|
|
Research Progress on Mott-Schottky Hydrogen Evolution Catalysts Based on Metal/Transition Metal Compounds
REN Xianpei, LI Chao, HU Qiwei, XIANG Hui, PENG Yuehong
2026 Vol. 41 (2): 137–149
 Abstract
Abstract(
317 )
 HTML
HTML(
8)
 PDF
PDF(5723KB)(
525
)
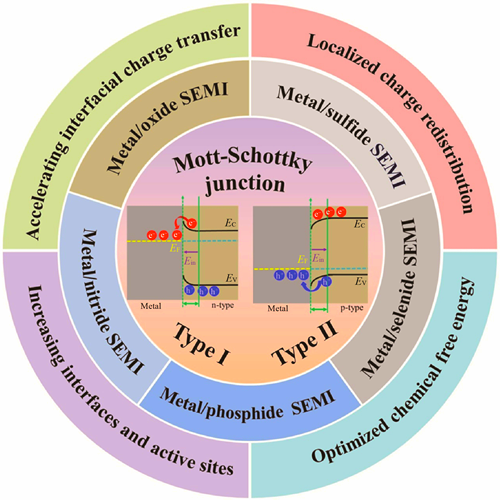
Hydrogen energy as an ideal energy carrier holds significant importance for promoting the transformation of energy structures. Electrolytic water splitting technology is crucial to achieve large-scale hydrogen production. The hydrogen evolution activity, stability and cost of catalysts are the key factors for its development. Transition metal compounds (TMCs) become popular candidates to replace noble-metal catalysts due to their low costs, abundant resources and tunable electronic structures. Mott-Schottky (M-S) junctions, constructed between transition metal compound semiconductors and metals, are considered to be an effective strategy to enhance catalytic activity. This review summarizes the research progress of metal/TMCs M-S junction catalysts, including their classification (metal/oxides, sulfides, selenides, phosphides, and nitrides, etc.), construction strategies (hydrothermal method, in-situ reduction, and phosphidation treatment, etc.), and enhancement mechanisms. Research findings indicate that the M-S junction optimizes their electronic structure and hydrogen adsorption free energy through interfacial charge rearrangement and their formation of built-in electric fields, thereby promoting charge separation and transfer and significantly enhancing hydrogen evolution activity. In addition, the review discusses the key issues that still need in-depth exploration and clarification regarding M-S junction catalysts, and provides an outlook on future research directions and development trends. It aims to offer theoretical guidance for design of efficient and cost-effective hydrogen evolution electrocatalysts and to promote sustainable development of hydrogen energy technology.
|
|
|
Recent Progress on Removal of Sr/Cs from Molten Salt in Dry Reprocessing
LIU Zhanyi, LI Mian, OUYANG Xiaoping, CHAI Zhifang, HUANG Qing
2026 Vol. 41 (2): 150–158
 Abstract
Abstract(
355 )
 HTML
HTML(
2)
 PDF
PDF(2260KB)(
98
)
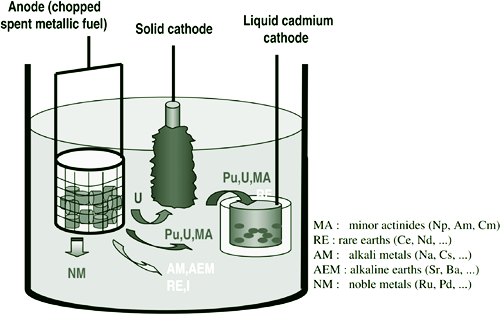
Dry reprocessing technology has advantages of irradiation resistance, proliferation resistance and simplified waste treatment, thereby rendering it a preferred technology for reprocessing of spent fuel of advanced fast reactors. Molten salt electrolytic refining serves as the core technology of dry reprocessing, primarily capitalizing on the difference in redox potential between actinides such as uranium and plutonium and other fissionable elements within a molten salt system. This technology facilitates separation and recovery of actinides. During the electrolytic refining process, lanthanide elements and fission elements, such as Sr and Cs, tend to accumulate within the molten salt, which can change the physicochemical properties of molten salt, thus seriously affecting the efficiency of electrolytic refining. In addition, fission products such as 90Sr and 137Cs are water-soluble and long-lived nuclides, posing significant environmental hazards if inadequately managed. Therefore, effective purification of fission elements such as Sr and Cs from molten salt is imperative, not only to improve the efficiency of dry reprocessing via molten salt electrolysis, but also as a crucial strategy to reduce the discharge of radioactive waste. This paper summarizes the current research status of Sr and Cs removal methods in molten salts, comparatively analyzes the separation principles and separation effects of different methods, such as electrolysis, crystallization, decompression distillation, precipitation, and ion exchange. Furthermore, it explores prospective direction of development and potential applicability of various material systems.
