
Journal of Inorganic Materials ›› 2019, Vol. 34 ›› Issue (1): 17-26.DOI: 10.15541/jim20180211
Special Issue: MAX相和MXene材料; 环境材料优选论文; 优秀作者论文集锦; 2019~2020年度优秀作者作品欣赏:环境材料
• REVIEW • Previous Articles Next Articles
WANG Xiang-Xue, YU Shu-Jun, WANG Xiang-Ke
Received:2018-05-07
Revised:2018-06-08
Published:2019-01-21
Online:2018-12-17
About author:WANG Xiang-Xue. E-mail: xxwang@ncepu.edu.cn
CLC Number:
WANG Xiang-Xue, YU Shu-Jun, WANG Xiang-Ke. Removal of Radionuclides by Metal-organic Framework-based Materials[J]. Journal of Inorganic Materials, 2019, 34(1): 17-26.

Fig. 1 (1) SEM images, (2) XRD patterns, (3) FT-IR spectra, (4) N2 sorption isotherms[26] of MIL-101 and its amino derivatives, (a) MIL-101; (b) MIL-101-NH2; (c) MIL-101-ED; (d) MIL-101-DETA

Fig. 2 (a) UV-Vis absorption spectra of TcO4- during the anion exchange; (b) Sorption kinetics of TcO4- by SCU-101 compared with Purolite A530E and A532E; (c) Sorption isotherms of ReO4- by SCU-101, Mg-Al-LDH, and NDTB-1; (d) Effect of competing anions on the removal percentage of TcO4- by SCU-101; (e) Effect of SO42- on the anion exchange of ReO4- by SCU-101; (f) Removal percentage of ReO4- after irradiation as compared with the original SCU-101 sample[28]
| Adsorbents | Radionuclides | (m/V)/(g·L-1) | C0/(mg·L-1) | t/h | pH | Qmax/(mg·g-1) | Interaction mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| MIL-101 | U(VI) | 0.4 | 100 | 2 | 5.5 | 20 | Surface complexation | [26] |
| MIL-101-NH2 | U(VI) | 0.4 | 100 | 2 | 5.5 | 90 | Surface complexation | [26] |
| MIL-101-ED | U(VI) | 0.4 | 100 | 2 | 5.5 | 200 | Surface complexation | [26] |
| MIL-101-DETA | U(VI) | 0.4 | 100 | 2 | 5.5 | 350 | Surface complexation | [26] |
| GO-COOH/UiO-66 | U(VI) | 0.5 | 95 | 4 | 8.0 | 188 | Surface complexation and ion exchange | [30] |
| SCU-101 | Re(IV) | 1.0 | 1000 | 0.2 | - | 217 | Ion exchange | [28] |
| SCU-100 | Re(IV) | 1.0 | 28 | 2 | - | 541 | Ion exchange | [29] |
| UiO-66-(COOH)2 | Th(IV) | 0.4 | 100 | 6 | 3.0 | 350 | Surface complexation | [31] |
| MOF-808-SO4 | Ba(II) | 1.0 | 42 | 0.1 | 5.8 | 131 | Surface complexation | [32] |
| UiO-66-Schiff | Co(II) | 0.1 | 10 | 5 | 8.4 | 256 | Surface complexation | [33] |
| FJSM-InMOF | Sr(II) | 2.5 | 18 | 12 | - | 44 | Ion exchange | [34] |
| FJSM-InMOF | Cs(I) | 2.5 | 90 | 3 | - | 199 | Ion exchange | [34] |
| LDO-C | U(VI) | 0.1 | 50 | 4 | 5.0 | 354 | Surface complexation and ion exchange | [35] |
| CS@LDH | U(VI) | 0.2 | 41 | 3 | 5.0 | 157 | Surface complexation | [36] |
| GO | Co(II) | 0.1 | 10 | 4 | 5.0 | 44 | Surface complexation | [37] |
| LDH | U(VI) | 0.2 | 50 | 6 | 4.5 | 69 | Surface complexation and electrostatic interaction | [38] |
| Na-montmorillonite | Ni(II) | 0.5 | 10 | 6 | 6.0 | 13 | Surface complexation and ion exchange | [39] |
