
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (6): 589-605.DOI: 10.15541/jim20220331
Special Issue: 【能源环境】储能电池(202506); 【能源环境】锂离子电池(202412)
• REVIEW • Next Articles
YANG Zhuo( ), LU Yong, ZHAO Qing, CHEN Jun(
), LU Yong, ZHAO Qing, CHEN Jun( )
)
Received:2022-06-14
Revised:2022-12-28
Published:2023-01-11
Online:2023-01-11
Contact:
CHEN Jun, professor. E-mail: chenabc@nankai.edu.cnAbout author:YANG Zhuo (1993-), male, PhD candidate. E-mail: yzhuo94@outlook.com.
Supported by:CLC Number:
YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries[J]. Journal of Inorganic Materials, 2023, 38(6): 589-605.
| Cathode material | Crystal structure | Space group | Cell parameter | Atom site | Theoretical specific capacity/(mAh·g-1) | Working voltage/ V (vs. Li+/Li) | |
|---|---|---|---|---|---|---|---|
| LiFePO4 | Olivine | Pnma | a≠b≠c | Li | 4a | 170 | 3.4 |
| Fe | 4c | ||||||
| O | 4c/8d | ||||||
| LiMn2O4 | Spinel | Fd-3m | a=b=c | Li | 8a | 148 | 4 |
| Mn | 16d | ||||||
| O | 32e | ||||||
| LiCoO2 | Layer | R-3m | a=b≠c | Li | 3a | 274 | 3.9 |
| Co | 3b | ||||||
| O | 6c | ||||||
| LiNixCoy(Mn/Al)1-x-yO2 | Layer | R-3m | a=b≠c | Li | 3a | 273-285 | 3.8 |
| Ni/Co/Mn/Al | 3b | ||||||
| O | 6c | ||||||
| xLi2MnO3·(1-x)LiMO2 (0<x<1, M=Ni, Co, Mn) | Layer | R-3m+ C2/m | a=b≠c | Li | 2b/2c/4h | 273-350 | 3.8 |
| Mn | 4g | ||||||
| O | 4i/4j | ||||||
Table 1 Structures and properties of common cathode materials for lithium-ion batteries[24⇓⇓⇓-28]
| Cathode material | Crystal structure | Space group | Cell parameter | Atom site | Theoretical specific capacity/(mAh·g-1) | Working voltage/ V (vs. Li+/Li) | |
