
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (3): 233-258.DOI: 10.15541/jim20230386
• REVIEW • Next Articles
Received:2023-08-28
Revised:2023-09-29
Published:2024-03-20
Online:2023-11-28
Contact:
LI Xijun, professor. E-mail: lixijun@westlake.edu.cnAbout author:BAO Ke (1984-), female, PhD. E-mail: baoke@westlake.edu.cn
Supported by:CLC Number:
BAO Ke, LI Xijun. Chemical Vapor Deposition of Vanadium Dioxide for Thermochromic Smart Window Applications[J]. Journal of Inorganic Materials, 2024, 39(3): 233-258.

Fig. 2 Schematics of the crystal structures and band structures of VO2 (a, b) Schematic depictions of the crystal structures of the high- temperature tetragonal rutile R phase (a) and low-temperature monoclinic M phase (b)[13]; (c, d) Schematic of the VO2 band structure in the metallic (c) and insulating (d) states[14]

Fig. 3 Structure and concept of an RCRT window[27] (a) Schematic structure of the RCRT window; (b, c) Working principle of RCRT window in summer (b) and winter (c)
| V valence | Compound/Molecular formula | Vapor pressure | Ref. |
|---|---|---|---|
| +3 | V(acac)3/V(C5H7O2)3 | Not volatile, sublimes@220 ℃ | [ |
| +3 | V(amd)3/C24H51N6V | 6.6 Pa@70 ℃, 2.6 Pa@120 ℃ | [ |
| +4 | TDMAV/C8H24N4V | 133 Pa@64 ℃ | [ |
| +4 | TEMAV/C12H32N4V | 13 Pa@25 ℃, 57 Pa@82 ℃, 133 Pa@107 ℃ | [ |
| +4 | VCl4 | 780 Pa@20 ℃, 13.3 kPa@100 ℃ | [ |
| +4 | VO(acac)2/VO(C5H7O2)2 | 0.21 Pa@96 ℃ | [ |
| +4 | VO(thd)2/VO(C11H19O2)2 | 0.27 Pa@96 ℃ | [ |
| +4 | VO(hfa)2/VO(C5H2F6O2)2 | 0.18 Pa@57 ℃ | [ |
| +5 | VO(OC3H7)3 | 6 Pa@20 ℃, 38.6 Pa@45 ℃, 268 Pa@82 ℃ | [[ |
| +5 | VOCl3 | 1.84 kPa@20 ℃, 9.3 kPa@55 ℃ | [ |
Table 1 Vapor pressure of the vanadium precursorscompounds used for the CVD of VO2 thin films
| V valence | Compound/Molecular formula | Vapor pressure | Ref. |
|---|---|---|---|
| +3 | V(acac)3/V(C5H7O2)3 | Not volatile, sublimes@220 ℃ | [ |
| +3 | V(amd)3/C24H51N6V | 6.6 Pa@70 ℃, 2.6 Pa@120 ℃ | [ |
| +4 | TDMAV/C8H24N4V | 133 Pa@64 ℃ | [ |
| +4 | TEMAV/C12H32N4V | 13 Pa@25 ℃, 57 Pa@82 ℃, 133 Pa@107 ℃ | [ |
| +4 | VCl4 | 780 Pa@20 ℃, 13.3 kPa@100 ℃ | [ |
| +4 | VO(acac)2/VO(C5H7O2)2 | 0.21 Pa@96 ℃ | [ |
| +4 | VO(thd)2/VO(C11H19O2)2 | 0.27 Pa@96 ℃ | [ |
| +4 | VO(hfa)2/VO(C5H2F6O2)2 | 0.18 Pa@57 ℃ | [ |
| +5 | VO(OC3H7)3 | 6 Pa@20 ℃, 38.6 Pa@45 ℃, 268 Pa@82 ℃ | [[ |
| +5 | VOCl3 | 1.84 kPa@20 ℃, 9.3 kPa@55 ℃ | [ |

Fig. 7 Vanadium oxide thin films grown by APCVD at 450 ℃ using different gas precursor ratios[90] (a) XRD patterns for the samples grown using VCl4: H2O ratios of 1 : 1, 1 : 2 and 1 : 3; (b-d) SEM images of as-deposited (b) V2O3, (c) VO2 and (d) V2O5 thin films

Fig. 8 FE-SEM images of VO2 films obtained via APCVD with VO(acac)2 and different oxygen sources at 500 ℃[96] VO2 obtained using (a) ethanol; (b) propanol; (c) O2 gas
| Vanadium precursor | Bubbler temperature/℃ | Oxygen source | Precursor ratio | Substrate temperature/℃ | Carrier gas flow (N2)/ (L·min-1) | *τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|---|
| VCl4 | 100 | H2O | 1 : 10 | 350-550 | 0.2 | 68 | [ |
| VCl4 | 80 | H2O | (0.43-1.08) : 1 | 350-450 | 12 | - | [ |
| VCl4 | 80 | C4H8O2 | 2 : 1 | 550 | 23.2 | 68 | [ |
| VOCl3 | 90 | H2O | 1.0 : 3.4 | 350-650 | 1.0 | 67 | [ |
| VO(acac)2 | 200 | O2 | - | 500-575 | 0.4 | 51.5 | [ |
| VO(OC3H7)3 | 50 | - | - | 450 | 3-4 | 65.5 | [ |
Table 2 Range of conditions for the vanadium oxide thin films prepared by APCVD
| Vanadium precursor | Bubbler temperature/℃ | Oxygen source | Precursor ratio | Substrate temperature/℃ | Carrier gas flow (N2)/ (L·min-1) | *τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|---|
| VCl4 | 100 | H2O | 1 : 10 | 350-550 | 0.2 | 68 | [ |
| VCl4 | 80 | H2O | (0.43-1.08) : 1 | 350-450 | 12 | - | [ |
| VCl4 | 80 | C4H8O2 | 2 : 1 | 550 | 23.2 | 68 | [ |