|
|
|
Research Progress on Zero-dimensional Metal Halide Scintillators towards Radiation Detection Applications
SUN Lian, ZHANG Leilei, XUE Zexu, WU Kun, CHEN Ye, LI Zhiyuan, WANG Lukai, WANG Zungang
2026 Vol. 41 (2): 159–176
 Abstract
Abstract(
263 )
 HTML
HTML(
5)
 PDF
PDF(11903KB)(
2509
)
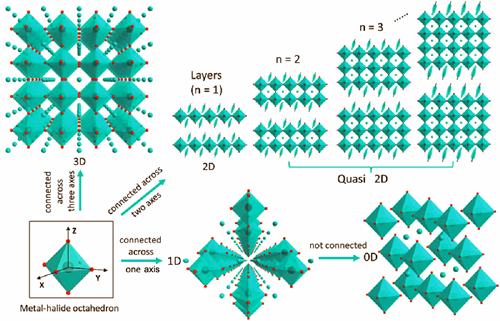
Scintillators are key materials for radiation detection field. They have wide applications in many fields such as high-energy physics, medical diagnostics, astronomy, radioactivity exploration, and homeland security. However, most of the reported scintillators (e.g. NaI:Tl and LaBr3:Ce) can hardly provide a perfect combination of high light yield and energy resolution, excellent ambient stability and low cost. It is urgent to discover novel scintillators with outstanding comprehensive performance and ideal cost. Zero-dimensional (0D) metal halides, possessing abundant advantages such as high light yield, weak self-absorption, strong ambient adaptability, and considerable stability under irradiation, are good candidates for the next-generation scintillators. This review consolidates the recent research progress on 0D metal halide scintillators towards applications in radiation detection. Firstly, the basic properties as well as the scintillation mechanism of 0D metal halides are analyzed at molecular scale, especially their unique self-trapped exciton emission characteristic. Then, some typical 0D metal halides which show excellent radiation detection properties are systematically introduced, containing Pb-, Cu-, Mn-, and Sn-based structures, and their key scintillation parameters are comprehensively compared. Furthermore, their applications in X-ray imaging, gamma-ray spectrum measurement, and neutron detection are well discussed. Last, the challenges and opportunities in development of 0D metal halide scintillators towards radiation detection are prospected. In the future, researchers should be committed to providing solutions to the issues such as growth of large-scale, defect-less and highly transparent single crystals, deep and reasonable explanation of scintillation mechanism, and creation of standard for the methods of performance measurement towards various kinds of scintillators.