|---|---|---|---|---|---|---|---|
| LiFePO4 | Olivine | Pnma | a≠b≠c | Li | 4a | 170 | 3.4 |
| Fe | 4c | ||||||
| O | 4c/8d | ||||||
| LiMn2O4 | Spinel | Fd-3m | a=b=c | Li | 8a | 148 | 4 |
| Mn | 16d | ||||||
| O | 32e | ||||||
| LiCoO2 | Layer | R-3m | a=b≠c | Li | 3a | 274 | 3.9 |
| Co | 3b | ||||||
| O | 6c | ||||||
| LiNixCoy(Mn/Al)1-x-yO2 | Layer | R-3m | a=b≠c | Li | 3a | 273-285 | 3.8 |
| Ni/Co/Mn/Al | 3b | ||||||
| O | 6c | ||||||
| xLi2MnO3·(1-x)LiMO2 (0<x<1, M=Ni, Co, Mn) | Layer | R-3m+ C2/m | a=b≠c | Li | 2b/2c/4h | 273-350 | 3.8 |
| Mn | 4g | ||||||
| O | 4i/4j | ||||||

Fig. 3 Schematic diagram of the crystal structures of cathode materials[25⇓⇓-28] (a) LiMPO4 (M=Fe, Mn)(© 2021, IOP Publishing)[25]; (b) LiMn2O4 (© 2022, MDPI)[26]; (c) LiMO2 (M=Ni, Co, Mn, Al) (© 2021, Elsevier Ltd.)[27]; (d) xLi2MnO3·(1-x)LiMO2 (M=Ni, Co, Mn) (© 2022, Springer Nature)[28] Colorful figures are available on website

Fig. 5 Structural evolution during high temperature synthesis of LiNi0.8Co0.2O2 (© 2017, Wiley-VCH)[41] (a) Concentration of the phases Ni(Co)O, Li2CO3, and LiNi0.8Co0.2O2 at different temperatures; (b) Ratio unit cell parameters (c/a), (c) content of Li+ occupying the 3a sites (Li sites), and (d) Ni-O and Li-O bond lengths as a function of temperature; (e) Schematic diagram of the structural evolution and variation of the interlayer distances of the Li and Ni(Co) slabs during the synthesis of LiNi0.8Co0.2O2. 1 Å=0.1 nm. Colorful figures are available on website
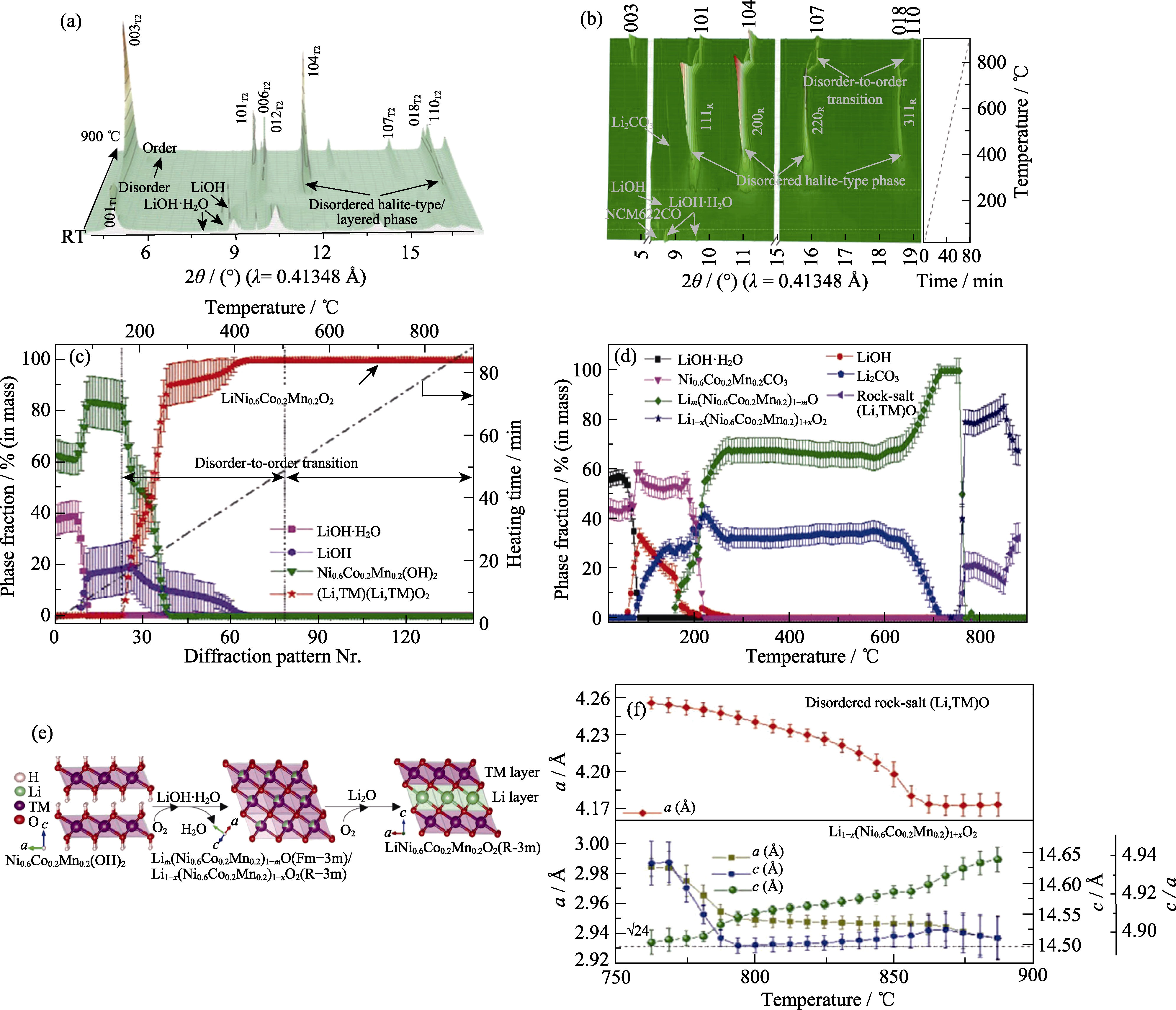