| VOCl3 | 90 | H2O | 1.0 : 3.4 | 350-650 | 1.0 | 67 | [ |
| VO(acac)2 | 200 | O2 | - | 500-575 | 0.4 | 51.5 | [ |
| VO(OC3H7)3 | 50 | - | - | 450 | 3-4 | 65.5 | [ |

Fig. 11 Substrates and VO2 films deposition via AACVD at 400-750 ℃ using different solutions of VO(acac)2[9] (a, c) Photographs (a) and XRD patterns (c) of VO2 films from methanol solution of VO(acac)2; (b, d) Photographs (b) and XRD patterns (d) of VO2 films from water solution of VO(acac)2

Fig. 12 SEM images of the VO2 films deposited via AACVD at 650 ℃ using a water solution of VO(acac)2[9] (a) Surface SEM image of the VO2 films at low magnification; (b) Surface SEM image of the VO2 films at high magnification; (c) Cross-sectional SEM image of the VO2 films

Fig. 14 VO2 films deposited via LPCVD from V(acac)3 at 350 ℃ for 30-120 min and then annealed at 350 ℃ for 2 h[103] (a) Raman spectra of VO2 films; (d) XRD patterns of VO2 films; (b, c, e, f) SEM photographs of VO2 films deposited for (b) 30, (c) 60, (e) 90, and (f) 120 min, respectively
| Vanadium precursor | Evaporator temperature/℃ | Reactor pressure/Pa | Substrate temperature/℃ | O2 flow | Carrier gas flow (Ar) | τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|---|
| VO(acac)2 | 150-175 | 2000 | 475-520 | 20-60 sccm | 50-100 sccm | 66-72 | [ |
| VO(acac)2/methanol | 150 | 101325 | 375-450 | 0.02-0.08 L/min | 0.98 L/min | 60 | [ |
| VO(acac)2 | 120-150 | 266.6 | 390-490 | Flow rate of O2 to Ar 0.2 | - | 62±1 | [ |
| VO(acac)2 | 170 | 399.9 | 200-750 | 150 sccm | 150 sccm | - | [ |
| VO(acac)2 | 185 | 2559.36 | 520-550 | 50 sccm | 100 sccm | 61.0-68.5 | [ |
| VO(hfa)2/H2O | 100-120 | 350 | 390-600 | - | 3.6-5.0 L/h | 60 | [ |
Table 3 Conditions for VO2 thin films deposited by MOCVD
| Vanadium precursor | Evaporator temperature/℃ | Reactor pressure/Pa | Substrate temperature/℃ | O2 flow | Carrier gas flow (Ar) | τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|---|
| VO(acac)2 | 150-175 | 2000 | 475-520 | 20-60 sccm | 50-100 sccm | 66-72 | [ |
| VO(acac)2/methanol | 150 | 101325 | 375-450 | 0.02-0.08 L/min | 0.98 L/min | 60 | [ |
| VO(acac)2 | 120-150 | 266.6 | 390-490 | Flow rate of O2 to Ar 0.2 | - | 62±1 | [ |
| VO(acac)2 | 170 | 399.9 | 200-750 | 150 sccm | 150 sccm | - | [ |
| VO(acac)2 | 185 | 2559.36 | 520-550 | 50 sccm | 100 sccm | 61.0-68.5 | [ |
| VO(hfa)2/H2O | 100-120 | 350 | 390-600 | - | 3.6-5.0 L/h | 60 | [ |

Fig. 17 Experimental details of VO2 film deposition based on MOCVD using reaction between VO(hfa)2 and H2O vapors[110-111] (a) Scheme of the MOCVD apparatus; (b) Scheme of pyrohydrolysis of VO(hfa)2 molecules resulting in the VO2 film growth
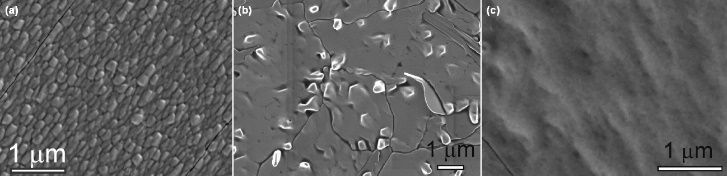
Fig. 18 SEM images of VO2 films deposited by MOCVD using VO(hfa)2/H2O[111] (a) As-deposited VO2 film at 390 ℃; (b, c) VO2 films after annealed at (b) 575 and (c) 600 ℃ for 60 min

Fig. 19 Schematic of ALD cycle[113] (a) Precursor A reacts with the substrate; (b) Excess precursor A and reaction byproducts are purged from the chamber; (c) Precursor B is pulsed into the chamber and reacts with the surface; (d) Excess precursor B and reaction products are purged from the chamber
| Vanadium precursor | Evaporator temperature/℃ | Oxidant | Substrate temperature/℃ | Annealing conditions | τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|
| VO(acac)2 | 150 | O2 | 400-475 | - | 66-70 | [ |
| VO(acac)2 | - | O2 | 400-450 | 550-850 ℃ for 3 min | - | [ |
| VO(OC3H7)3 | RT | H2O | 60-90 | Ar plasma-annealing at 550 ℃ in vacuum | 68 | [ |
| TEMAV | 105 | O3 | 150 | 450 ℃ in He/O2 | 68 | [ |
| TEMAV | 105 | O3 | 150 | 2 h at 585 ℃ in 1.333×10-3 Pa O2 | 68 | [ |
| TEMAV | 105 | H2O | 150 | 450 ℃ in O2/Ar (13.33 Pa) | 70.1 | [ |
| TDMAV | RT | H2O/O3 | 50-200 | 550-800 ℃ in N2 for 2 h | - | [ |
| TDMAV | 60 | H2O | 150-200 | 475 ℃ in Ar for 100 min | 72 | [ |