| Fe3O4@TNS | U(VI) | 0.2 | 20 | 8 | 5.0 | 83 | Ion exchange | [40] |
Table 1 Radionuclides adsorption on different materials
| Adsorbents | Radionuclides | (m/V)/(g·L-1) | C0/(mg·L-1) | t/h | pH | Qmax/(mg·g-1) | Interaction mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| MIL-101 | U(VI) | 0.4 | 100 | 2 | 5.5 | 20 | Surface complexation | [26] |
| MIL-101-NH2 | U(VI) | 0.4 | 100 | 2 | 5.5 | 90 | Surface complexation | [26] |
| MIL-101-ED | U(VI) | 0.4 | 100 | 2 | 5.5 | 200 | Surface complexation | [26] |
| MIL-101-DETA | U(VI) | 0.4 | 100 | 2 | 5.5 | 350 | Surface complexation | [26] |
| GO-COOH/UiO-66 | U(VI) | 0.5 | 95 | 4 | 8.0 | 188 | Surface complexation and ion exchange | [30] |
| SCU-101 | Re(IV) | 1.0 | 1000 | 0.2 | - | 217 | Ion exchange | [28] |
| SCU-100 | Re(IV) | 1.0 | 28 | 2 | - | 541 | Ion exchange | [29] |
| UiO-66-(COOH)2 | Th(IV) | 0.4 | 100 | 6 | 3.0 | 350 | Surface complexation | [31] |
| MOF-808-SO4 | Ba(II) | 1.0 | 42 | 0.1 | 5.8 | 131 | Surface complexation | [32] |
| UiO-66-Schiff | Co(II) | 0.1 | 10 | 5 | 8.4 | 256 | Surface complexation | [33] |
| FJSM-InMOF | Sr(II) | 2.5 | 18 | 12 | - | 44 | Ion exchange | [34] |
| FJSM-InMOF | Cs(I) | 2.5 | 90 | 3 | - | 199 | Ion exchange | [34] |
| LDO-C | U(VI) | 0.1 | 50 | 4 | 5.0 | 354 | Surface complexation and ion exchange | [35] |
| CS@LDH | U(VI) | 0.2 | 41 | 3 | 5.0 | 157 | Surface complexation | [36] |
| GO | Co(II) | 0.1 | 10 | 4 | 5.0 | 44 | Surface complexation | [37] |
| LDH | U(VI) | 0.2 | 50 | 6 | 4.5 | 69 | Surface complexation and electrostatic interaction | [38] |
| Na-montmorillonite | Ni(II) | 0.5 | 10 | 6 | 6.0 | 13 | Surface complexation and ion exchange | [39] |
| Fe3O4@TNS | U(VI) | 0.2 | 20 | 8 | 5.0 | 83 | Ion exchange | [40] |
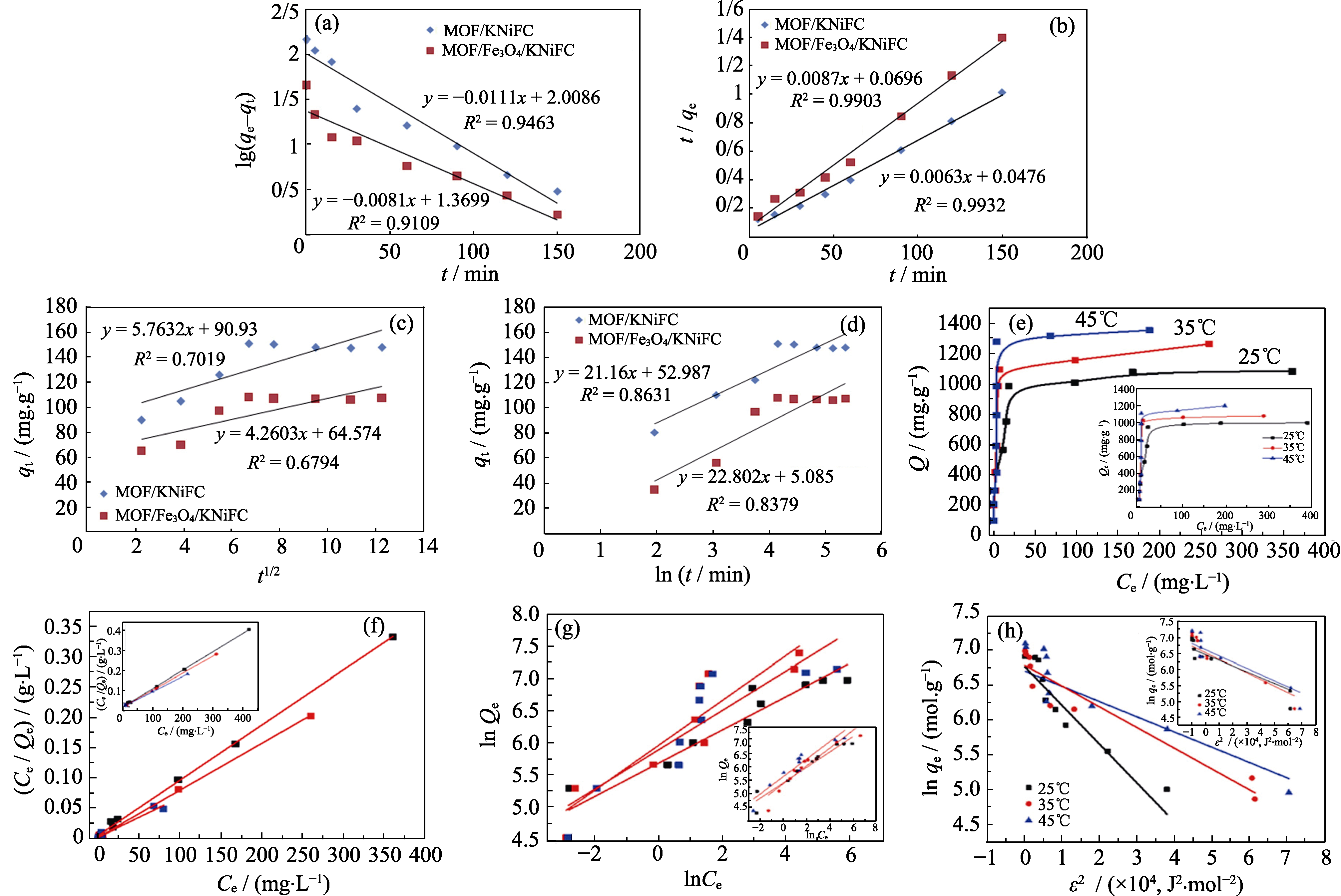
Fig. 3 Linear pseudo-first-order kinetic (a), pseudo-second-order (b), intraparticle diffusion (c) and elovich equation (d) for adsorption of Cs+ on MOF/KNiFC and MOF/Fe3O4/KNiFC[44]; (e) Isotherm model of U(VI) adsorption on UiO-66 (inset) and GO-COOH/UiO-66 composites; (f) Langmuir model, (g) Freundlich model, and (h) Dubinin-Radushkevich model[30]
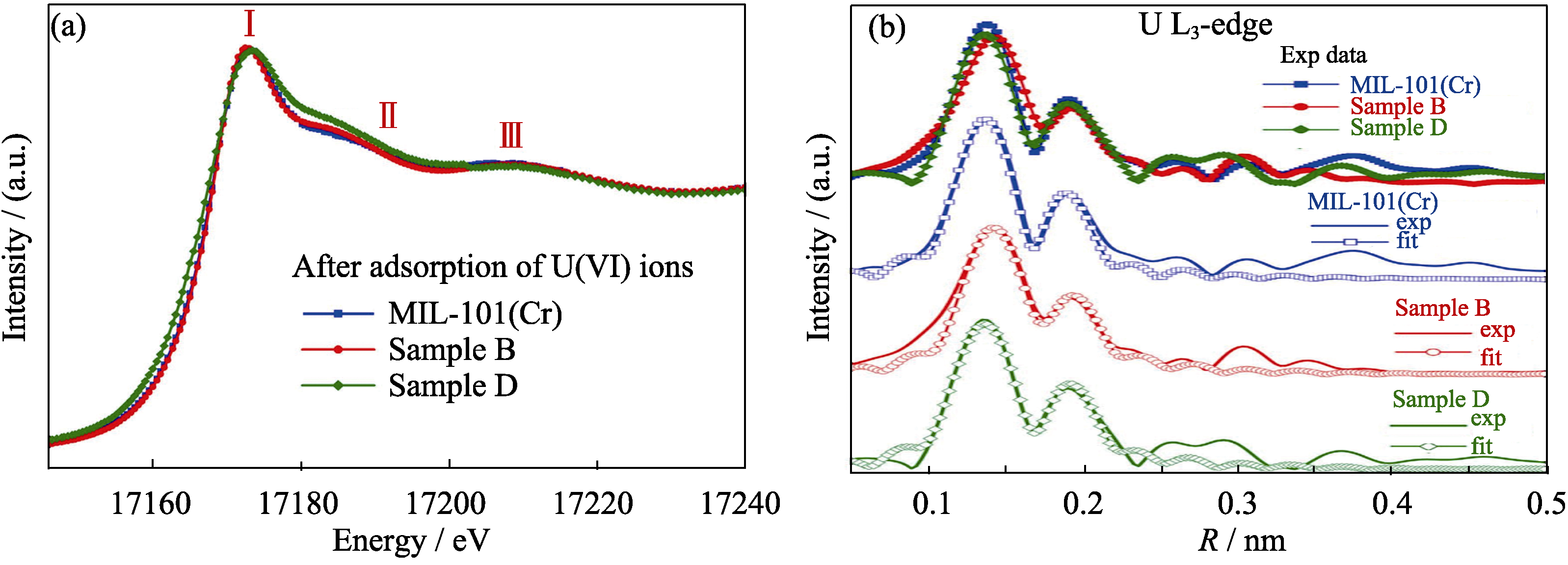
Fig. 4 (a) Comparison of experimental U L3-edge XANES spectra for pristine MIL-101(Cr), and different ED contents grafting ED-MIL-101(Cr) samples after the adsorption of U(VI), (b) Experimental Fourier transform of the U L3-edge EXAFS data for different samples and their corresponding fits[54]
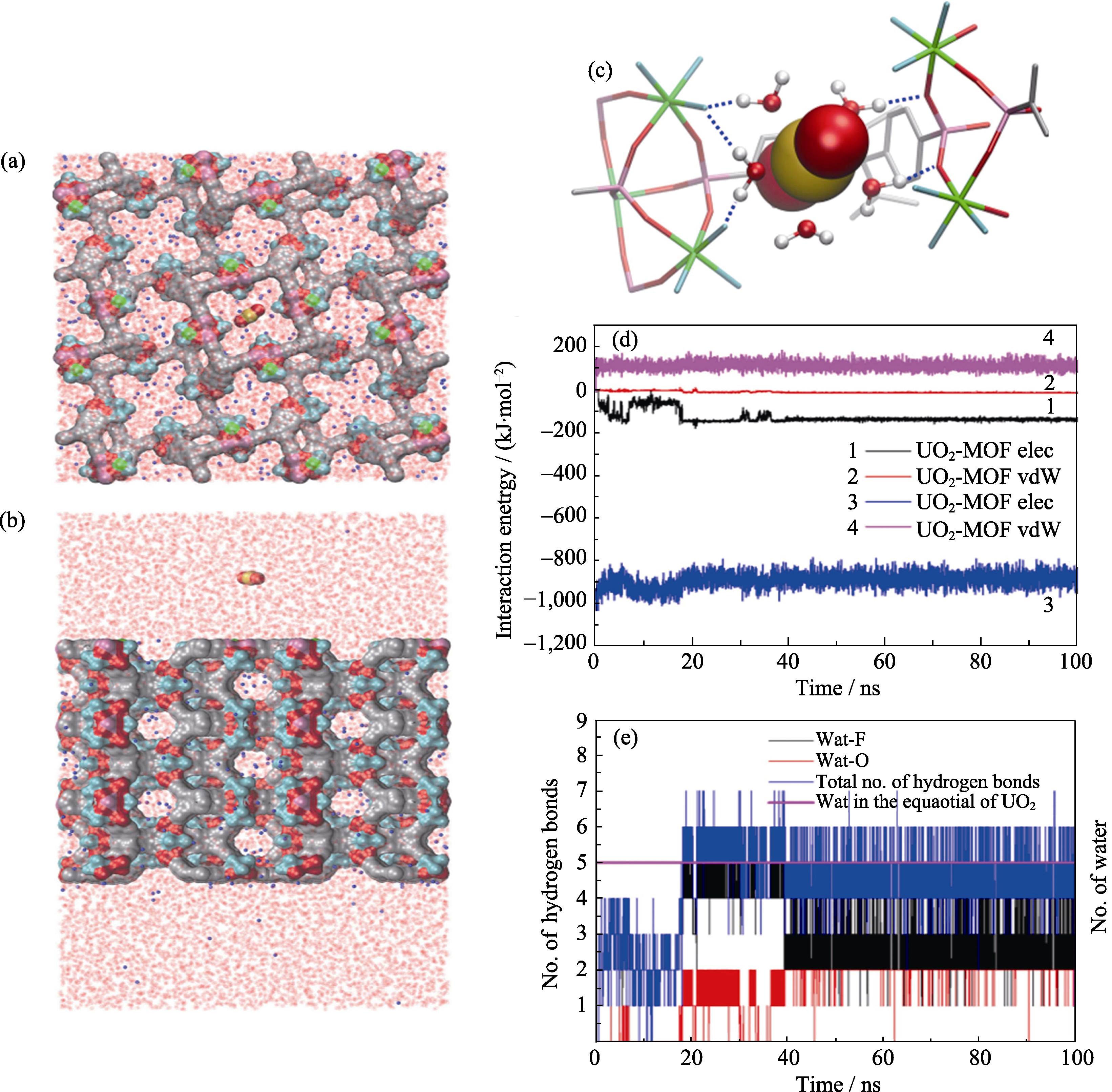
