn wound healing. Applied Materials Today, 2021, 23: 101065. |
| [57] | LADEMANN J, KNORR F, RICHTER H, et al. Hair follicles as a target structure for nanoparticles. Journal of Innovative Optical Health Sciences, 2015, 8(4): 1530004. |
| [58] | LADEMANN J, RICHTER H, TEICHMANN A, et al. Nanoparticles - an efficient carrier for drug delivery into the hair follicles. European Journal of Pharmaceutics and Biopharmaceutics, 2007, 66(2): 159. |
| [59] |
MAK W C, PATZELT A, RICHTER H, et al. Triggering of drug release of particles in hair follicles. Journal of Controlled Release, 2012, 160(3): 509.
DOI PMID |
| [60] |
PATZELT A, RICHTER H, KNORR F, et al. Selective follicular targeting by modification of the particle sizes. Journal of Controlled Release, 2011, 150(1): 45.
DOI PMID |
| [61] | DARVIN M E, KÖNIG K, KELLNER-HOEFER M, et al. Safety assessment by multiphoton fluorescence/second harmonic generation/ hyper-rayleigh scattering tomography of ZnO nanoparticles used in cosmetic products. Skin Pharmacology and Physiology, 2012, 25(4): 219. |
| [62] | FILIPE P, SILVA J N, SILVA R, et al. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacology and Physiology, 2009, 22(5): 266. |
| [63] |
YANG D, CHEN M, SUN Y, et al. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomaterialia, 2021, 121: 119.
DOI PMID |
| [64] | YANG G, CHEN G, GU Z. Transdermal drug delivery for hair regrowth. Molecular Pharmaceutics, 2021, 18(2): 483. |
| [65] | HU C, CHU C, LIU L, et al. Dissecting the microenvironment around biosynthetic scaffolds in murine skin wound healing. Science Advances, 2021, 7(22): eabf0787. |
| [66] | OCAMPO-GARZA S S, FABBROCINI G, OCAMPO-CANDIANI J, et al. Micro needling: a novel therapeutic approach for androgenetic alopecia, a review of literature. Dermatologic Therapy, 2020, 33(6): e14267. |
| [67] | KIM S, EUM J, YANG H, et al. Transdermal finasteride delivery via powder-carrying microneedles with a diffusion enhancer to treat androgenetic alopecia. Journal of Controlled Release, 2019, 316: 1. |
| [68] | FANG J H, LIU C H, HSU R S, et al. Transdermal composite microneedle composed of mesoporous iron oxide nanoraspberry and PVA for androgenetic alopecia treatment. Polymers, 2020, 12(6): 1392. |
| [69] | KARGOZAR S, SINGH R K, KIM H W, et al. “Hard” ceramics for “soft” tissue engineering: paradox or opportunity. Acta Biomaterialia, 2020, 115: 1. |
| [70] |
BHARDWAJ N, KUNDU S C. Electrospinning: a fascinating fiber fabrication technique. Biotechnology Advances, 2010, 28(3): 325.
DOI PMID |
| [71] | XIE X, CHEN Y, WANG X, et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. Journal of Materials Science & Technology, 2020, 59: 243. |
| [72] |
JAHROMI M A M, ZANGABAD P S, BASRI S M M, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Advanced Drug Delivery Reviews, 2018, 123: 33.
DOI PMID |
| [73] | DO A V, KHORSAND B, GEARY S M, et al. 3D printing of scaffolds for tissue regeneration applications. Advanced Healthcare Materials, 2015, 4(12): 1742. |
| [74] | WANG X, TANG M. Bioceramic materials with ion-mediated multifunctionality for wound healing. Smart Medicine, 2022, 1(1): e20220032. |
| [75] |
MURPHY S V, ATALA A. 3D bioprinting of tissues and organs. Nature Biotechnology, 2014, 32(8): 773.
DOI PMID |
| [76] |
MANDRYCKY C, WANG Z J, KIM K, et al. 3D bioprinting for engineering complex tissues. Biotechnology Advances, 2016, 34(4): 422.