|
|
|
2D/2D Coupled ZnIn2S4/TiO2 Heterojunction and Its Enhanced Photocatalytic Reduction of CO2
ZHU Jianhua, YANG Xin, RU Lingjie
2026 Vol. 41 (2): 177–185
 Abstract
Abstract(
297 )
 HTML
HTML(
7)
 PDF
PDF(1801KB)(
1437
)
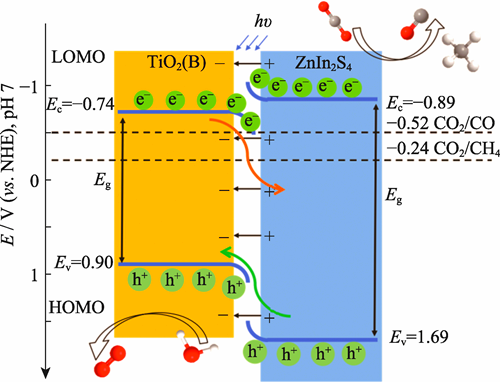
As a typical photocatalytic material, titanium dioxide (TiO2) has been widely applied in environmental remediation and energy conversion due to its excellent chemical stability, non-toxicity and low cost. However, its wide bandgap structure restricts light absorption to ultraviolet wavelengths, and severe recombination of photogenerated electron-hole pairs in bulk materials limits quantum efficiency. Here, a hydrothermal method was used to construct a novel 2D/2D coupled ZnIn2S4 (ZIS)@TiO2 composite material. This heterojunction consists of ultrathin TiO2 nanocages composited with ZIS nanosheets, exhibiting a unique hollow core-shell morphology. ZIS-T20 catalyst with composite 20 mg TiO2 nanocages demonstrates significantly enhanced light absorption across a broad wavelength range of 400-720 nm. Under influence of the built-in electric field, photo-generated electrons cannot migrate from conduction band (CB) of ZIS to that of TiO2, whereas the transfer of holes from valence band (VB) of ZIS to that of TiO2 proceeds unimpededly. This spatial separation effect preserves electrons with high reduction potential in ZIS, overcoming inherent drawback of reduced redox capability in conventional type-I heterojunctions. In photocatalytic CO2 reduction (PCR) reaction, ZIS@TiO2 exhibits improved performance, achieving CO and CH4 production rates of 58.87 and 12.03 μmol·g-1·h-1, respectively, with CO selectivity as high as 83.03%. Compared to individual components, the CO yield is 6.15 and 1.96 times higher than that of pristine ZIS and TiO2, respectively. This work not only provides a new strategy for designing efficient 2D/2D heterojunction photocatalysts, but also offers valuable insights into understanding interfacial charge transfer mechanisms.
|
|
|
Regulating Perovskite Film Crystallization via Organic Amine Salts for Enhanced Photoelectric Conversion Efficiency and Stability
JIANG Jun, YANG Gonglü, YANG Yufan, LI Yi, YUAN Ningyi, DING Jianning
2026 Vol. 41 (2): 186–192
 Abstract
Abstract(
328 )
 HTML
HTML(
3)
 PDF
PDF(6136KB)(
371
)
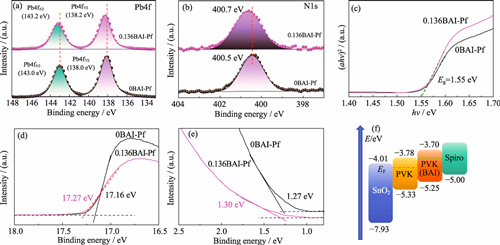
Currently, photoelectric conversion efficiency (PCE) of perovskite solar cells (PSCs) has reached 27%, yet their industrialization remains constrained by film quality and stability issues in perovskite layers. This study employed butylammonium iodide (BAI) as additive to regulate crystal orientation in perovskite films through retarded crystallization kinetics and induced preferred orientation growth, resulting in significantly enlarged grain sizes and reduced defect densities. Benefiting from the intrinsic high hydrophobicity of organic ammonium salts, optimized films demonstrated enhanced environmental tolerance. Consequently, achieved PCE of rigid devices improved from 22.32% to 23.46% with notably suppressed hysteresis, while that of flexible perovskite solar cells (F-PSCs) improved from 21.51% to 22.26%, confirming the strategy's universality across substrates. Stability tests demonstrated that treatment of perovskite film with BAI led to simultaneous improvements in the environmental stability, thermal stability and light stability of PSCs, as well as the mechanical stability of F-PSCs. This work provides a novel solution for enhancing crystallization control and stability in perovskite films, offering new insights for developing high-performance perovskite photovoltaics with significant industrial application potential.