Fig. 5 MD simulations on the process of uranyl sorption into SZ-2. The top (a) and side (b) view of the simulation system-1 (uranyl cation approaching along the c axis); (c) The final snapshot (at t ¼ 100 ns) of run 1 (out of total 6) to show the importance of equatorial water of uranyl cation in mediating its binding to the SZ-2 (the blue dash line indication the hydrogen bond between equatorial water molecules and the dangling hydrogen bond acceptors); (d) Time evolution of the electrostatic and vdW interaction energies of uranyl cation with SZ-2 and water; (e) The number of equatorial water molecules of uranyl cation (pink curve) and the number of hydrogen bonds formed between equatorial coordinating water molecules and other acceptors (including F and O in main framework) as the function of simulation time[58]
| 技术 | 主要目的 | 优点 | 缺点 |
|---|---|---|---|
| 宏观实验 | 反应达到平衡所需时间, 最大吸附量, 选择性和影响因素[ | 非常直观得到实验结果, 方便和有效 | 无法得到分子和原子水平上的作用机理 |
| XPS分析 | 元素氧化态、元素种类和几乎所有元素的键合关系(除了H和He) | 定量分析、元素组成分析、高表面灵敏度检测(1~10 nm) | 在真空中进行的测量, 可能改变样品的性质; 在元素个数比值高于0.05%~ 1.0%条件下进行, 依赖于元素的性质 |
| XAFS分析 | 氧化态、配位数、原子间键距离以及目标离子周围的离子状态[ | 特定的元素, 并且总是可以检测到的, 对于研究非晶体材料是有用的; 吸附物种的分析 | 无法区分原子能相差较小的原子(C、N、O或S、Cl、Mn或Fe)[ |
| FT-IR分析 | 对微米范围内吸附行为的研究(光密度≥10-5) | 灵敏检测官能团和极性键[ | 定性而不是定量, 灵敏度低 |
| DFT计算 | 键能、键长、轨道和系统电荷密度[ | 对局部环境的吸附描述和原子级吸附过程的描述[ | 优化结构之间的能量与长时间模拟结果较不准确 |
| 分子动力学模拟 | 位置、势能和宏观现象的预测[ | 吸附过程的快照在几秒内发生[ | 长时间的计算时间, 依赖于计算的性能 |
Table 2 The main purpose, advantages and disadvantages of main adsorption characterization techniques mentioned above
| 技术 | 主要目的 | 优点 | 缺点 |
|---|---|---|---|
| 宏观实验 | 反应达到平衡所需时间, 最大吸附量, 选择性和影响因素[ | 非常直观得到实验结果, 方便和有效 | 无法得到分子和原子水平上的作用机理 |
| XPS分析 | 元素氧化态、元素种类和几乎所有元素的键合关系(除了H和He) | 定量分析、元素组成分析、高表面灵敏度检测(1~10 nm) | 在真空中进行的测量, 可能改变样品的性质; 在元素个数比值高于0.05%~ 1.0%条件下进行, 依赖于元素的性质 |
| XAFS分析 | 氧化态、配位数、原子间键距离以及目标离子周围的离子状态[ | 特定的元素, 并且总是可以检测到的, 对于研究非晶体材料是有用的; 吸附物种的分析 | 无法区分原子能相差较小的原子(C、N、O或S、Cl、Mn或Fe)[ |
| FT-IR分析 | 对微米范围内吸附行为的研究(光密度≥10-5) | 灵敏检测官能团和极性键[ | 定性而不是定量, 灵敏度低 |
| DFT计算 | 键能、键长、轨道和系统电荷密度[ | 对局部环境的吸附描述和原子级吸附过程的描述[ | 优化结构之间的能量与长时间模拟结果较不准确 |
| 分子动力学模拟 | 位置、势能和宏观现象的预测[ | 吸附过程的快照在几秒内发生[ | 长时间的计算时间, 依赖于计算的性能 |
| [1] | AI Y J, LIU Y, LAN W Y, et al.The effect of pH on the U(VI) sorption on graphene oxide (GO): a theoretical study. Chemical Engineering Journal, 2018, 343: 460-466. |
| [2] | PANG H W, WANG X X, YAO W, et al.Removal of radionuclides by metal oxide materials and mechanism research. Scientia Sinica Chimica, 2018, 48: 58-73. |
| [3] | YANG S Y, WANG X X, CHEN Z S, et al.Synthesis of Fe3O4-based nanomaterials and their application in the removal of radionuclides and heavy metal ions. Progress in Chemistry, 2018, 30(2/3): 225-242. |
| [4] | YU S J, WANG X X, PANG H W, et al.Boron nitride-based materials for the removal of pollutants from aqueous solutions: a review. Chemical Engineering Journal, 2018, 333: 343-360. |
| [5] | YU S J, WANG X X, YANG S T, et al.Interaction of radionuclides with natural and manmade materials using XAFS technique. Science China Chemistry, 2016, 60(2): 170-187. |