DOI PMID |
| [77] | MATAI I, KAUR G, SEYEDSALEHI A, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials, 2020, 226: 119536. |
| [78] | CHOUHAN D, DEY N, BHARDWAJ N, et al. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials, 2019, 216: 119267. |
| [79] | CUBO N, GARCIA M, DEL CANIZO J F, et al. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication, 2017, 9: 015006. |
| [80] | PEDDE R D, MIRANI B, NAVAEI A, et al. Emerging biofabrication strategies for engineering complex tissue constructs. Advanced Materials, 2017, 29(19): 201606061. |
| [81] |
AUGUSTINE R. Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks. Progress in Biomaterials, 2018, 7(2): 77.
DOI PMID |
| [82] | CORREIA M, LOPES J, LOPES D, et al. Nanotechnology-based techniques for hair follicle regeneration. Biomaterials, 2023, 302: 122348. |
| [83] |
PATZELT A, LADEMANN J. Recent advances in follicular drug delivery of nanoparticles. Expert Opinion on Drug Delivery, 2020, 17(1): 49.
DOI |
| [84] | COSTA C, CAVACO-PAULO A, MATAMÁ T. Mapping hair follicle-targeted delivery by particle systems: what has science accomplished so far. International Journal of Pharmaceutics, 2021, 610: 121273. |
| [85] | DONG R, GUO B. Smart wound dressings for wound healing. Nano Today, 2021, 41: 101290. |
| [86] | MA J, WU C. Bioactive inorganic particles-based biomaterials for skin tissue engineering. Exploration, 2022, 2(5): 20210083. |
| [1] | YU Shengyang, SU Haijun, JIANG Hao, YU Minghui, YAO Jiatong, YANG Peixin. A Review of Pore Defects in Ultra-high Temperature Oxide Ceramics by Laser Additive Manufacturing: Formation and Suppression [J]. Journal of Inorganic Materials, 2025, 40(9): 944-956. |
| [2] | LIU Jiangping, GUAN Xin, TANG Zhenjie, ZHU Wenjie, LUO Yongming. Research Progress on Catalytic Oxidation of Nitrogen-containing Volatile Organic Compounds [J]. Journal of Inorganic Materials, 2025, 40(9): 933-943. |
| [3] | XIAO Xiaolin, WANG Yuxiang, GU Peiyang, ZHU Zhenrong, SUN Yong. Advances in Regulation of Damaged Skin Regeneration by Two-dimensional Inorganic Materials [J]. Journal of Inorganic Materials, 2025, 40(8): 860-870. |
| [4] | ZHANG Hongjian, ZHAO Ziyi, WU Chengtie. Inorganic Biomaterials on Regulating Neural Cell Function and Innervated Tissue Regeneration: A Review [J]. Journal of Inorganic Materials, 2025, 40(8): 849-859. |
| [5] | AI Minhui, LEI Bo. Micro-nanoscale Bioactive Glass: Functionalized Design and Angiogenic Skin Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 921-932. |
| [6] | WANG Yutong, CHANG Jiang, XU He, WU Chengtie. Advances in Silicate Bioceramic/Bioglass for Wound Healing: Effects, Mechanisms and Application Ways [J]. Journal of Inorganic Materials, 2025, 40(8): 911-920. |
| [7] | MA Wenping, HAN Yahui, WU Chengtie, LÜ Hongxu. Application of Inorganic Bioactive Materials in Organoid Research [J]. Journal of Inorganic Materials, 2025, 40(8): 888-900. |
| [8] | LUO Xiaomin, QIAO Zhilong, LIU Ying, YANG Chen, CHANG Jiang. Inorganic Bioactive Materials Regulating Myocardial Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 871-887. |
| [9] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [10] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [11] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [12] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [13] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [14] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [15] | CHEN Xi, YUAN Yuan, TAN Yeqiang, LIU Changsheng. Strategic Study on the Development of Inorganic Non-metallic Biomaterials [J]. Journal of Inorganic Materials, 2025, 40(5): 449-456. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||