|
|
|
High-entropy Doping Modification of High-nickel Single-crystal Layered Ternary Cathode Material
HOU Jiabing, HU Qiang, CUI Jiuzhi, HUANG Yundi, WANG Xinrui, LIU Xingquan
2026 Vol. 41 (2): 193–200
 Abstract
Abstract(
258 )
 HTML
HTML(
4)
 PDF
PDF(5471KB)(
859
)
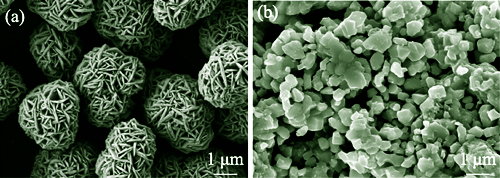
LiNixCoyMn1-x-yO2 (NCM, x≥0.8), a promising cathode material for lithium-ion batteries, possesses high energy density and excellent discharge performance. However, it also appears drawbacks such as severe cation disorder, poor cycling performance and insecurity at high temperatures and high cut-off voltages. In this study, a high-entropy (HE) doping modification strategy was introduced into a high-nickel and low-cobalt LiNi0.86Co0.04Mn0.1O2 cathode material, and a high-nickel single-crystal layered ternary cathode material was synthesized by a high-temperature solid-state reaction method. The results show that at 0.1C (1C=180 mA·g-1) and 25 ℃, the battery equipped with modified material has a reversible discharge capacity of 197 mAh·g-1 and exhibits excellent discharge performance at high temperatures and high cut-off voltages. At 0.5C, 55 ℃ and 4.3 V, the discharge capacity reaches 281 mAh·g-1, while at 0.5C, 25 ℃ and 4.5 V, the discharge capacity is as high as 194 mAh·g-1. This material has a good layered structure with a uniform arrangement of nanoscale primary particles at microscale and a smaller total impedance. This work significantly improves cycling performance and safety of high-nickel ternary cathode materials at high temperatures and high cut-off voltages, providing a good modification method for the cobalt-free, high-nickel and practical application of ternary materials.
|
|
|
Organic-Inorganic Composite Scintillators Loaded with LiF-CaF2:Eu Eutectic Powder: Preparation and Characterization
ZHOU Qi, LI Xiang, ZHANG Kaihui, WANG Zeliang, DENG Mingxue, JIA Wenbao, WANG Ke, CHEN Junfeng
2026 Vol. 41 (2): 201–207
 Abstract
Abstract(
198 )
 HTML
HTML(
4)
 PDF
PDF(2101KB)(
220
)
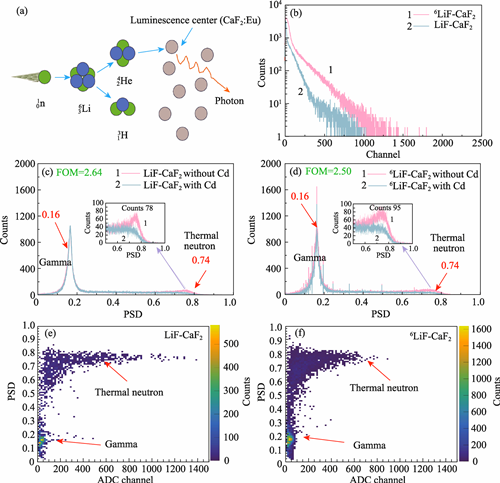
Thermal neutron technology is widely applied in many fields, such as homeland security, nuclear non-proliferation, nuclear energy development, industrial nondestructive testing, and nuclear physics research. However, existing commercially available thermal neutron detection materials currently still face many developmental challenges in terms of detection performance and manufacturing costs. In this study, a series of organic-inorganic composite scintillators sensitive to thermal neutrons was prepared via solid-state reaction by uniformly dispersing 0-20% (in mass) of LiF-CaF2:Eu eutectic powder in organic polystyrene matrixes. X-ray diffraction patterns, scanning electron microscope morphologies, and elemental distributions of the synthesized LiF-CaF2:Eu eutectic powder were analyzed. The radioluminescence and optical transmittance of the prepared composite scintillators were evaluated, and comparisons were made between LiF-CaF2:Eu eutectic powder and LiF-CaF2:Eu mixed powder as inorganic additives. Using cadmium difference method and pulse shape discrimination technologies, the thermal neutron detection performance of composite scintillators loaded with LiF-CaF2:Eu (natural Li abundance) and 6LiF-CaF2:Eu (95% enriched 6Li) eutectic powder was systematically investigated. Compared to composites filled with LiF-CaF2:Eu mixed powder, those filled with LiF-CaF2:Eu eutectic powder exhibited better optical transmittance and higher radioluminescence intensity, which increased with the addition of more eutectic powder. Increasing the abundance of 6Li can effectively improve thermal neutron detection efficiency, and figure of merit for thermal neutron/gamma discrimination in composite scintillators can reach 2.64. As a promising novel scintillator for thermal neutron detection, the composite scintillators loaded with LiF-CaF2:Eu eutectic powder show excellent thermal neutron detection and thermal neutron/gamma discrimination.