| VCl4 | - | H2O | 350 | ≥500 ℃ in 90% N2/10% H2 for 60 min | 68 | [ |
Table 4 Summary of the ALD parameters used for the growth of VOx thin films
| Vanadium precursor | Evaporator temperature/℃ | Oxidant | Substrate temperature/℃ | Annealing conditions | τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|
| VO(acac)2 | 150 | O2 | 400-475 | - | 66-70 | [ |
| VO(acac)2 | - | O2 | 400-450 | 550-850 ℃ for 3 min | - | [ |
| VO(OC3H7)3 | RT | H2O | 60-90 | Ar plasma-annealing at 550 ℃ in vacuum | 68 | [ |
| TEMAV | 105 | O3 | 150 | 450 ℃ in He/O2 | 68 | [ |
| TEMAV | 105 | O3 | 150 | 2 h at 585 ℃ in 1.333×10-3 Pa O2 | 68 | [ |
| TEMAV | 105 | H2O | 150 | 450 ℃ in O2/Ar (13.33 Pa) | 70.1 | [ |
| TDMAV | RT | H2O/O3 | 50-200 | 550-800 ℃ in N2 for 2 h | - | [ |
| TDMAV | 60 | H2O | 150-200 | 475 ℃ in Ar for 100 min | 72 | [ |
| VCl4 | - | H2O | 350 | ≥500 ℃ in 90% N2/10% H2 for 60 min | 68 | [ |
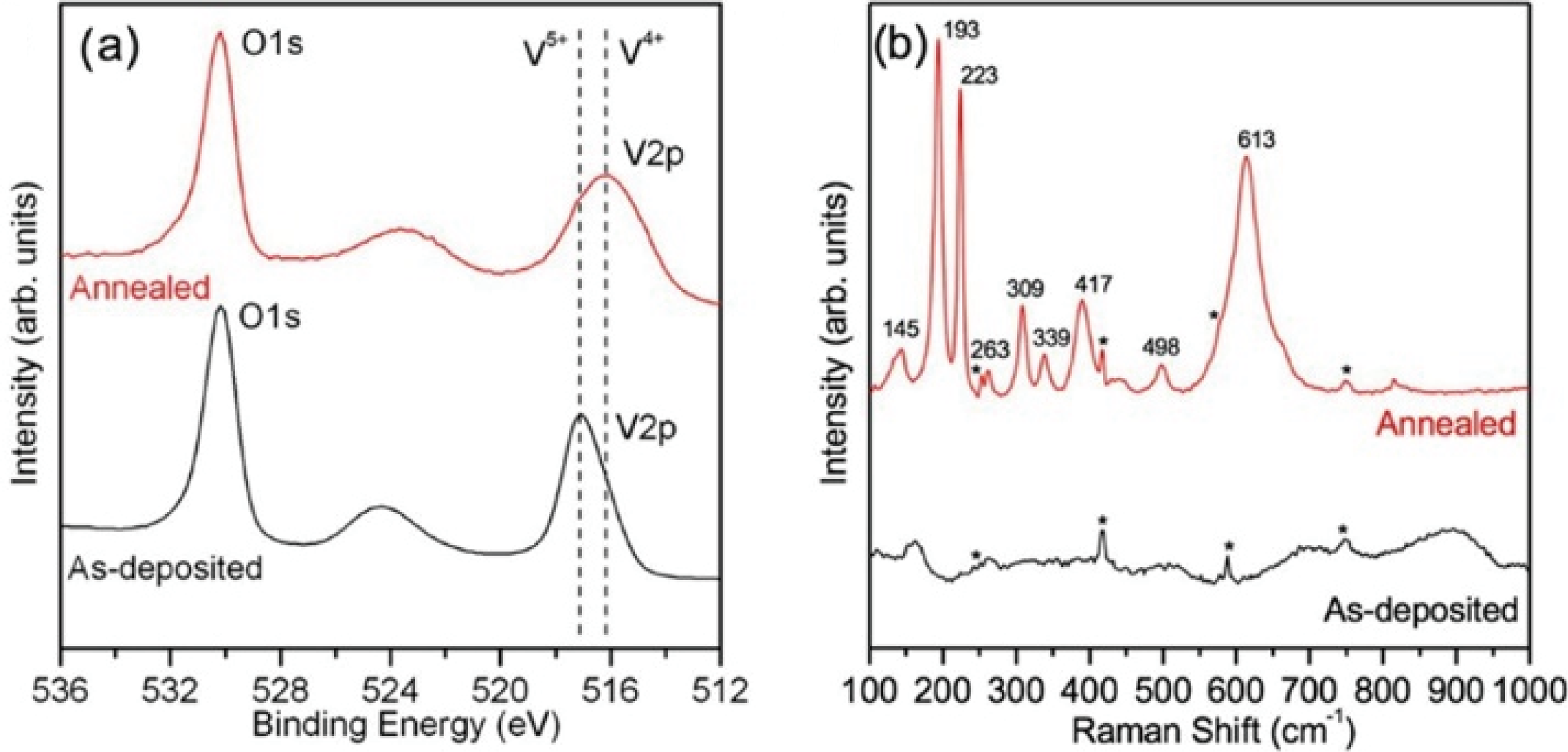
Fig. 21 XPS spectra of ALD VO2 films using TEMAV/O3 as reactants on c-Al2O3[123] (a) XPS spectra of as-deposited (black) and annealed (red) VO2 films; (b) Raman spectra of as-deposited (black) and annealed (red) VO2 films

Fig. 23 Schematic of SP and MP modes employed in the ALD process[80] (a) Typical sequence of the SP and MP modes of the ALD process; (b) Resistivity as a function of temperature and its derivative for the annealed VO2 thickness indicating the transition temperatures during heating and cooling cycle for depositions in SP and MP modes

Fig. 24 ALD VOx films deposited with TDMAV/H2O at 50 ℃[119] (a) XRD patterns of VOx film before and after 2 h annealing at 800 ℃ under N2; (b) SEM images of as-deposited and annealed VOx films
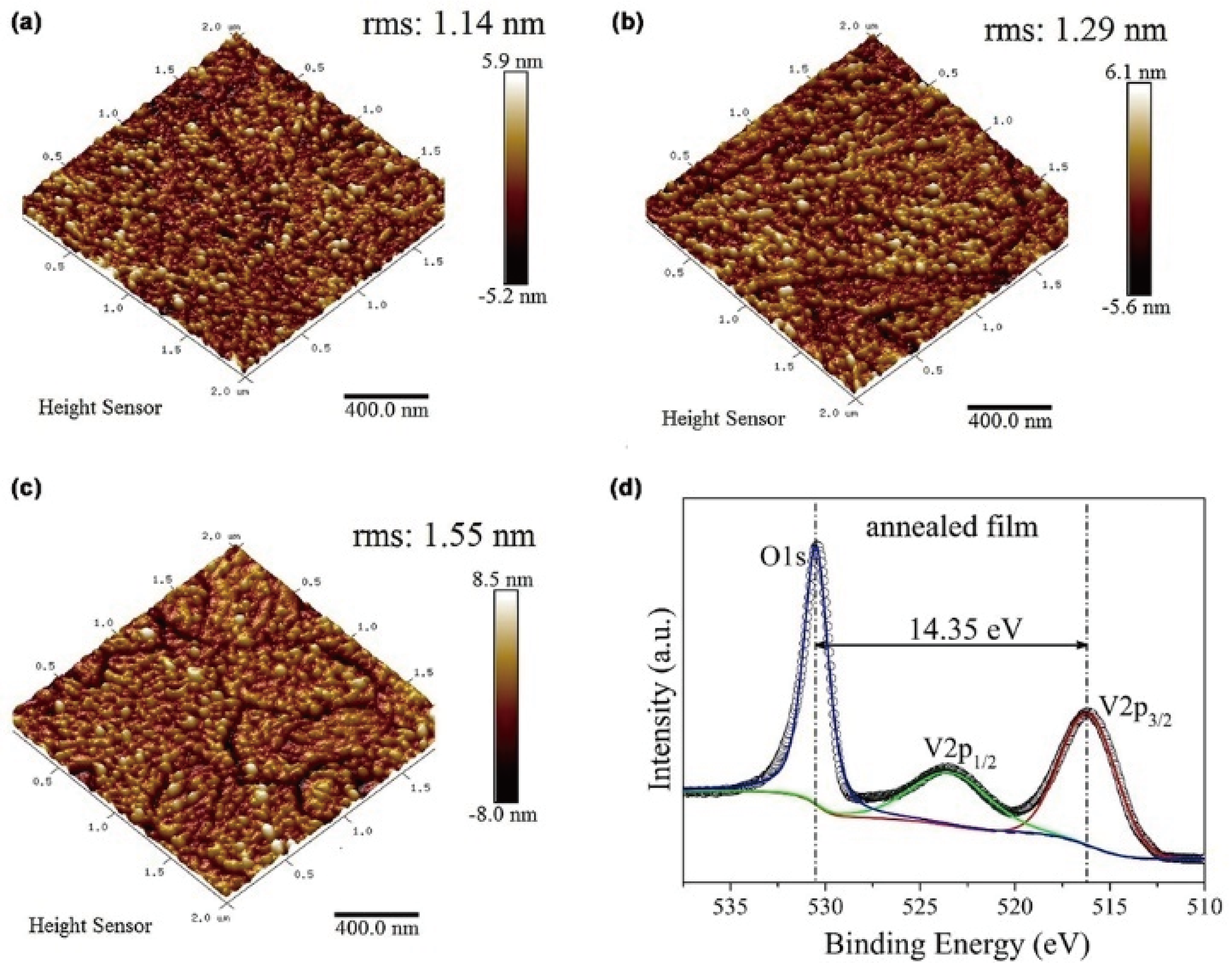
Fig. 25 ALD VO2 films deposited from TDMAV/H2O at 150-200 ℃ and annealed at 475 ℃ for 100 min in Ar[124] (a-c) AFM images of VO2 films deposited at (a) 150, (b) 175 and (c) 200 ℃; (d) V2p XPS spectrum for the annealed VO2 film deposited at 200 ℃
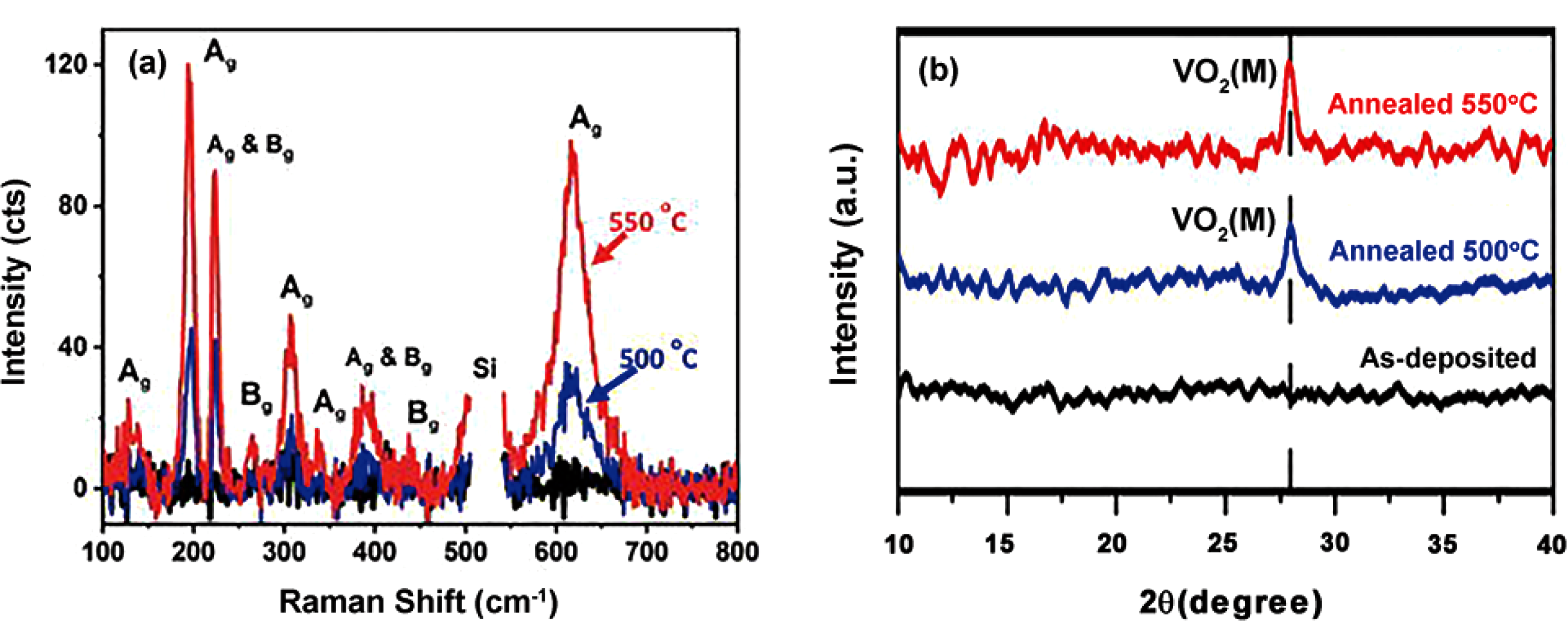
Fig. 26 ALD VO2 films deposited from VCl4/H2O and subsequently annealed at 500 and 550 ℃[125] (a) Raman spectra of as-deposited sample (black) and annealed samples at 500 (blue) and 550 ℃ (red); (b) XRD patterns of as-deposited and annealed VO2 films

Fig. 29 Crystalline structures of the WxV1-xO2[158] (a) Crystal structure relationship diagram of R and M1 phases; (b) Schematic diagram of local rutile structure around W dopant
| Vanadium precursor | Oxygen source | Dopant precursor | CVD process | Substrate/ temperature/℃ | Doping level/% (in atomic) | τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|---|
| VOCl3 | H2O | WCl6 | APCVD | Glass/500-650 ℃ | 1.9 | 29 | [ |
| VCl4 | H2O | W(OC2H5)6 | APCVD | Glass/500-600 ℃ | 0.3 | 50 | [ |
| VCl4 | H2O | WCl6 | APCVD | Glass/550 ℃ | 0.12-1.75 | 55.0-5.5 | [ |
| VO(acac)2 | 2O2/98N2 | WCl6 | APCVD | Glass/525 ℃ | 0.5 | 55 | [ |
| VO(acac)2 | O2 | W(OC2H5)5 | MOCVD | Glass/450 ℃ | 2 | 35 | [ |
| VO(acac)2 | - | W(OC2H5)5 | AACVD | Glass/550 ℃ | 0.175-1.98 | 47-28 | [ |
| TEMAV | O2 | W(CO)6 | ALD | Si/200 ℃ | 1.63 | 32 | [ |
Table 5 Summary of the CVD conditions for the synthesis of W-doped VO2 thin films
| Vanadium precursor | Oxygen source | Dopant precursor | CVD process | Substrate/ temperature/℃ | Doping level/% (in atomic) | τc of VO2/℃ | Ref. |
|---|---|---|---|---|---|---|---|
| VOCl3 | H2O | WCl6 | APCVD | Glass/500-650 ℃ | 1.9 | 29 | [ |
| VCl4 | H2O | W(OC2H5)6 | APCVD | Glass/500-600 ℃ | 0.3 | 50 | [ |
| VCl4 | H2O | WCl6 | APCVD | Glass/550 ℃ | 0.12-1.75 | 55.0-5.5 | [ |
| VO(acac)2 | 2O2/98N2 | WCl6 | APCVD | Glass/525 ℃ | 0.5 | 55 | [ |
| VO(acac)2 | O2 | W(OC2H5)5 | MOCVD | Glass/450 ℃ | 2 | 35 | [ |
| VO(acac)2 | - | W(OC2H5)5 | AACVD | Glass/550 ℃ | 0.175-1.98 | 47-28 | [ |