| [6] | LI X, LIU Y, ZHANG C L, et al.Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chemical Engineering Journal, 2018, 336: 241-252. |
| [7] | LIANG Y, GU P C, YAO W, et al.Adsorption of radionuclide uranium onto carbon-based nanomaterials from aqueous systems. Process in Chemistry, 2017, 29(9): 1062-1071. |
| [8] | DU Y C, WANG X K, HOU R Q, et al.In-situ growth of Nb2O5 nanorods on diatomite and highly effective removal of Cr(VI). Journal of Inorganic Materials, 2018, 33(5): 557-564. |
| [9] | CHEN H J, HUANG S Y, ZHANG Z B, et al.Synthesis of functional nanoscale zero-valent iron composites for the application of radioactive uranium enrichment from environment: a review. Acta Chimica Sinica, 2017, 75(6): 560-574. |
| [10] | DU Y, WANG J, WANG H Q, et al.Research on sorption mechanism of radionuclides by manufactured nanomaterials. Journal of Agro-Environment Science, 2016, 35: 1837-1847. |
| [11] | WANG X N, MENG H, MA F Y, et al.Influence of preparation method on oxidation degree of graphene oxide and adsorption for Th(IV) and U(VI). Journal of Inorganic Materials, 2016, 31(5): 454-460. |
| [12] | GU P C, XING J L, WEN T, et al.Experimental and theoretical calculation investigation on efficient Pb(II) adsorption on etched Ti3AlC2 nanofibers and nanosheets. Environmental Science: Nano, 2018, 5(4): 946-955. |
| [13] | YAO W, WU Y H, PANG H W, et al.In-situ reduction synthesis of manganese dioxide@polypyrrole core/shell nanomaterial for highly efficient enrichment of U(VI) and Eu(III). Science China Chemistry, 2018(7): 1-12. |
| [14] | SONG S, YIN L, WANG X X, et al.Interaction of U(VI) with ternary layered double hydroxides by combined batch experiments and spectroscopy study. Chemical Engineering Journal, 2018, 338: 579-590. |
| [15] | WANG P Y, YIN L, WANG X X, et al.L-cysteine intercalated layered double hydroxide for highly efficient capture of U(VI) from aqueous solutions. Journal of Environmental Management, 2018, 217: 468-477. |
| [16] | LIU W, DAI X, BAI Z L, et al.Highly sensitive and selective uranium detection in natural water systems using a luminescent mesoporous metal-organic framework equipped with abundant lewis basic sites: a combined batch, X-ray absorption spectroscopy, and first principles simulation investigation. Environmental Science & Technology, 2017, 51(7): 3911-3921. |
| [17] | LI J, WANG X X, ZHAO G X, et al.Metal-organic framework- based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chemical Society Reviews, 2018, 47(7): 2322-2356. |
| [18] | WU Y H, PANG H W, YAO W, et al.Synthesis of rod-like metal-organic framework (MOF-5) nanomaterial for efficient removal of U(VI): batch experiments and spectroscopy study. Science Bulletin, 2018, 63(13): 831-839. |