|
|
|
3D Network-structured Fly Ash Microbeads@Carbon Nanotubes Composites for Electromagnetic Wave Absorption
ZHANG Xiaomin, TONG Liangyu, GAO Hongjie, CHEN Xu, YAN Huhu, GAO Yang
2026 Vol. 41 (2): 208–216
 Abstract
Abstract(
309 )
 HTML
HTML(
2)
 PDF
PDF(7557KB)(
121
)
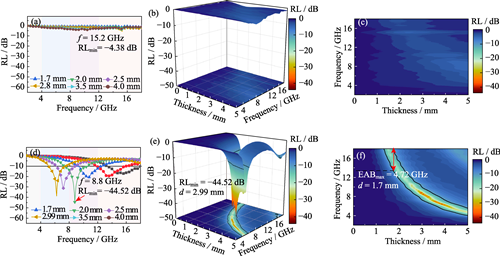
With rapid development of 5G communication and miniaturization of electronic devices, development of lightweight, broadband and high-efficiency electromagnetic wave absorbing materials has emerged as a critical solution to challenges posed by electromagnetic pollution and information leakage. However, traditional absorbing materials face significant limitations, such as high density, narrow absorption bandwidth and poor environmental compatibility, while the resource utilization of industrial solid waste provides an innovative path for the design of high-performance absorbing materials with both economic and ecological benefits. Here, magnetic fly ash (MFA) microbeads were derived from solid waste of coal-fired power plants through magnetic separation. Additionally, magnetic fly ash microbeads@carbon nanotubes (MFA@CNTs) composite wave-absorbing materials with a three-dimensional interpenetrating network structure were successfully prepared by chemical vapor deposition (CVD) using in situ-loaded Fe-based nanoparticles on surface of the MFA microbeads as catalysts. Microstructural characterization showed that the bamboo-like CNTs grown on surface of the MFA microbeads formed a porous structure by inter tubular winding and bridging with silicate framework. The composite material achieves a minimum reflection loss (RLmin) of -44.52 dB at 8.8 GHz (with a thickness of 2.99 mm) and an effective absorption bandwidth (EAB, RL<-10 dB) covering 4.72 GHz (with a thickness of 1.7 mm). The performance enhancement mechanism can be attributed to the following aspects. (1) The magnetic component (Fe3O4) in the MFA microbeads interacts with conductive network of the CNTs, thereby establishing a magneto-electrical coupling effect to optimize the impedance matching. (2) The defective structure of bamboo-like CNTs induces multiple polarized relaxation (including interfacial and dipole polarizations), significantly enhancing the dielectric loss. (3) The 3D porous network extends the propagation path of electromagnetic wave, thereby promoting multiple reflection and scattering loss. This study not only provides a new paradigm for high-value utilization of industrial solid waste, but also lays a theoretical and technical foundation for the design of lightweight and broadband wave-absorbing materials.
|
|
|
Influence of Grain Size on Weibull Distribution of Fracture Strength in Atmospheric-pressure Solid-phase Sintered SiC Ceramics
CAO Juan, WU Xishi, LIU Zehua, PEI Bingbing, HAN Jianshen, LIU Huan, YANG Yitian, WU Haibo, HUANG Zhengren
2026 Vol. 41 (2): 217–224
 Abstract
Abstract(
241 )
 HTML
HTML(
2)
 PDF
PDF(3068KB)(
129
)
Silicon carbide (SiC) ceramics have found extensive application in strategic fields, such as semiconductor technology, nuclear energy, aerospace engineering, and marine engineering, due to their remarkable properties, which encompass excellent mechanical properties, resistance to high-temperature creep, acid and alkali corrosion, and high thermal conductivity. However, the fracture strength of these brittle ceramic materials typically exhibits significant discreteness, which adversely affects reliability and limits their wider application as engineering structural materials. In this work, reliability of fracture strength in solid-state sintered silicon carbide (SSiC) ceramics was enhanced through regulation of grain size. The influence of grain size on mechanical properties, Weibull distribution of fracture strength, and crack extension resistance curve (R-curve) characteristics of SSiC ceramics was systematically evaluated. Reliability regulatory mechanism for fracture strength of SSiC ceramics was analyzed. The results indicated that, with an increase in sintering temperature from 2100 ℃ to 2200 ℃, average grain size of SSiC ceramics increased from 3.01 µm to 8.45 µm, while coefficient of grain size distribution uniformity dropped from 0.70 to 0.62. As the average grain size was reduced from 8.45 µm to 3.01 µm, Weibull modulus of fracture strength for SSiC ceramics increased gradually from 8.5 to 12.2, representing a 44% increment. This clearly indicates the positive impact of grain refinement on reliability of fracture strength. Enhancement in Weibull modulus of fracture strength as a result of grain refinement can primarily be attributed to high-density grain boundary network, which effectively mitigates stress concentration via crack bifurcation and bridging mechanisms. Additionally, uniformity of grain distribution and reduced defect size contribute to an elevated energy threshold for crack propagation, leading to an ascending R-curve behavior. This work achieves a significant improvement in the fracture strength reliability of SiC ceramics through regulating grain size, which is expected to promote the wider engineering application of SiC ceramic materials.