| TEMAV | O2 | W(CO)6 | ALD | Si/200 ℃ | 1.63 | 32 | [ |
| Dopant (s), (in atom) | τc | ΔTsol | Tlum/% | Ref. |
|---|---|---|---|---|
| W6+/0.6% | ~21.6 ℃/% | 11.4% | 50.8 | [ |
| Mo6+ | ~5 ℃/% | - | - | [ |
| Nb5+/10% | 52.2 ℃ | - | - | [ |
| Ta5+/4% | 24.8 ℃ | 6.8% | 47.1 | [ |
| Zr4+/9.8% | 64.3 ℃ | 14.1% | 60.4 | [ |
| Mg2+/7% | ~3 ℃/% | 4.8% | 51 | [ |
| Co2+/10% | 44 ℃ | 3% | 79 | [ |
| Tb3+/2% | 65 ℃ | 8.3% | 54 | [ |
| La3+/4% | ~1.1 ℃/% | 10.3 | 50.1 | [ |
| Eu3+/4% | ~6.5 ℃/% | 6.7% | 54 | [ |
| Si4+/3% | 63.1 ℃ | 13.9% | 54.7 | [ |
| Fe3+/Mg2+ | 38.2 ℃ | 12.8% | 42.1 | [ |
| Mg2+/W6+ | 35 ℃ | 4.3% | 81.3 | [ |
| Tb3+/W6+ | 40.8 ℃ | 6.3% | 40 | [ |
| Zr3+/W6+ | 28.6 ℃ | 4.9% | 48.6 | [ |
Table 6 Effect of dopants on the thermochromic performance of VO2 thin films
| Dopant (s), (in atom) | τc | ΔTsol | Tlum/% | Ref. |
|---|---|---|---|---|
| W6+/0.6% | ~21.6 ℃/% | 11.4% | 50.8 | [ |
| Mo6+ | ~5 ℃/% | - | - | [ |
| Nb5+/10% | 52.2 ℃ | - | - | [ |
| Ta5+/4% | 24.8 ℃ | 6.8% | 47.1 | [ |
| Zr4+/9.8% | 64.3 ℃ | 14.1% | 60.4 | [ |
| Mg2+/7% | ~3 ℃/% | 4.8% | 51 | [ |
| Co2+/10% | 44 ℃ | 3% | 79 | [ |
| Tb3+/2% | 65 ℃ | 8.3% | 54 | [ |
| La3+/4% | ~1.1 ℃/% | 10.3 | 50.1 | [ |
| Eu3+/4% | ~6.5 ℃/% | 6.7% | 54 | [ |
| Si4+/3% | 63.1 ℃ | 13.9% | 54.7 | [ |
| Fe3+/Mg2+ | 38.2 ℃ | 12.8% | 42.1 | [ |
| Mg2+/W6+ | 35 ℃ | 4.3% | 81.3 | [ |
| Tb3+/W6+ | 40.8 ℃ | 6.3% | 40 | [ |
| Zr3+/W6+ | 28.6 ℃ | 4.9% | 48.6 | [ |
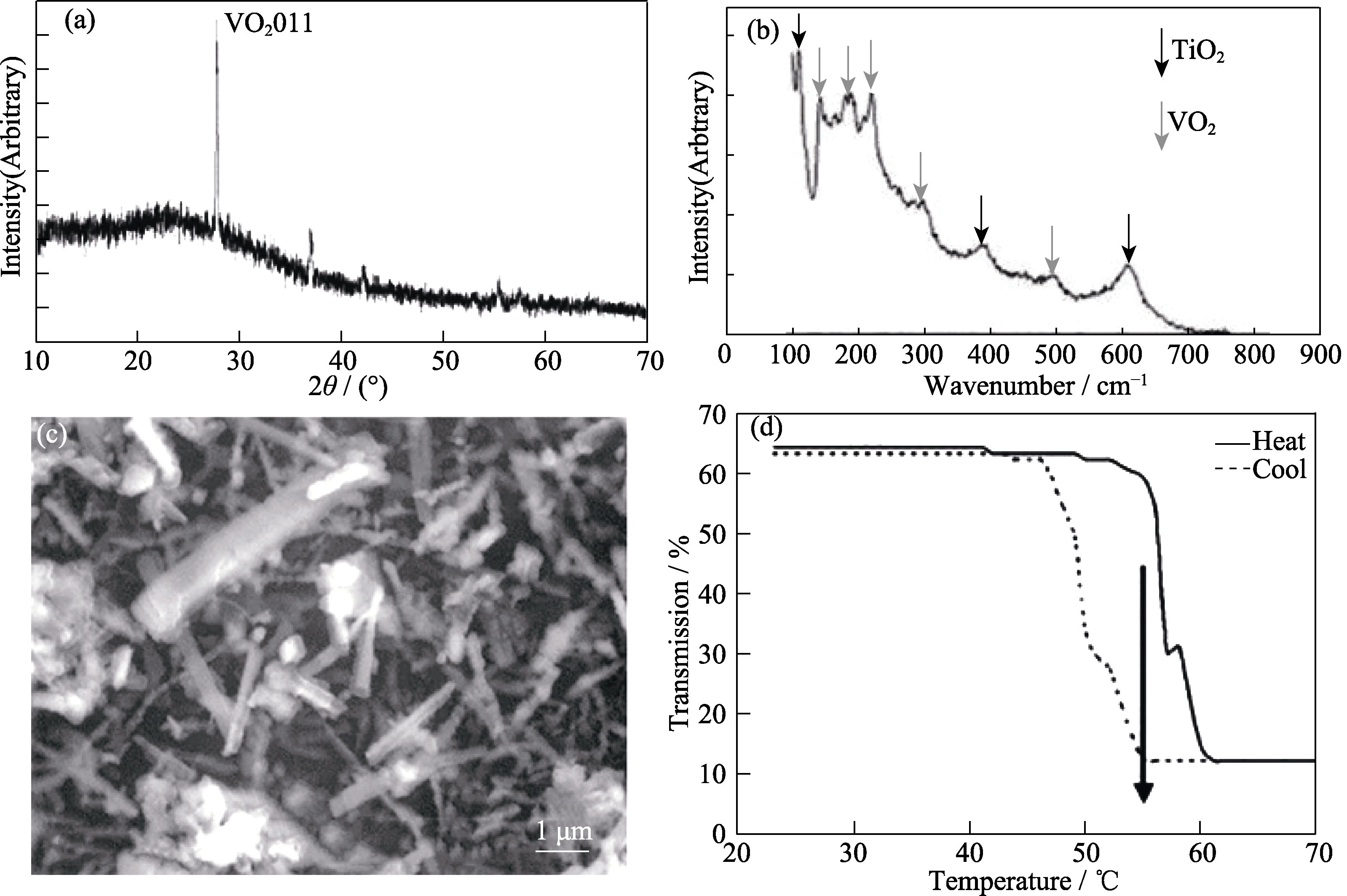
Fig. 30 Characterization of VO2/TiO2 film formed by the APCVD reaction of VOCl3/TTIP/H2O at 650 ℃[183] (a) XRD pattern; (b) Raman spectrum; (c) SEM image; (d) Variable temperature transmission plot at 2.5 μm
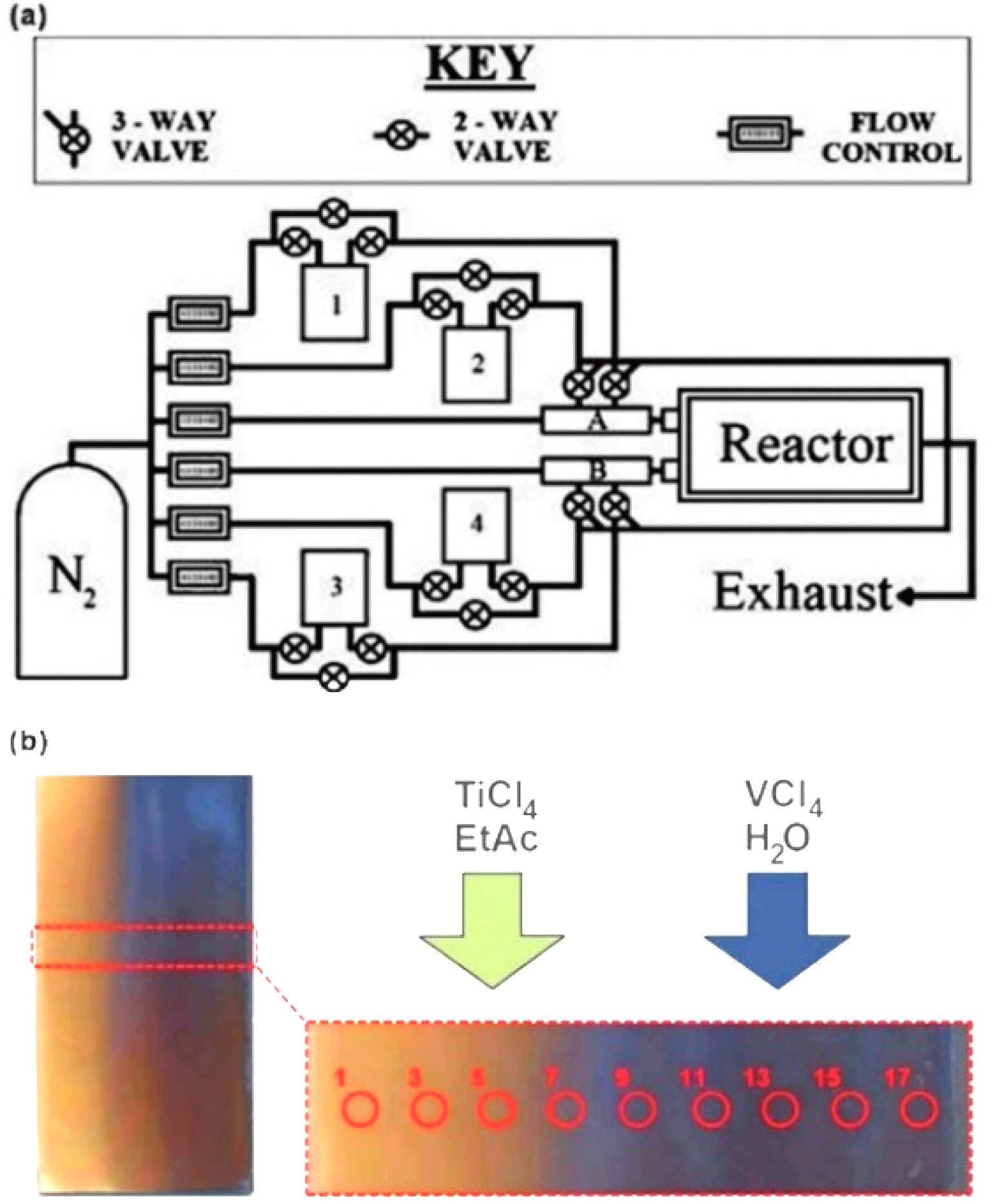
Fig. 31 Combinatorial APCVD for the synthesis of VO2/TiO2 composite film[184] (a) Schematic of the APCVD apparatus; (b) Picture of the entire graded VO2/TiO2 composite film formed
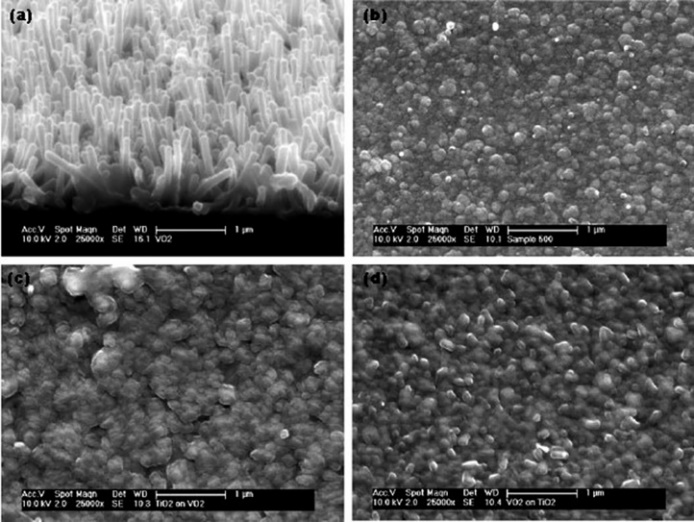
Fig. 33 SEM images of the monolayer and multilayer samples prepared by APCVD[193] (a) VO2 film; (b) TiO2 film; (c) Multilayer of TiO2 over VO2; (d) Multilayer of VO2 over TiO2
| Layer | Precursor | Bubbler temperature /℃ | N2 flow rate /(L·min-1) | Thickness /nm |
|---|---|---|---|---|
| VO2 | VCl4/C4H8O4 | 80/40 | 0.7/0.2 | ~300 |
| SiO2 | SiC8H20O4/C4H8O4 | 130/40 | 0.7/0.2 | ~1300 |
| TiO2 | TiCl4/C4H8O4 | 75/40 | 0.6/0.6 | ~100 |
Table 7 APCVD conditions of the VO2/SiO2/TiO2 films[195]
| Layer | Precursor | Bubbler temperature /℃ | N2 flow rate /(L·min-1) | Thickness /nm |
|---|---|---|---|---|
| VO2 | VCl4/C4H8O4 | 80/40 | 0.7/0.2 | ~300 |
| SiO2 | SiC8H20O4/C4H8O4 | 130/40 | 0.7/0.2 | ~1300 |
| TiO2 | TiCl4/C4H8O4 | 75/40 | 0.6/0.6 | ~100 |

Fig. 34 SEM images of multi-layered VO2/SiO2/TiO2 films using APCVD[195] (a) Typical VO2 coating on glass; (b) Porous structure of the SiO2 interlayer, as deposited on VO2 coating; (c) Typical surface morphology of the TiO2 layer in the VO2/SiO2/TiO2 system; (d) Side-on SEM image of VO2/SiO2/TiO2 film
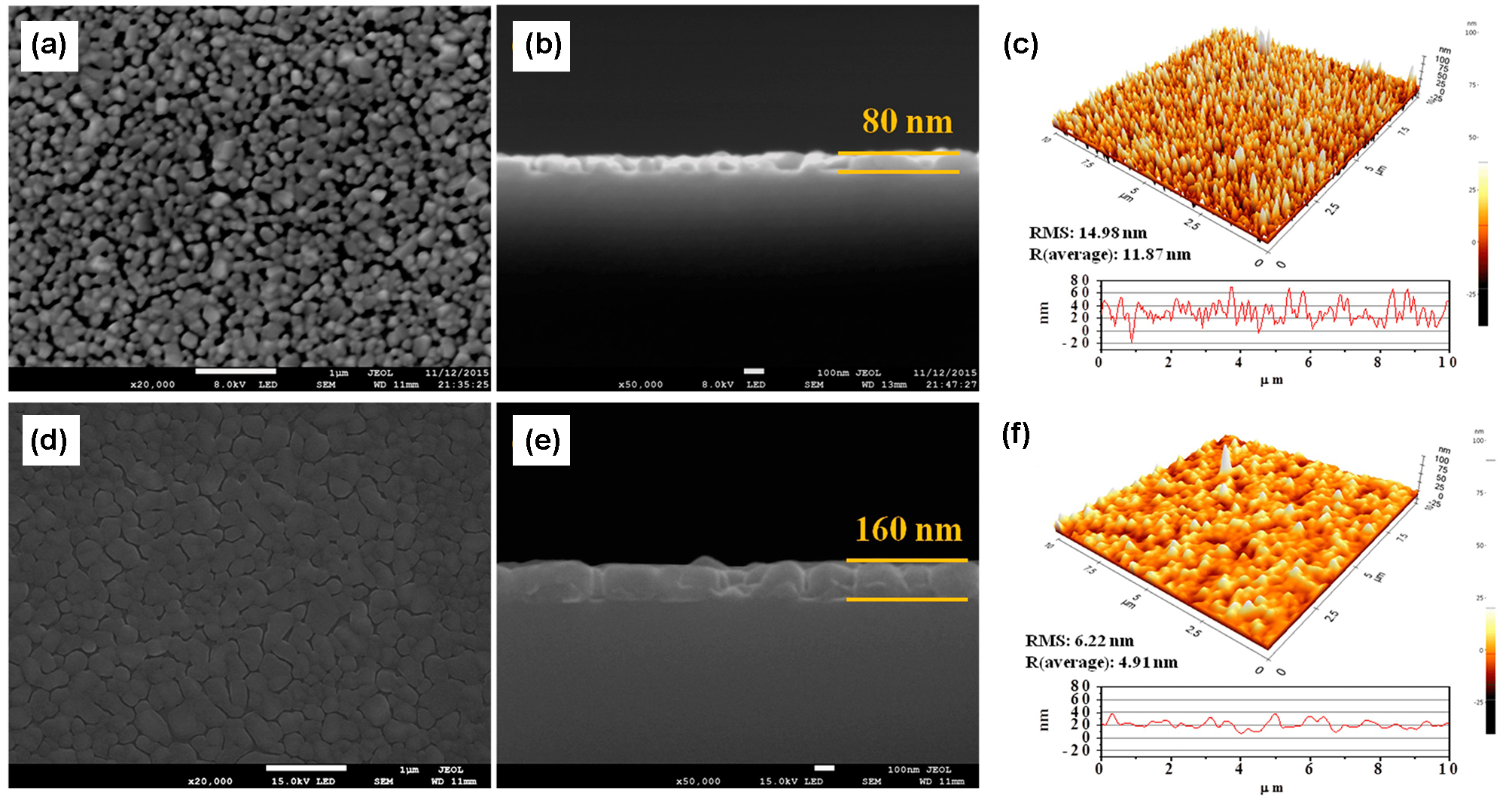
Fig. 35 SEM and AFM images of VO2 and SiO2/VO2 thin films[196] (a, b) SEM and (c) AFM images of the pristine VO2 thin film; (d, e) SEM and (f) AFM images of SiO2/VO2 thin film
| [1] |
BERARDI U. A cross-country comparison of the building energy consumptions and their trends. Resources, Conservation and Recycling, 2017, 123: 230.
DOI URL |
| [2] |
DEFOREST N, SHEHABI A, SELKOWITZ S, et al. A comparative energy analysis of three electrochromic glazing technologies in commercial and residential buildings. Applied Energy, 2017, 192: 95.
DOI URL |
| [3] |
KE Y, ZHOU C, ZHOU Y, et al. Emerging thermal-responsive materials and integrated techniques targeting the energy-efficient smart window application. Advanced Functional Materials, 2018, 28(22): 1800113.