| [19] | DROUT R J, OTAKE K, HOWARTH A J, et al.Efficient capture of perrhenate and pertechnetate by a mesoporous Zr metal-organic framework and examination of anion binding motifs. Chemistry of Materials, 2018, 30(4): 1277-1284. |
| [20] | WANG Y L, LIU Z Y, LI Y X, et al.Umbellate distortions of the uranyl coordination environment result in a stable and porous polycatenated framework that can effectively remove cesium from aqueous solutions. Journal of America Chemistry Society, 2015, 137(19): 6144-6147. |
| [21] | BANERJEE D, KIM D, SCHWEIGER M J, et al.Removal of TcO4- ions from solution: materials and future outlook. Chemical Society Reviews, 2016, 45(10): 2724-2739. |
| [22] | PANG H W, HUANG S Y, WU Y H, et al.Efficient elimination of U(VI) by polyethyleneimine decorated fly ash. Inorganic Chemistry Frontiers, 2018, 5: 2399-2407 |
| [23] | YU S J, WANG X X, TAN X L, et al.Sorption of radionuclides from aqueous systems onto graphene oxide-based materials: a review. Inorganic Chemistry Frontiers, 2015, 2(7): 593-612. |
| [24] | DUAN L F, ZHANG Y, WANG L M, et al.Synthesis and characterization of MnFe2O4 with different morphologies and their application in water treatment. Journal of Inorganic Materials, 2014, 29(7): 763-768. |
| [25] | CARBONI M, ABNEY C W, LIU S B, et al.Highly porous and stable metal-organic frameworks for uranium extraction. Chemical Science, 2013, 4(6): 2396-2402. |
| [26] | BAI Z Q, YUAN L Y, ZHU L, et al.Introduction of amino groups into acid-resistant MOFs for enhanced U(VI) sorption. Journal of Materials Chemistry A, 2015, 3(2): 525-534. |
| [27] | LI L N, MA W, SHEN S S, et al.A combined experimental and theoretical study on the extraction of uranium by amino-derived metal-organic frameworks through post-synthetic strategy. ACS Applied Materials & Interfaces, 2016, 8(45): 31032-31041. |
| [28] | ZHU L, SHENG D P, XU C, et al.Identifying the recognition site for selective trapping of 99TcO4- in a hydrolytically stable and radiation resistant cationic metal-organic framework. Journal of America Chemistry Society, 2017, 139(42): 14873-14876. |
| [29] | SHENG D P, ZHU L, XU C, et al.Efficient and selective uptake of TcO4- by a cationic metal-organic framework material with open Ag+ sites. Environmental Science & Technology, 2017, 51(6): 3471-3479. |
| [30] | YANG P P, LIU Q, LIU J Y, et al.Interfacial growth of a metal-organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(VI). Journal of Materials Chemistry A, 2017, 5(34): 17933-17942. |
| [31] | ZHANG N, YUAN L Y, GUO W L, et al.Extending the use of highly porous and functionalized MOFs to Th(IV) capture. ACS Applied Materials & Interfaces, 2017, 9(30): 25216-25224. |