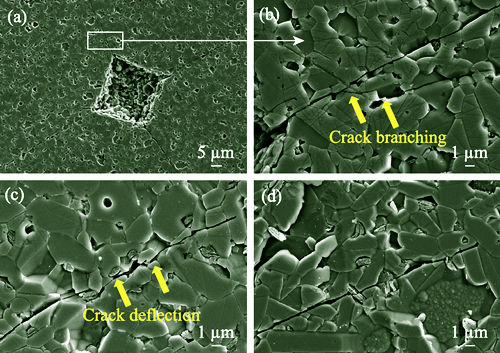
|
|
|
Functionalized Quantum Dot Fluorescent Probes with Dopamine Quinone: Construction and pH Response
WANG Zheng, HOU Xiaoqi, LIU Xuanyong
2026 Vol. 41 (2): 225–233
 Abstract
Abstract(
217 )
 HTML
HTML(
2)
 PDF
PDF(6045KB)(
301
)
Quantum dots (QDs) have demonstrated significant potential in the field of biological detection due to their excellent photoluminescence properties. As one of the key parameters in regulating physiological functions, pH response with high sensitivity is of great significance. However, conventional pH fluorescent probes are frequently limited by insufficient sensitivity or poor stability. In this work, band gap engineering was used to design and construct a CdSe/CdS/ZnS core-shell structured QD to improve the fluorescence quantum yield and stability. Additionally, the modification with mercaptoethylamine (MEA) and dopamine-isothiocyanate (DA-ITC) results in improved QD fluorescent probe exhibiting a highly sensitive pH response. Experimental results indicate that the amino-protected CdSe/CdS/ZnS QDs possess excellent optical properties. Following modification with DA-ITC, the probe exhibits highly sensitive pH response. The underlying response mechanism is attributed to fluorescence quenching effect of the dopamine quinone (DQ), which is formed by oxidation in surface ligands on the QDs under alkaline conditions. For 1 nmol QDs, when the input amount of DA-ITC is in the range of 4-40 μg/nmol, the fluorescence intensity of the probe reveals a linear decreasing trend with increasing pH from 5.0 to 10.0 (R2>0.90). Notably, when the addition of DA-ITC is set at 20 μg/nmol, the probe demonstrates optimal pH responsiveness (R2=0.9869). Moreover, this probe has good cytocompatibility, allowing for effective application in fluorescence imaging for monitoring of cell pH.