DOI URL |
| [4] |
SVENSSON J S E M, GRANQVIST C G. Electrochromic coatings for “smart windows”. Solar Energy Materials, 1985, 12(6): 391.
DOI URL |
| [5] |
WANG Y, RUNNERSTROM E L, MILLIRON D J. Switchable materials for smart windows. Annual Review of Chemical and Biomolecular Engineering, 2016, 7(1): 283.
DOI PMID |
| [6] |
KE Y, CHEN J, LIN G, et al. Smart windows: electro-, thermo-, mechano-, photochromics, and beyond. Advanced Energy Materials, 2019, 9(39): 1902066.
DOI URL |
| [7] |
WARWICK M E A, BINIONS R. Advances in thermochromic vanadium dioxide films. Journal of Materials Chemistry A, 2014, 2(10): 3275.
DOI URL |
| [8] |
CUI Y, KE Y, LIU C, et al. Thermochromic VO2 for energy-efficient smart windows. Joule, 2018, 2(9): 1707.
DOI URL |
| [9] |
MATAMURA Y, IKENOUE T, MIYAKE M, et al. Mist CVD of vanadium dioxide thin films with excellent thermochromic properties using a water-based precursor solution. Solar Energy Materials and Solar Cells, 2021, 230: 111287.
DOI URL |
| [10] |
SJOBERG S G. Nils Gabriel Sefstrom and the discovery of vanadium. Journal of Chemical Education, 1951, 28(6): 294.
DOI URL |
| [11] |
MORIN F J. Oxides which show a metal-to-insulator transition at the neel temperature. Physical Review Letters, 1959, 3(1): 34.
DOI URL |
| [12] |
GOODENOUGH J B. The two components of the crystallographic transition in VO2. Journal of Solid State Chemistry, 1971, 3(4): 490.
DOI URL |
| [13] |
WHITTAKER L, PATRIDGE C J, BANERJEE S. Microscopic and nanoscale perspective of the metal-insulator phase transitions of VO2: some new twists to an old tale. The Journal of Physical Chemistry Letters, 2011, 2(7): 745.
DOI URL |
| [14] |
AETUKURI N B, GRAY A X, DROUARD M, et al. Control of the metal-insulator transition in vanadium dioxide by modifying orbital occupancy. Nature Physics, 2013, 9(10): 661.
DOI |
| [15] |
BABULANAM S M, ERIKSSON T S, NIKLASSON G A, et al. Thermochromic VO2 films for energy-efficient windows. Solar Energy Materials, 1987, 16(5): 347.
DOI URL |
| [16] |
BARIMAH E K, BOONTAN A, STEENSON D P, et al. Infrared optical properties modulation of VO2 thin film fabricated by ultrafast pulsed laser deposition for thermochromic smart window applications. Scientific Reports, 2022, 12: 11421.
DOI |
| [17] |
CHANG T, CAO X, DEDON L R, et al. Optical design and stability study for ultrahigh-performance and long-lived vanadium dioxide-based thermochromic coatings. Nano Energy, 2018, 44: 256.
DOI URL |
| [18] |
LIU K, LEE S, YANG S, et al. Recent progresses on physics and applications of vanadium dioxide. Materials Today, 2018, 21(8): 875.
DOI URL |
| [19] | CHEN S, WANG Z, REN H, et al. Gate-controlled VO2 phase transition for high-performance smart windows. Science Advances, 2019, 5(3): 6815. |
| [20] |
VU T D, CHEN Z, ZENG X, et al. Physical vapour deposition of vanadium dioxide for thermochromic smart window applications. Journal of Materials Chemistry C, 2019, 7(8): 2121.
DOI URL |
| [21] |
YAO L, QU Z, PANG Z, et al. Three-layered hollow nanospheres based coatings with ultrahigh-performance of energy-saving, antireflection, and self-cleaning for smart windows. Small, 2018, 14(34): 1801661.
DOI URL |
| [22] |
ABURAS M, SOEBARTO V, WILLIAMSON T, et al. Thermochromic smart window technologies for building application: a review. Applied Energy, 2019, 255: 113522.
DOI URL |
| [23] |
KE Y, YIN Y, ZHANG Q, et al. Adaptive thermochromic windows from active plasmonic elastomers. Joule, 2019, 3(3): 858.
DOI |
| [24] |
CAO X, CHANG T, SHAO Z, et al. Challenges and opportunities toward real application of VO2-based smart glazing. Matter, 2020, 2(4): 862.
DOI URL |
| [25] |
ZENG W, CHEN N, XIE W. Research progress on the preparation methods for VO2 nanoparticles and their application in smart windows. CrystEngComm, 2020, 22(5): 851.
DOI URL |
| [26] |
CHEN Z, TANG Y, JI A, et al. Large-scale preparation of durable VO2 nanocomposite coatings. ACS Applied Nano Materials, 2021, 4(4): 4048.
DOI URL |
| [27] |
WANG S, JIANG T, MENG Y, et al. Scalable thermochromic smart windows with passive radiative cooling regulation. Science, 2021, 374(6574): 1501.
DOI PMID |
| [28] | CAO C, HU B, TU G, et al. Sputtering flexible VO2 films for effective thermal modulation. ACS Applied Materials & Interfaces, 2022, 14(24): 28105. |
| [29] |
SUN K, WHEELER C, HILLIER J A, et al. Room temperature phase transition of W-doped VO2 by atomic layer deposition on 200 mm Si wafers and flexible substrates. Advanced Optical Materials, 2022, 10(23): 2201326.
DOI URL |
| [30] |
VU T D, XIE H, WANG S, et al. Durable vanadium dioxide with 33-year service life for smart windows applications. Materials Today Energy, 2022, 26: 100978.
DOI URL |
| [31] | TANG K, DONG K, LI J, et al. Temperature-adaptive radiative coating for all-season household thermal regulation. 2021, 374(6574): 1504. |
| [32] |
SHEN N, CHEN S, HUANG R, et al. Vanadium dioxide for thermochromic smart windows in ambient conditions. Materials Today Energy, 2021, 21: 100827.
DOI URL |
| [33] |
KIM J, PAIK T. Recent advances in fabrication of flexible, thermochromic vanadium dioxide films for smart windows. Nanomaterials (Basel), 2021, 11(10): 2674.
DOI URL |
| [34] |
LI X, CAO C, LIU C, et al. Self-rolling of vanadium dioxide nanomembranes for enhanced multi-level solar modulation. Nature Communications, 2022, 13: 7819.
DOI PMID |
| [35] |
LI S Y, NIKLASSON G A, GRANQVIST C G. Thermochromic fenestration with VO2-based materials: three challenges and how they can be met. Thin Solid Films, 2012, 520(10): 3823.
DOI URL |
| [36] |
WANG S, OWUSU K A, MAI L, et al. Vanadium dioxide for energy conservation and energy storage applications: synthesis and performance improvement. Applied Energy, 2018, 211: 200.
DOI URL |
| [37] |
SHAO Z, CAO X, LUO H, et al. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Materials, 2018, 10(7): 581.
DOI |
| [38] |
HU P, HU P, VU T D, et al. Vanadium oxide: phase diagrams, structures, synthesis, and applications. Chemical Reviews, 2023, 123(8): 4353.
DOI URL |
| [39] |
KOSUGE K. The phase diagram and phase transition of the V2O3-V2O5 system. Journal of Physics and Chemistry of Solids, 1967, 28(8): 1613.
DOI URL |
| [40] |
WRIEDT H A. The O-V (oxygen-vanadium) system. Bulletin of Alloy Phase Diagrams, 1989, 10(3): 271.
DOI URL |
| [41] |
CHOI Y, JUNG Y, KIM H. Low-temperature deposition of thermochromic VO2 thin films on glass substrates. Thin Solid Films, 2016, 615: 437.
DOI URL |
| [42] |
ZHANG D P, ZHU M D, LIU Y, et al. High performance VO2 thin films growth by DC magnetron sputtering at low temperature for smart energy efficient window application. Journal of Alloys and Compounds, 2016, 659: 198.
DOI URL |
| [43] |
AIJAZ A, JI Y X, MONTERO J, et al. Low-temperature synthesis of thermochromic vanadium dioxide thin films by reactive high power impulse magnetron sputtering. Solar Energy Materials and Solar Cells, 2016, 149: 137.
DOI URL |
| [44] |
TAHA M, WALIA S, AHMED T, et al. Insulator-metal transition in substrate-independent VO2 thin film for phase-change devices. Scientific Reports, 2017, 7: 17899.
DOI |
| [45] |
VU T D, LIU S, ZENG X, et al. High-power impulse magnetron sputtering deposition of high crystallinity vanadium dioxide for thermochromic smart windows applications. Ceramics International, 2020, 46(6): 8145.
DOI URL |
| [46] |
ZHU M, WANG H, WANG B, et al. New route for modification of thermochromic properties of vanadium dioxide films via high-energy X-ray irradiation. Ceramics International, 2019, 45(2, Part A): 1661.
DOI URL |
| [47] |
LIU B, GONG M, ZHANG J, et al. Comparative study of the metal insulator transition of a VO2 film with simultaneous infrared thermography and electric measurements. AIP Advances, 2021, 11(3): 035026.
DOI URL |
| [48] |
CASE F C. Low temperature deposition of VO2 thin films. Journal of Vacuum Science & Technology A, 1990, 8(3): 1395.
DOI URL |
| [49] |
LEE M H, KIM M G. RTA and stoichiometry effect on the thermochromism of VO2 thin films. Thin Solid Films, 1996, 286(1): 219.
DOI URL |
| [50] |
THéRY V, BOULLE A, CRUNTEANU A, et al. Structural and electrical properties of large area epitaxial VO2 films grown by electron beam evaporation. Journal of Applied Physics, 2017, 121(5): 055303.
DOI URL |
| [51] |
MANOUSOU D K, GARDELIS S, CALAMIOTOU M, et al. VO2 thin films fabricated by reduction of thermal evaporated V2O5 under N2 flow. Materials Letters, 2021, 299: 130086.
DOI URL |
| [52] |
GUO X, TAN Y, HU Y, et al. High quality VO2 thin films synthesized from V2O5 powder for sensitive near-infrared detection. Scientific Reports, 2021, 11: 21749.
DOI |
| [53] |
PóSA L, MOLNÁR G, KALAS B, et al. A rational fabrication method for low switching-temperature VO2. Nanomaterials (Basel), 2021, 11(1): 212.
DOI URL |
| [54] |
KIM D H, KWOK H S. Pulsed laser deposition of VO2 thin films. Applied Physics Letters, 1994, 65(25): 3188.
DOI URL |
| [55] |
ZHU P R, YAMAMOTO S, MIYASHITA A, et al. Pulsed laser deposition of VO2 single crystal thin films on sapphire substrates. Chinese Physics Letters, 1998, 15(12): 904.
DOI URL |
| [56] |
LIU H, VASQUEZ O, SANTIAGO V R, et al. Novel pulsed-laser-deposition—VO2 thin films for ultrafast applications. Journal of Electronic Materials, 2005, 34(5): 491.
DOI URL |
| [57] |
FU D, LIU K, TAO T, et al. Comprehensive study of the metal-insulator transition in pulsed laser deposited epitaxial VO2 thin films. Journal of Applied Physics, 2013, 113(4): 043707.
DOI URL |
| [58] |
MATHEVULA L, NGOM B D, KOTSEDI L, et al. Thermochromic VO2 on zinnwaldite mica by pulsed laser deposition. Applied Surface Science, 2014, 314: 476.
DOI URL |
| [59] |
KUMI-BARIMAH E, ANAGNOSTOU D E, JOSE G. Phase changeable vanadium dioxide (VO2) thin films grown from vanadium pentoxide (V2O5) using femtosecond pulsed laser deposition. AIP Advances, 2020, 10(6): 065225.