| [32] | PENG Y G, HUANG H L, LIU D H, et al.Radioactive barium ion trap based on metal-organic framework for efficient and irreversible removal of barium from nuclear wastewater. ACS Applied Materials & Interfaces, 2016, 8(13): 8527-8535. |
| [33] | YUAN G Y, TIAN Y, LIU J, et al.Schiff base anchored on metal-organic framework for Co(II) removal from aqueous solution. Chemical Engineering Journal, 2017, 326: 691-699. |
| [34] | GAO Y J, FENG M L, ZHANG B, et al.An easily synthesized microporous framework material for the selective capture of radioactive Cs+ and Sr2+ ions. Journal of Materials Chemistry A, 2018, 6(9): 3967-3976. |
| [35] | YAO W, WANG X X, LIANG Y, et al.Synthesis of novel flower-like layered double oxides/carbon dots nanocomposites for U(VI) and 241Am(III) efficient removal: batch and EXAFS studies. Chemical Engineering Journal, 2018, 332: 775-786. |
| [36] | WANG X X, YU S J, WU Y H, et al.The synergistic elimination of uranium(VI) species from aqueous solution using bi-functional nanocomposite of carbon sphere and layered double hydroxide. Chemical Engineering Journal, 2018, 342: 321-330. |
| [37] | WANG X X, LIU Y, PANG H W, et al.Effect of graphene oxide surface modification on the elimination of Co(II) from aqueous solutions. Chemical Engineering Journal, 2018, 344: 380-390. |
| [38] | YU S J, WANG J, SONG S, et al.One-pot synthesis of graphene oxide and Ni-Al layered double hydroxides nanocomposites for the efficient removal of U(VI) from wastewater. Science China Chemistry, 2017, 60(3): 415-422. |
| [39] | YU S J, WANG X X, CHEN Z S, et al.Interaction mechanism of radionickel on Na-montmorillonite: influences of pH, electrolyte cations, humic acid and temperature. Chemical Engineering Journal, 2016, 302: 77-85. |
| [40] | YIN L, SONG S, WANG X X, et al.Rationally designed core-shell and yolk-shell magnetic titanate nanosheets for efficient U(VI) adsorption performance. Environmental Pollution, 2018, 238: 725-738. |
| [41] | GU P C, ZHANG S, LI X, et al.Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environmental Pollution, 2018, 240: 493-505. |
| [42] | SONG W C, WANG X X, CHEN Z S, et al.Enhanced immobilization of U(VI) on Mucor circinelloides in presence of As(V): batch and XAFS investigation. Environmental Pollution, 2018, 237: 228-236. |
| [43] | TAN X L, FANG M, TAN L Q, et al.Core-shell hierarchical C@Na2Ti3O7·9H2O nanostructures for the efficient removal of radionuclides. Environmental Science: Nano, 2018, 5(5): 1140-1149. |
| [44] | NAEIMI S, FAGHIHIAN H.Performance of novel adsorbent prepared by magnetic metal-organic framework MOF modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution. Separation and Purification Technology, 2017, 175: 255-265. |