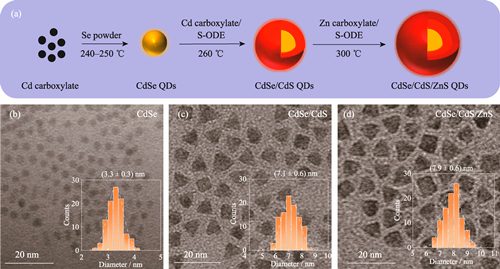
|
|
|
Ti3C2Tx Piezoelectric Composite Hydrogels for Bacterial-infected Skin Wound Healing
NIE Xiaoshuang, LI Dandan, WANG Fang, OUYANG Liping, LI Heng, QIU Jiajun
2026 Vol. 41 (2): 234–244
 Abstract
Abstract(
280 )
 HTML
HTML(
1)
 PDF
PDF(23694KB)(
796
)
Effective control of bacterial infection, alongside the simultaneous promotion of angiogenesis, represents a significant challenge for accelerating healing of infected wounds. Development of a multifunctional hydrogel wound dressing with both antibacterial and angiogenesis-promoting functions is of great research value. In this study, Ti3C2Al was used as the precursor, and Ti3C2Tx MXene nanosheets were prepared by selective chemical etching. These nanosheets were then integrated into a dynamic crosslinking network of injectable poly(vinyl alcohol) (PVA)/cationic guar gum (CGG) hydrogel to construct a PVA/CGG/MXene (PCM) composite hydrogel with ultrasound response properties. Experimental results showed that the quaternary ammonium cationic group in the CGG molecular chain significantly enhanced antibacterial performance of the PCM hydrogel through electrostatic effect, whose antibacterial rates against S. aureus and E. coli reached 97.34% and 95.40%, respectively. The MXene nanosheets endowed the PCM hydrogel with stable electrical conductivity and piezoelectric properties. Under low-frequency ultrasound stimulation, the PCM hydrogel could generate an electrical signal, which in turn promoted cell proliferation, migration and vascular regeneration. Rat whole-layer skin infection wound model confirmed that PCM hydrogel significantly accelerated the wound healing process by increasing antibacterial, promoting angiogenesis and collagen deposition, even almost completely healing within 10 d. This study presents a smart hydrogel dressing that integrates ultrasound-responsive electrical stimulation, antibacterial and angiogenesis-promoting, offering an innovative new therapeutic strategy for repairing infected wounds.
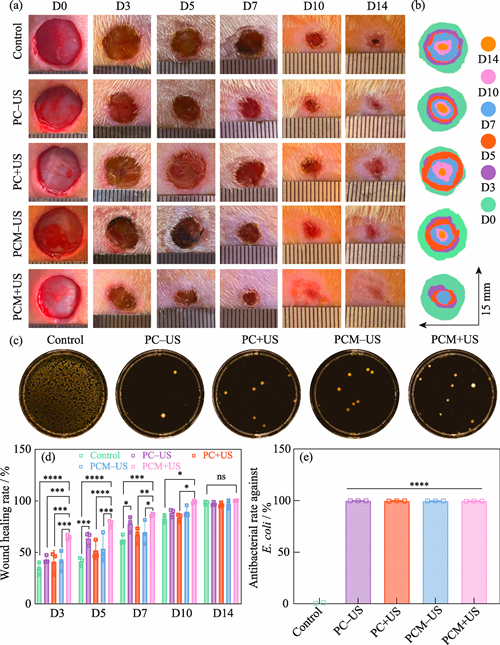
|
|
|
CeO2 Clusterzymes: Biomimetic Synthesis and Treatment for Acute Liver Injury
YAN Mijia, ZHANG Jiale, ZHANG Qiuhong, CHEN Hangrong
2026 Vol. 41 (2): 245–252
 Abstract
Abstract(
199 )
 HTML
HTML(
3)
 PDF
PDF(2104KB)(
319
)
Acetaminophen (APAP) overdose is a common cause of acute liver injury (ALI) in clinical settings, characterized by accumulation of reactive oxygen species (ROS) and infiltration of inflammatory cells. In this study, CeO2 clusterzymes (named as CeCs) with ultra-small size (around 1.3 nm) was synthesized by a biomimetic mineralization process. The obtained CeCs presented a high oxygen vacancy content (52.6%) and a high Ce3+/Ce4+ ratio (1.06), showing excellent adsorption and removal abilities to various ROS (including free radicals), which could be used for hepatocyte protection. In vivo animal experiments further confirmed that CeCs provided highly effective therapeutic intervention for APAP-induced ALI, extending the therapeutic window, and reversing sterile inflammation, underscoring their potential for clinical application.