DOI URL |
| [60] |
BUKHARI S A, KUMAR S, KUMAR P, et al. The effect of oxygen flow rate on metal-insulator transition (MIT) characteristics of vanadium dioxide (VO2) thin films by pulsed laser deposition (PLD). Applied Surface Science, 2020, 529: 146995.
DOI URL |
| [61] |
YIN D, XU N, ZHANG J, et al. High quality vanadium dioxide films prepared by an inorganic Sol-Gel method. Materials Research Bulletin, 1996, 31(3): 335.
DOI URL |
| [62] |
LU S, HOU L, GAN F. Structure and optical property changes of sol-gel derived VO2 thin films. Advanced Materials, 1997, 9(3): 244.
DOI URL |
| [63] |
LU S, HOU L, GAN F. Surface analysis and phase transition of gel-derived VO2 thin films. Thin Solid Films, 1999, 353(1): 40.
DOI URL |
| [64] |
YAN J, HUANG W, ZHANG Y, et al. Characterization of preferred orientated vanadium dioxide film on muscovite (001) substrate. Physica Status Solidi (a), 2008, 205(10): 2409.
DOI URL |
| [65] |
LIU D, CHENG H, ZHENG W, et al. Infrared thermochromic properties of VO2 thin films prepared through aqueous Sol-Gel process. Journal of Wuhan University of Technology-Mater. Sci. Ed., 2012, 27(5): 861.
DOI URL |
| [66] |
WU Y F, FAN L L, CHEN S M, et al. Spectroscopic analysis of phase constitution of high quality VO2 thin film prepared by facile Sol-Gel method. AIP Advances, 2013, 3(4): 042132.
DOI URL |
| [67] | KANG L, GAO Y, LUO H. A novel solution process for the synthesis of VO2 thin films with excellent thermochromic properties. ACS Applied Materials & Interfaces, 2009, 1(10): 2211. |
| [68] | KANG L, GAO Y, LUO H, et al. Nanoporous thermochromic VO2 films with low optical constants, enhanced luminous transmittance and thermochromic properties. ACS Applied Materials & Interfaces, 2011, 3(2): 135. |
| [69] |
YUE F, HUANG W, SHI Q, et al. Phase transition properties of vanadium oxide films deposited by polymer-assisted deposition. Journal of Sol-Gel Science and Technology, 2014, 72(3): 565.
DOI URL |
| [70] |
BRECKENFELD E, KIM H, GORZKOWSKI E P, et al. Laser-processing of VO2 thin films synthesized by polymer-assisted-deposition. Applied Surface Science, 2017, 397: 152.
DOI URL |
| [71] |
DEVTHADE V, LEE S. Synthesis of vanadium dioxide thin films and nanostructures. Journal of Applied Physics, 2020, 128(23): 231101.
DOI URL |
| [72] |
SUN L, YUAN G, GAO L, et al. Chemical vapour deposition. Nature Reviews Methods Primers, 2021, 1(1): 5.
DOI |
| [73] |
WARWICK M E A, BINIONS R. Chemical vapour deposition of thermochromic vanadium dioxide thin films for energy efficient glazing. Journal of Solid State Chemistry, 2014, 214: 53.
DOI URL |
| [74] | DROSOS C, VERNARDOU D. Perspectives of energy materials grown by APCVD. Solar Energy Materials and Solar Cells, 2015, 140: 1. |
| [75] |
MARUYAMA T, IKUTA Y. Vanadium dioxide thin films prepared by chemical vapour deposition from vanadium(III) acetylacetonate. Journal of Materials Science, 1993, 28(18): 5073.
DOI URL |
| [76] |
LIM B S, RAHTU A, PARK J S, et al. Synthesis and characterization of volatile, thermally stable, reactive transition metal amidinates. Inorganic Chemistry, 2003, 42(24): 7951.
PMID |
| [77] |
PRASADAM V P, BAHLAWANE N, MATTELAER F, et al. Atomic layer deposition of vanadium oxides: process and application review. Materials Today Chemistry, 2019, 12: 396.
DOI URL |
| [78] |
WEIMER M S, KIM I S, GUO P, et al. Oxidation state discrimination in the atomic layer deposition of vanadium oxides. Chemistry of Materials, 2017, 29(15): 6238.
DOI URL |
| [79] |
PRASADAM V P, DEY B, BULOU S, et al. Study of VO2 thin film synthesis by atomic layer deposition. Materials Today Chemistry, 2019, 12: 332.
DOI URL |
| [80] |
NIANG K M, BAI G, ROBERTSON J. Influence of precursor dose and residence time on the growth rate and uniformity of vanadium dioxide thin films by atomic layer deposition. Journal of Vacuum Science & Technology A, 2020, 38(4): 042401.
DOI URL |
| [81] |
BAI G, NIANG K M, ROBERTSON J. Preparation of atomic layer deposited vanadium dioxide thin films using tetrakis (ethylmethylamino) vanadium as precursor. Journal of Vacuum Science & Technology A, 2020, 38(5): 052402.
DOI URL |
| [82] |
THOMAS T, BLACKMAN C S, PARKIN I P, et al. Atmospheric pressure chemical vapour deposition of vanadium arsenide thin films via the reaction of VCl4 or VOCl3 with tBuAsH2. Thin Solid Films, 2013, 537: 171.
DOI URL |
| [83] |
MALKEROVA I P, MAKAREVICH A M, ALIKHANYAN A S, et al. Volatility and thermal stability of vanadyl β-diketonate complexes. Russian Journal of Inorganic Chemistry, 2017, 62(6): 818.
DOI URL |
| [84] |
KANAI H, YOSHIKAWA T, SONE T, et al. Preparation and characterization of highly dispersed V2O5/SiO2 prepared by a CVD method. Reaction Kinetics and Catalysis Letters, 2002, 75(2): 213.
DOI URL |
| [85] |
BADOT J C. Atomic layer epitaxy of vanadium oxide thin films and electrochemical behavior in presence of lithium ions. Electrochemical and Solid-State Letters, 1999, 3(10): 485.
DOI URL |
| [86] |
MANNING T D, PARKIN I P, CLARK R J H, et al. Intelligent window coatings: atmospheric pressure chemical vapour deposition of vanadium oxides. Journal of Materials Chemistry, 2002, 12(10): 2936.
DOI URL |
| [87] |
DROSOS C, VERNARDOU D. Advancements, challenges and prospects of chemical vapour pressure at atmospheric pressure on vanadium dioxide structures. Materials, 2018, 11(3): 384.
DOI URL |
| [88] |
FIELD M N, PARKIN I P. Atmospheric pressure chemical vapour deposition of vanadium(V) oxide films on glass substrates from reactions of VOCl3 and VCl4 with water. Journal of Materials Chemistry, 2000, 10(8): 1863.
DOI URL |
| [89] |
MANNING T D, PARKIN I P. Vanadium(IV) oxide thin films on glass and silicon from the atmospheric pressure chemical vapour deposition reaction of VOCl3 and water. Polyhedron, 2004, 23(18): 3087.
DOI URL |
| [90] |
VERNARDOU D, PATERAKIS P, DROSOS H, et al. A study of the electrochemical performance of vanadium oxide thin films grown by atmospheric pressure chemical vapour deposition. Solar Energy Materials and Solar Cells, 2011, 95(10): 2842.
DOI URL |
| [91] |
MALARDE D, POWELL M J, QUESADA-CABRERA R, et al. Optimized atmospheric-pressure chemical vapor deposition thermochromic VO2 thin films for intelligent window applications. ACS Omega, 2017, 2(3): 1040.
DOI URL |
| [92] |
GASKELL J M, AFZAAL M, SHEEL D W, et al. Optimised atmospheric pressure CVD of monoclinic VO2 thin films with picosecond phase transition. Surface and Coatings Technology, 2016, 287: 160.
DOI URL |
| [93] |
VERNARDOU D, PEMBLE M E, SHEEL D W. The growth of thermochromic VO2 films on glass by atmospheric-pressure CVD: a comparative study of precursors, CVD methodology, and substrates. Chemical Vapor Deposition, 2006, 12(5): 263.
DOI URL |
| [94] |
VERNARDOU D, LOULOUDAKIS D, SPANAKIS E, et al. Thermochromic amorphous VO2 coatings grown by APCVD using a single-precursor. Solar Energy Materials and Solar Cells, 2014, 128: 36.
DOI URL |
| [95] |
VERNARDOU D, PEMBLE M E, SHEEL D W. In-situ Fourier transform infrared spectroscopy gas phase studies of vanadium (IV) oxide coating by atmospheric pressure chemical vapour deposition using vanadyl (IV) acetylacetonate. Thin Solid Films, 2008, 516(14): 4502.
DOI URL |
| [96] | VERNARDOU D, BEI A, LOULOUDAKIS D, et al. Oxygen source-oriented control of atmospheric pressure chemical vapor deposition of VO2 for capacitive applications. Journal of Electrochemical Science and Engineering, 2016, 6(2): 165. |
| [97] |
WARWICK M E A, RIDLEY I, BINIONS R. Thermochromic vanadium dioxide thin films prepared by electric field assisted atmospheric pressure chemical vapour deposition for intelligent glazing application and their energy demand reduction properties. Solar Energy Materials and Solar Cells, 2016, 157: 686.
DOI URL |
| [98] |
MATHUR S, RUEGAMER T, GROBELSEK I. Phase-selective CVD of vanadium oxide nanostructures. Chemical Vapor Deposition, 2007, 13(1): 42.
DOI URL |
| [99] |
PAPADIMITROPOULOS G, KOSTIS I, TRANTALIDIS S, et al. Investigation of structural, morphological and electrical properties of APCVD vanadium oxide thin films. Physica Status Solidi C, 2015, 12(7): 964.
DOI URL |
| [100] |
JIAMPRASERTBOON A, POWELL M J, DIXON S C, et al. Photocatalytic and electrically conductive transparent Cl-doped ZnO thin films via aerosol-assisted chemical vapour deposition. Journal of Materials Chemistry A, 2018, 6(26): 12682.
DOI URL |
| [101] |
PICCIRILLO C, BINIONS R, PARKIN I P. Synthesis and functional properties of vanadium oxides: V2O3, VO2, and V2O5 deposited on glass by aerosol-assisted CVD. Chemical Vapor Deposition, 2007, 13(4): 145.
DOI URL |
| [102] |
WARWICK M E A, BINIONS R. Thermochromic vanadium dioxide thin films from electric field assisted aerosol assisted chemical vapour deposition. Solar Energy Materials and Solar Cells, 2015, 143: 592.
DOI URL |
| [103] |
GUO B, CHEN L, SHI S, et al. Low temperature fabrication of thermochromic VO2 thin films by low-pressure chemical vapor deposition. RSC Advances, 2017, 7(18): 10798.
DOI URL |
| [104] |
WRIGHT P J, CROSBIE M J, LANE P A, et al. Metal organic chemical vapor deposition (MOCVD) of oxides and ferroelectric materials. Journal of Materials Science: Materials in Electronics, 2002, 13(11): 671.
DOI URL |
| [105] |
YAKOVKINA L V, MUTILIN S V, PRINZ V Y, et al. MOCVD growth and characterization of vanadium dioxide films. Journal of Materials Science, 2017, 52(7): 4061.
DOI URL |
| [106] |
SPANÒ S F, TORO R G, CONDORELLI G G, et al. Phase- selective route to V-O film formation: a systematic MOCVD study into the effects of deposition temperature on structure and morphology. Chemical Vapor Deposition, 2015, 21: 319.