| [45] | ZHANG C L, LIU Y, LI X, et al.Highly uranium elimination by crab shells-derived porous graphitic carbon nitride: batch, EXAFS and theoretical calculations. Chemical Engineering Journal, 2018, 346: 406-415. |
| [46] | HU Y Z, WANG X X, ZOU Y D, et al.Superior sorption capacities of Ca-Ti and Ca-Al bimetallic oxides for U(VI) from aqueous solutions. Chemical Engineering Journal, 2017, 316: 419-428. |
| [47] | CHEN Z S, WANG J, PU Z X, et al.Synthesis of magnetic Fe3O4/CFA composites for the efficient removal of U(VI) from wastewater. Chemical Engineering Journal, 2017, 320: 448-457. |
| [48] | ZHANG C L, LI X, CHEN Z S, et al.Synthesis of ordered mesoporous carbonaceous materials and its highly efficient capture of uranium from solutions. Science China Chemistry, 2018, 61(3): 281-293. |
| [49] | SUN Y B, LU S H, WANG X X, et al.Plasma-facilitated synthesis of amidoxime/carbon nanofiber hybrids for effective enrichment of 238U(VI) and 241Am(III). Environmental Science & Technology, 2017, 51(21): 12274-12282. |
| [50] | WANG J, WANG X X, ZHAO G X, et al.Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium(VI) from aqueous solutions. Chemical Engineering Journal, 2018, 334: 569-578. |
| [51] | YANG D X, WANG X X, SONG G, et al.One-pot synthesis of arginine modified hydroxyapatite carbon microsphere composites for efficient removal of U(VI) from aqueous solutions. Science Bulletin, 2017, 62(23): 1609-1618. |
| [52] | SONG S, HUANG S Y, ZHANG R, et al.Simultaneous removal of U(VI) and humic acid on defective TiO2-x investigated by batch and spectroscopy techniques. Chemical Engineering Journal, 2017, 325: 576-587. |
| [53] | TAN L Q, TAN X L, MEI H Y, et al.Coagulation behavior of humic acid in aqueous solutions containing Cs+, Sr2+ and Eu3+: DLS, EEM and MD simulation. Environmental Pollution, 2018, 235: 835-843. |
| [54] | ZHANG J Y, ZHANG N, ZHANG L J, et al. Adsorption of uranyl ions on amine-functionalization of MIL-101(Cr) nanoparticles by a facile coordination-based post-synthetic strategy and X-ray absorption spectroscopy studies. Scientific Reports, 2015, 5: 13514- 1-10. |
| [55] | WEN H, PAN Z Z, GIAMMAR D E, et al.Enhanced uranium immobilization by phosphate amendment under variable geochemical and flow conditions: insights from reactive transport modeling. Environmental Science & Technology, 2018, 52(10): 5841-5850. |
| [56] | ZHANG Y J, LAN J H, WANG L, et al.Adsorption of uranyl species on hydroxylated titanium carbide nanosheet: a first-principles study. Journal of Hazardous Materials, 2016, 308: 402-410. |
| [57] | WANG L, YUAN L Y, CHEN K, et al.Loading actinides in multilayered structures for nuclear waste treatment: the first case study of uranium capture with vanadium carbide MXene. ACS Applied Materials & Interfaces, 2016, 8(25): 16396-16403. |