|
|
|
A Rectifier Bridge Circuit Based on Metal-semiconductor-metal Fin Tunneling Diode for High-frequency Application
DENG Hengyang, QIN Cuijie, HAO Shenglan, FENG Guangdi, ZHU Qiuxiang, TIAN Bobo, CHU Junhao, DUAN Chungang
2026 Vol. 41 (2): 253–261
 Abstract
Abstract(
283 )
 HTML
HTML(
1)
 PDF
PDF(1951KB)(
165
)
Tunneling diodes hold significant promise for future rectification in the terahertz (THz) and visible light spectra, thanks to their femtosecond-scale transit-time tunneling capabilities. In this work, TiN/ZnO/Pt fin tunneling diodes (FTDs) with tunneling distances of 10 and 5 nm are fabricated, which demonstrate remarkable characteristics, including ultrahigh asymmetry (1.6×104 for 10 nm device and 1.6×103 for 5 nm device), high responsivity (25.3 V-1 for 10 nm device and 28.3 V-1 for 5 nm device) at zero bias, surpassing the thermal voltage limit of conventional Schottky diodes, and low turn-on voltage (Von) of approximately 100 mV for both devices, making them ideal for power conversion applications. Using technology computer-aided design (TCAD) simulations, the observed asymmetry in electronic transport is attributed to the transition between Fowler-Nordheim tunneling (FNT) and trap-assisted tunneling (TAT) under different biasing conditions, as illustrated by the corresponding energy band profiles. Furthermore, by integrating the FTDs, a rectifier bridge circuit is designed and exhibits full-wave rectification behavior, validated through SPICE simulations for THz-band operations. This advancement offers a highly efficient solution for THz-band energy conversion and effective detection applications.
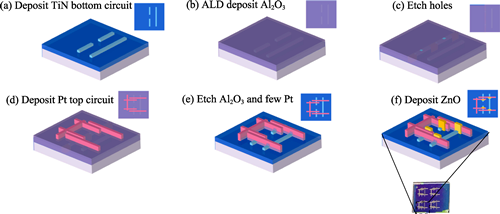
|
|
|
High Temperature Resistant Calcium-doped Silica Aerogels with Enhanced Thermal Insulation via Sol-Gel Hydrothermal Route
LI Hao, QI Yuan, GAO Xiangdong, ZHANG Xingxing, WANG Jinmin
2026 Vol. 41 (2): 262–272
 Abstract
Abstract(
509 )
 HTML
HTML(
3)
 PDF
PDF(5252KB)(
139
)
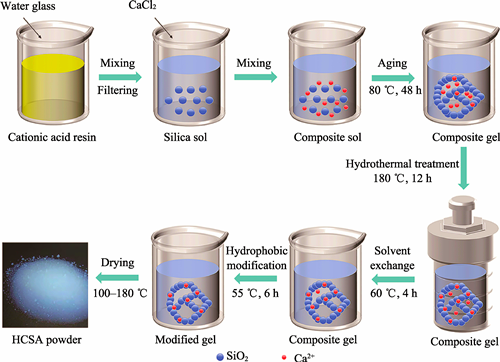
Silica aerogel has broad applications in the field of high-temperature thermal insulation due to its low density, low thermal conductivity and high stability. However, its thermal insulation performance deteriorates significantly at elevated temperatures exceeding 600 ℃, primarily due to the collapse of pore structure. Meanwhile, the shielding capacity of SiO2 aerogel to the infrared radiation at high temperature is rather low due to the intrinsic properties of SiO2. Herein, a strategy for improving the high-temperature stability and infrared shielding properties of SiO2 aerogel via Ca doping was explored. Calcium-doped silica aerogel (CSA) powders were prepared by Sol-Gel, hydrothermal, and ambient pressure drying (APD) techniques using water glass and anhydrous calcium chloride as precursors and trimethylchlorosilane as a hydrophobic modifier. The effects of Ca/Si molar ratio in the precursor and hydrothermal conditions (temperature and pH) on the crystalline properties, microscopic morphology and pore structure of CSAs were investigated. The results show that the Ca/Si molar ratio and hydrothermal treatment have significant effects on the microstructure and heat resistance of CSAs in the temperature range of 400-1000 ℃. The samples sintered at 1000 ℃ have a high specific surface area of 100.1 m2/g and a pore volume of 0.8705 cm3/g, indicating that the CSA has good heat resistance. One-side insulation tests at temperatures up to 600 ℃ show that the sample with a Ca/Si molar ratio of 1.0 has the best insulation performance, with a cold surface temperature of 450 ℃, which is 27 ℃ lower than that of the pure silica aerogel.
|
|