DOI URL |
| [107] |
SAHANA M B, DHARMAPRAKASH M S, SHIVASHANKAR S A. Microstructure and properties of VO2 thin films deposited by MOCVD from vanadyl acetylacetonate. Journal of Materials Chemistry, 2002, 12(2): 333.
DOI URL |
| [108] |
VERNARDOU D, PEMBLE M E, SHEEL D W. Vanadium oxides prepared by liquid injection MOCVD using vanadyl acetylacetonate. Surface and Coatings Technology, 2004, 188-189: 250.
DOI URL |
| [109] |
RAJESWARAN B, UMARJI A M. Defect engineering of VO2 thin films synthesized by chemical vapor deposition. Materials Chemistry and Physics, 2020, 245: 122230.
DOI URL |
| [110] |
MAKAREVICH A M, SADYKOV I I, SHAROVAROV D I, et al. Chemical synthesis of high quality epitaxial vanadium dioxide films with sharp electrical and optical switch properties. Journal of Materials Chemistry C, 2015, 3(35): 9197.
DOI URL |
| [111] |
MAKAREVICH A M, SOBOL A G, SADYKOV I I, et al. Delicate tuning of epitaxial VO2 films for ultra-sharp electrical and intense IR optical switching properties. Journal of Alloys and Compounds, 2021, 853: 157214.
DOI URL |
| [112] |
LESKELä M, RITALA M. Atomic layer deposition (ALD): from precursors to thin film structures. Thin Solid Films, 2002, 409(1): 138.
DOI URL |
| [113] |
ASUNDI A S, RAIFORD J A, BENT S F. Opportunities for atomic layer deposition in emerging energy technologies. ACS Energy Letters, 2019, 4(4): 908.
DOI URL |
| [114] |
SUNTOLA T. Atomic layer epitaxy. Materials Science Reports, 1989, 4(5): 261.
DOI URL |
| [115] |
ØSTRENG E, NILSEN O, FJELLVÅG H. Optical properties of vanadium pentoxide deposited by ALD. The Journal of Physical Chemistry C, 2012, 116(36): 19444.
DOI URL |
| [116] |
ØSTRENG E, GANDRUD K B, HU Y, et al. High power nano- structured V2O5 thin film cathodes by atomic layer deposition. Journal of Materials Chemistry A, 2014, 2(36): 15044.
DOI URL |
| [117] |
CHEN X, POMERANTSEVA E, BANERJEE P, et al. Ozone-based atomic layer deposition of crystalline V2O5 films for high performance electrochemical energy storage. Chemistry of Materials, 2012, 24(7): 1255.
DOI URL |
| [118] |
BLANQUART T, NIINISTÖ J, GAVAGNIN M, et al. Atomic layer deposition and characterization of vanadium oxide thin films. RSC Advances, 2013, 3(4): 1179.
DOI URL |
| [119] |
WANG X, GUO Z, GAO Y, et al. Atomic layer deposition of vanadium oxide thin films from tetrakis(dimethylamino)vanadium precursor. Journal of Materials Research, 2017, 32(1): 37.
DOI URL |
| [120] | DAGUR P, MANE A U, SHIVASHANKAR S A. Thin films of VO2 on glass by atomic layer deposition: microstructure and electrical properties. Journal of Crystal Growth, 2005, 275(1): 1223. |
| [121] |
JUAN P C, LIN K C, CHO W H, et al. Atomic layer deposition of vanadium oxides using vanadyl acetylacetonate as the precursor. Thin Solid Films, 2021, 725: 138639.
DOI URL |
| [122] |
RAMPELBERG G, DEDUYTSCHE D, DE SCHUTTER B, et al. Crystallization and semiconductor-metal switching behavior of thin VO2 layers grown by atomic layer deposition. Thin Solid Films, 2014, 550: 59.
DOI URL |
| [123] |
KOZEN A C, JORESS H, CURRIE M, et al. Structural characterization of atomic layer deposited vanadium dioxide. The Journal of Physical Chemistry C, 2017, 121(35): 19341.
DOI URL |
| [124] |
LV X, CAO Y, YAN L, et al. Atomic layer deposition of VO2 films with tetrakis-dimethyl-amino vanadium (IV) as vanadium precursor. Applied Surface Science, 2017, 396: 214.
DOI URL |
| [125] |
GANESAN J P, DEV D, KRISHNAPRASAD A, et al. Semiconductor- to-metal transition in atomic layer deposition (ALD) of VO2 films using VCl4 and water. Applied Physics Letters, 2021, 118(26): 261902.
DOI URL |
| [126] |
KERÄNEN J, AUROUX A, EK-HÄRKÖNEN S, et al. Calorimetric measurements of the acidity of supported vanadium oxides prepared by ALE and impregnation. Thermochimica Acta, 2001, 379(1): 233.
DOI URL |
| [127] |
MUSSCHOOT J, DEDUYTSCHE D, POELMAN H, et al. Comparison of thermal and plasma-enhanced ALD/CVD of vanadium pentoxide. Journal of the Electrochemical Society, 2009, 156(7): P122.
DOI URL |
| [128] |
DAUBERT J S, LEWIS N P, GOTSCH H N, et al. Effect of meso- and micro-porosity in carbon electrodes on atomic layer deposition of pseudocapacitive V2O5 for high performance supercapacitors. Chemistry of Materials, 2015, 27(19): 6524.
DOI URL |
| [129] |
DAUBERT J S, WANG R, OVENTAL J S, et al. Intrinsic limitations of atomic layer deposition for pseudocapacitive metal oxides in porous electrochemical capacitor electrodes. Journal of Materials Chemistry A, 2017, 5(25): 13086.
DOI URL |
| [130] |
RAMPELBERG G, SCHAEKERS M, MARTENS K, et al. Semiconductor-metal transition in thin VO2 films grown by ozone based atomic layer deposition. Applied Physics Letters, 2011, 98(16): 162902.
DOI URL |
| [131] | ZHANG K, TANGIRALA M, NMINIBAPIEL D, et al. Synthesis of VO2 thin films by atomic layer deposition with TEMAV as precursor. ECS Transactions, 2013, 50(13): 175. |
| [132] |
PREMKUMAR P A, TOELLER M, RADU I P, et al. Process study and characterization of VO2 thin films synthesized by ALD using TEMAV and O3 precursors. ECS Journal of Solid State Science and Technology, 2012, 1(4): P169.
DOI URL |
| [133] |
PETER A P, MARTENS K, RAMPELBERG G, et al. Metal- insulator transition in ALD VO2 ultrathin films and nanoparticles: morphological control. Advanced Functional Materials, 2015, 25(5): 679.
DOI URL |
| [134] |
MATTELAER F, GERYL K, RAMPELBERG G, et al. Atomic layer deposition of vanadium oxides for thin-film lithium-ion battery applications. RSC Advances, 2016, 6(115): 114658.
DOI URL |
| [135] |
CURRIE M, MASTRO M A, WHEELER V D. Characterizing the tunable refractive index of vanadium dioxide. Optical Materials Express, 2017, 7(5): 1697.
DOI URL |
| [136] | CURRIE M, MASTRO M A, WHEELER V D. Atomic layer deposition of vanadium dioxide and a temperature-dependent optical model. Journal of Visualized Experiments, 2018, 135: 57103. |
| [137] |
GAO Y, SHAO Y, YAN L, et al. Efficient charge injection in organic field-effect transistors enabled by low-temperature atomic layer deposition of ultrathin VOx interlayer. Advanced Functional Materials, 2016, 26(25): 4456.
DOI URL |
| [138] |
SILVERSMIT G, DEPLA D, POELMAN H, et al. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). Journal of Electron Spectroscopy and Related Phenomena, 2004, 135(2): 167.
DOI URL |
| [139] |
MATERO R, RAHTU A, RITALA M, et al. Effect of water dose on the atomic layer deposition rate of oxide thin films. Thin Solid Films, 2000, 368(1): 1.
DOI URL |
| [140] |
OKIMURA K, WATANABE T, SAKAI J. Stress-induced VO2 films with M2 monoclinic phase stable at room temperature grown by inductively coupled plasma-assisted reactive sputtering. Journal of Applied Physics, 2012, 111(7): 073514.
DOI URL |
| [141] |
LEE W J, CHANG Y H. Growth without postannealing of monoclinic VO2 thin film by atomic layer deposition using VCl4 as precursor. Coatings, 2018, 8(12): 431.
DOI URL |
| [142] |
DUN H, PAN P, WHITE F R, et al. Mechanisms of plasma- enhanced silicon nitride deposition using SiH4/N2 mixture. Journal of the Electrochemical Society, 1981, 128(7): 1555.
DOI |
| [143] | MARTINU L, ZABEIDA O, KLEMBERG-SAPIEHA J E. Plasma- enhanced Chemical Vapor Deposition of Functional Coatings. // MARTIN P M. Handbook of Deposition Technologies for Films and Coatings (Third Edition). Boston: William Andrew Publishing, 2010: 392-465. |
| [144] | YASAMAN H, PRATHYUSHAKRISHNA M, TIMOTHY J B, et al. Plasma-enhanced Chemical Vapor Deposition: Where We are and the Outlook for the Future. // SUDHEER N. Chemical Vapor Deposition. Rijeka: IntechOpen, 2016: 247. |
| [145] | KONUMA M. Chemical Vapor Deposition under Plasma Conditions. // KONUMA M. Film Deposition by Plasma Techniques. Berlin, Heidelberg: Springer Berlin Heidelberg, 1992: 149-184. |
| [146] |
PAN Y, LIU H, WANG Z, et al. Optical constant and conformality analysis of SiO2 thin films deposited on linear array microstructure substrate by PECVD. Coatings, 2021, 11(5): 510.
DOI URL |
| [147] |
OH S J, MA B S, YANG C, et al. Intrinsic mechanical properties of free-standing SiNx thin films depending on PECVD conditions for controlling residual stress. ACS Applied Electronic Materials, 2022, 4(8): 3980.
DOI URL |
| [148] |
GARCIA-BARRIENTOS A, BERNAL-PONCE J L, PLAZA- CASTILLO J, et al. Analysis, synthesis and characterization of thin films of a-Si:H (n-type and p-type) deposited by PECVD for solar cell applications. Materials (Basel), 2021, 14(21): 6349.
DOI URL |
| [149] |
ASCHWANDEN R, KÖTHEMANN R, ALBERT M, et al. Optical properties of silicon oxynitride films grown by plasma-enhanced chemical vapor deposition. Thin Solid Films, 2021, 736: 138887.
DOI URL |
| [150] |
KUYPERS A D, SPEE C I M A, LINDEN J L, et al. Plasma- enhanced CVD of electrochromic materials. Surface and Coatings Technology, 1995, 74-75: 1033.
DOI URL |
| [151] |
ZHANG J G, LIU P, TURNER J A, et al. Highly stable vanadium oxide cathodes prepared by plasma-enhanced chemical vapor deposition. Journal of the Electrochemical Society, 1998, 145(6): 1889.
DOI |
| [152] |
BARRECA D, ARMELAO L, CACCAVALE F, et al. Highly oriented V2O5 nanocrystalline thin films by plasma-enhanced chemical vapor deposition. Chemistry of Materials, 2000, 12(1): 98.
DOI URL |
| [153] |
FUERST T F, REESE M O, WOLDEN C A. PECVD synthesis of flexible optical coatings for renewable energy applications. Plasma Processes and Polymers, 2016, 13(1): 184.