| [58] | ZHENG T, YANG Z X, GUI D X, et al. Overcoming the crystallization and designability issues in the ultrastable zirconium phosphonate framework system. Nature Communications, 2017, 8: 15369-1-11. |
| [59] | SHENG G D, SHAO D D, FAN Q H D, et al. Effect of pH and ionic strength on sorption of Eu(III) to MX-80 bentonite: batch and XAFS study. Radiochimica Acta, 2009, 97(11): 621-630. |
| [60] | TAN X L, REN X M, CHEN C L, et al.Analytical approaches to the speciation of lanthanides at solid-water interfaces. TrAC Trends in Analytical Chemistry, 2014, 61: 107-132. |
| [61] | LUO F, CHEN J L, DANG L L, et al.High-performance Hg2+ removal from ultra-low-concentration aqueous solution using both acylamide-and hydroxyl-functionalized metal-organic framework. Journal of Materials Chemistry A, 2015, 3(18): 9616-9620. |
| [62] | BANERJEE D, XU W Q, NIE Z M, et al.Zirconium-based metal-organic framework for removal of perrhenate from water. Inorganic Chemistry, 2016, 55(17): 8241-8243. |
| [63] | HOSKINS B F, ROBSON R.Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. Journal of the American Chemical Society, 1989, 111(15): 5962-5964. |
| [64] | NALAPARAJU A, JIANG J W.Ion exchange in metal-organic framework for water purification: insight from molecular simulation. The Journal of Physical Chemistry C, 2012, 116(12): 6925-6931. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | WEI Jianwen, ZHANG Lijuan, GENG Linlin, LI Yu, LIAO Lei, WANG Dunqiu. Novel CO2 Adsorbent Prepared with ZSM-5/MCM-48 as Support: High Adsorption Property and Its Mechanism [J]. Journal of Inorganic Materials, 2025, 40(7): 833-839. |
| [3] | JIANG Zongyu, HUANG Honghua, QING Jiang, WANG Hongning, YAO Chao, CHEN Ruoyu. Aluminum Ion Doped MIL-101(Cr): Preparation and VOCs Adsorption Performance [J]. Journal of Inorganic Materials, 2025, 40(7): 747-753. |
| [4] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [5] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [6] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [7] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [8] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [9] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [10] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [11] | HONG Peiping, LIANG Long, WU Lian, MA Yingkang, PANG Hao. Structure Regulation of ZIF-67 and Adsorption Properties for Chlortetracycline Hydrochloride [J]. Journal of Inorganic Materials, 2025, 40(4): 388-396. |
| [12] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [13] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [14] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [15] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||