DOI URL |
| [154] | MONNE M A, ZAID A, MIA D, et al. Anti-reflective Coating for Flexible Devices Using Plasma Enhanced Chemical Vapor Deposition Technique. 2018 International Conference on Optical MEMS and Nanophotonics (OMN), Lausanne, Switzerland, 2018: 1-2. |
| [155] |
XU F, JIN P S, LUO H J, et al. VO2 thermochromic smart window: status, challenges and prospects. Journal of Inorganic Materials, 2021, 36(10): 1013.
DOI URL |
| [156] |
PAN G, YIN J, JI K, et al. Synthesis and thermochromic property studies on W doped VO2 films fabricated by Sol-Gel method. Scientific Reports, 2017, 7: 6132.
DOI |
| [157] |
TAN X, YAO T, LONG R, et al. Unraveling metal-insulator transition mechanism of VO2 triggered by tungsten doping. Scientific Reports, 2012, 2: 466.
DOI |
| [158] |
WU Y, FAN L, HUANG W, et al. Depressed transition temperature of WxV1-xO2: mechanistic insights from the X-ray absorption fine structure (XAFS) spectroscopy. Physical Chemistry Chemical Physics, 2014, 16(33): 17705.
DOI URL |
| [159] |
MANNING T D, PARKIN I P. Atmospheric pressure chemical vapour deposition of tungsten doped vanadium(IV) oxide from VOCl3, water and WCl6. Journal of Materials Chemistry, 2004, 14(16): 2554.
DOI URL |
| [160] |
MANNING T D, PARKIN I P, BLACKMAN C, et al. APCVD of thermochromic vanadium dioxide thin films—solid solutions V2-xMxO2 (M = Mo, Nb) or composites VO2: SnO2. Journal of Materials Chemistry, 2005, 15(42): 4560.
DOI URL |
| [161] |
MANNING T D, PARKIN I P, PEMBLE M E, et al. Intelligent window coatings:atmospheric pressure chemical vapor deposition of tungsten-doped vanadium dioxide. Chemistry of Materials, 2004, 16(4): 744.
DOI URL |
| [162] |
BLACKMAN C S, PICCIRILLO C, BINIONS R, et al. Atmospheric pressure chemical vapour deposition of thermochromic tungsten doped vanadium dioxide thin films for use in architectural glazing. Thin Solid Films, 2009, 517(16): 4565.
DOI URL |
| [163] |
BINIONS R, HYETT G, PICCIRILLO C, et al. Doped and un-doped vanadium dioxide thin films prepared by atmospheric pressure chemical vapour deposition from vanadyl acetylacetonate and tungsten hexachloride: the effects of thickness and crystallographic orientation on thermochromic properties. Journal of Materials Chemistry, 2007, 17(44): 4652.
DOI URL |
| [164] |
VERNARDOU D, PEMBLE M E, SHEEL D W. Tungsten-doped vanadium oxides prepared by direct liquid injection MOCVD. Chemical Vapor Deposition, 2007, 13(4): 158.
DOI URL |
| [165] |
PICCIRILLO C, BINIONS R, PARKIN I P. Synthesis and characterisation of W-doped VO2 by aerosol assisted chemical vapour deposition. Thin Solid Films, 2008, 516(8): 1992.
DOI URL |
| [166] |
GRANQVIST C G. Recent progress in thermochromics and electrochromics: a brief survey. Thin Solid Films, 2016, 614: 90.
DOI URL |
| [167] |
MLYUKA N R, NIKLASSON G A, GRANQVIST C G. Mg doping of thermochromic VO2 films enhances the optical transmittance and decreases the metal-insulator transition temperature. Applied Physics Letters, 2009, 95(17): 171909.
DOI URL |
| [168] |
WANG N, LIU S, ZENG X T, et al. Mg/W-codoped vanadium dioxide thin films with enhanced visible transmittance and low phase transition temperature. Journal of Materials Chemistry C, 2015, 3(26): 6771.
DOI URL |
| [169] |
GUO B, WAN D, WANG J, et al. Mo-Al co-doped VO2(B) thin films: CVD synthesis, thermal sensitive properties, synchrotron radiation photoelectron and absorption spectroscopy study. Journal of Alloys and Compounds, 2018, 745: 247.
DOI URL |
| [170] |
SHEN N, CHEN S, SHI R, et al. Phase transition hysteresis of tungsten doped VO2 synergistically boosts the function of smart windows in ambient conditions. ACS Applied Electronic Materials, 2021, 3(8): 3648.
DOI URL |
| [171] |
DANG Y, WANG D, ZHANG X, et al. Structure and thermochromic properties of Mo-doped VO2 thin films deposited by Sol-Gel method. Inorganic and Nano-Metal Chemistry, 2019, 49(4): 120.
DOI URL |
| [172] |
LI H, WANG J, YOU Z, et al. Nb-doped VO2 thin films with enhanced thermal sensing performance for uncooled infrared detection. Materials Research Bulletin, 2022, 146: 111615.
DOI URL |
| [173] |
LI B, TIAN S, WANG Z, et al. Thermochromic Ta doped VO2 films: enhanced luminous transmittance, significantly depressed phase transition temperature and hysteresis width. Applied Surface Science, 2021, 568: 150959.
DOI URL |
| [174] |
SHEN N, CHEN S, CHEN Z, et al. The synthesis and performance of Zr-doped and W-Zr-codoped VO2 nanoparticles and derived flexible foils. Journal of Materials Chemistry A, 2014, 2(36): 15087.
DOI URL |
| [175] | GENG X, CHANG T, FAN J, et al. Tuning phase transition and thermochromic properties of vanadium dioxide thin films via cobalt doping. ACS Applied Materials & Interfaces, 2022, 14(17): 19736. |
| [176] |
WANG N, DUCHAMP M, DUNIN-BORKOWSKI R E, et al. Terbium-doped VO2 thin films: reduced phase transition temperature and largely enhanced luminous transmittance. Langmuir, 2016, 32(3): 759.
DOI URL |
| [177] |
WANG N, TAN N C S, DUCHAMP M, et al. Effect of lanthanum doping on modulating the thermochromic properties of VO2 thin films. RSC Advances, 2016, 6(54): 48455.
DOI URL |
| [178] |
CAO X, WANG N, MAGDASSI S, et al. Europium doped vanadium dioxide material: reduced phase transition temperature, enhanced luminous transmittance and solar modulation. Science of Advanced Materials, 2014, 6(3): 558.
DOI URL |
| [179] |
ZOU Z, ZHANG Z, XU J, et al. Phase transition mechanism and application of silicon-doped VO2 thin films to smart windows. Journal of Materials Science: Materials in Electronics, 2021, 32(19): 23825.
DOI |
| [180] |
JI C, WU Z, LU L, et al. High thermochromic performance of Fe/Mg co-doped VO2 thin films for smart window applications. Journal of Materials Chemistry C, 2018, 6(24): 6502.
DOI URL |
| [181] |
WANG N, GOH Q S, LEE P L, et al. One-step hydrothermal synthesis of rare earth/W-codoped VO2 nanoparticles: reduced phase transition temperature and improved thermochromic properties. Journal of Alloys and Compounds, 2017, 711: 222.
DOI URL |
| [182] |
QURESHI U, MANNING T D, PARKIN I P. Atmospheric pressure chemical vapour deposition of VO2 and VO2/TiO2 films from the reaction of VOCl3, TiCl4 and water. Journal of Materials Chemistry, 2004, 14(7): 1190.
DOI URL |
| [183] |
QURESHI U, MANNING T D, BLACKMAN C, et al. Composite thermochromic thin films: (TiO2)-(VO2) prepared from titanium isopropoxide, VOCl3 and water. Polyhedron, 2006, 25(2): 334.
DOI URL |
| [184] |
WILKINSON M, KAFIZAS A, BAWAKED S M, et al. Combinatorial atmospheric pressure chemical vapor deposition of graded TiO2-VO2 mixed-phase composites and their dual functional property as self-cleaning and photochromic window coatings. ACS Combinatorial Science, 2013, 15(6): 309.
DOI URL |
| [185] |
CHEN Z, CAO C, CHEN S, et al. Crystallised mesoporous TiO2(A)-VO2(M/R) nanocomposite films with self-cleaning and excellent thermochromic properties. Journal of Materials Chemistry A, 2014, 2(30): 11874.
DOI URL |
| [186] | ZHU J, ZHOU Y, WANG B, et al. Vanadium dioxide nanoparticle- based thermochromic smart coating: high luminous transmittance, excellent solar regulation efficiency, and near room temperature phase transition. ACS Applied Materials & Interfaces, 2015, 7(50): 27796. |
| [187] |
KANG J, LIU J, SHI F, et al. Facile fabrication of VO2/SiO2 aerogel composite films with excellent thermochromic properties for smart windows. Applied Surface Science, 2022, 573: 151507.
DOI URL |
| [188] |
ZHANG Z, ZHANG L, ZHOU Y, et al. Thermochromic energy efficient windows: fundamentals, recent advances, and perspectives. Chemical Reviews, 2023, 123(11): 7025.
DOI PMID |
| [189] | RAYLEIGH L. On reflection of vibrations at the confines of two media between which the transition is gradual. Proceedings of the London Mathematical Society, 1879, 1-11(1): 51. |
| [190] |
XU G, JIN P, TAZAWA M, et al. Optimization of antireflection coating for VO2-based energy efficient window. Solar Energy Materials and Solar Cells, 2004, 83(1): 29.
DOI URL |
| [191] |
LIU C, WANG S, ZHOU Y, et al. Index-tunable anti-reflection coatings: maximizing solar modulation ability for vanadium dioxide-based smart thermochromic glazing. Journal of Alloys and Compounds, 2018, 731: 1197.
DOI URL |
| [192] |
ZHAN Y, LU Y, XIAO X, et al. Tuning thermochromic performance of VOx-based multilayer films by controlling annealing pressure. Ceramics International, 2020, 46(2): 2079.
DOI URL |
| [193] |
EVANS P, PEMBLE M E, SHEEL D W, et al. Multi-functional self-cleaning thermochromic films by atmospheric pressure chemical vapour deposition. Journal of Photochemistry and Photobiology A: Chemistry, 2007, 189(2): 387.
DOI URL |
| [194] | BRECKENFELD E, KIM H, BURGESS K, et al. Strain effects in epitaxial VO2 thin films on columnar buffer-layer TiO2/Al2O3 virtual substrates. ACS Applied Materials & Interfaces, 2017, 9(2): 1577. |
| [195] |
POWELL M J, QUESADA-CABRERA R, TAYLOR A, et al. Intelligent multifunctional VO2/SiO2/TiO2 coatings for self-cleaning, energy-saving window panels. Chemistry of Materials, 2016, 28(5): 1369.
DOI URL |
| [196] |
YU J H, NAM S H, LEE J W, et al. Enhanced visible transmittance of thermochromic VO2 thin films by SiO2 passivation layer and their optical characterization. Materials, 2016, 9(7): 556.
DOI URL |
| [197] |
KOUDOUMAS E, LE K T, VERNARDOU D. Recent advances of chemical vapor deposited thermochromic vanadium dioxide materials. Energy Nexus, 2023, 11: 100237.
DOI URL |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | DONG Chenyu, ZHENG Weijie, MA Yifan, ZHENG Chunyan, WEN Zheng. Characterizations by Piezoresponse Force Microscopy on Relaxor Properties of Pb(Mg,Nb)O3-PbTiO3 Ultra-thin Films [J]. Journal of Inorganic Materials, 2025, 40(6): 675-682. |
| [4] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [5] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [6] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [7] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [11] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [12] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [13] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [14] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [15] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