Fig. 6 Temperature-resolved XRD characterization of synthesis process of cathode material LiNi0.6Co0.2Mn0.2O2 (© 2021, Wiley-VCH)[43] (a) In situ XRD patterns of the mixture of Ni0.6Co0.2Mn0.2(OH)2 and LiOH·H2O during heating and (c) corresponding weight fractions of different phases as a function of temperature; (b) 3D profiles of in situ XRD patterns, corresponding (d) evolution of phase fraction and (f) lattice parameters of the samples as a function of heating temperature starting from mixture of the Ni0.6Co0.2Mn0.2CO3 and LiOH·H2O; (e) Schematic illustration of structural evolution during synthesis of LiNi0.6Co0.2Mn0.2O2; In (a, b), the subscripts T1, T2 and R represent Ni0.6Co0.2Mn0.2(OH)2 (P-3m1, T1 phase), LiNi0.6Co0.2Mn0.2O2 (R-3m, T2 phase) and the rock-salt-type, respectively. 1 Å=0.1 nm Colorful figures are available on website

Fig. 7 In-situ XRD characterization of microwave (MW) hydrothermal synthesis of NCM111(© 2020, AAAS) [44] (a) Schematic illustration of the experimental setup specialized for fast synchrotron X-ray probing of the microwave hydrothermal synthesis; (b) Time resolved synchrotron XRD patterns during MW hydrothermal synthesis of NCM111; (c) Lattice parameter c of the Ni1/3Co1/3Mn1/3(OH)2 precursor (green) as a function of temperature during solid-state synthesis (black), hydrothermal synthesis (blue), and MW hydrothermal synthesis (red). 1 Å=0.1 nm. Colorful figures are available on website
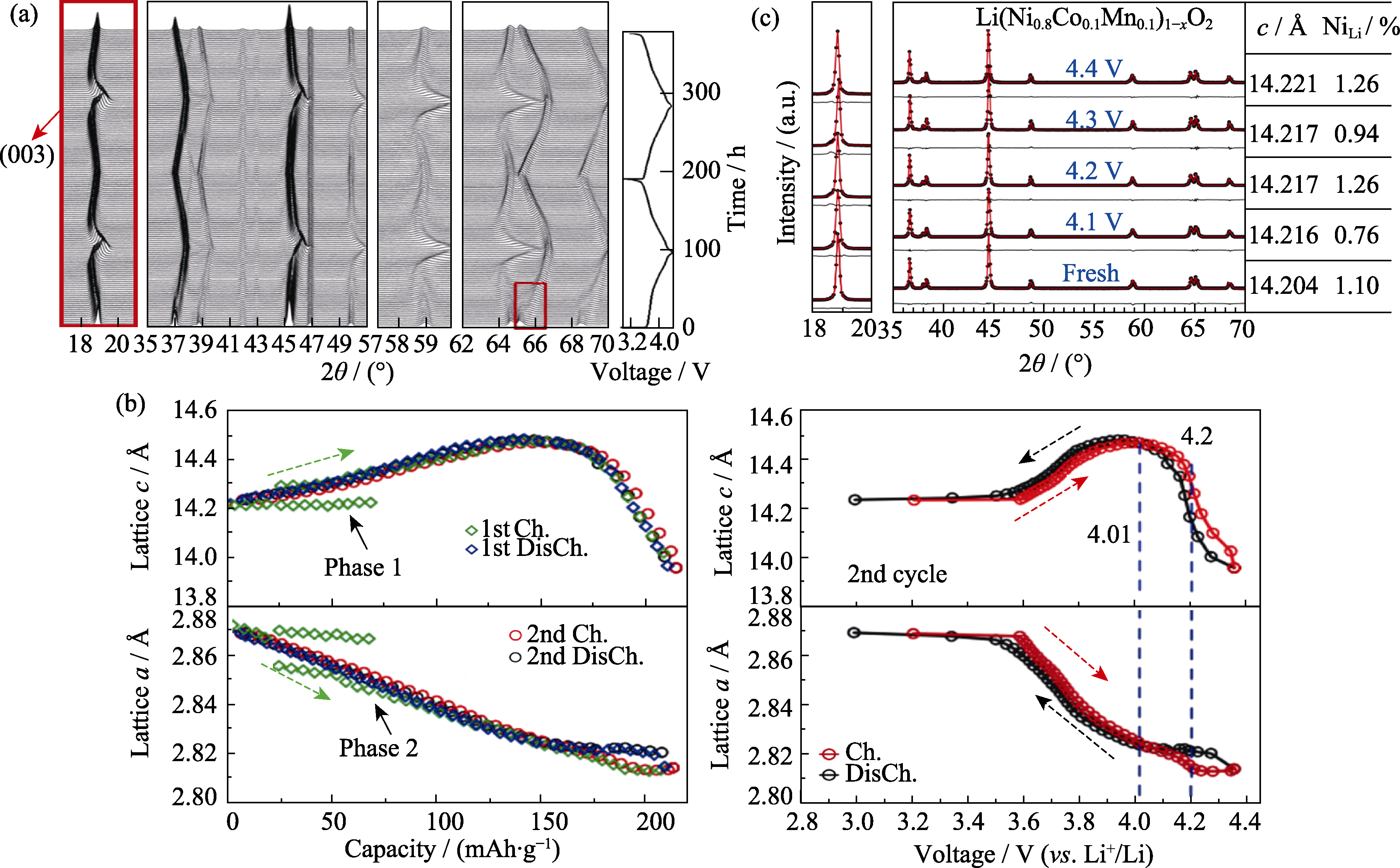
Fig. 8 In situ XRD characterization of Ni-rich cathode LiNi0.8Co0.1Mn0.1O2(NCM811) during charge-discharge (© 2015, ECS)[49] (a) In-situ XRD patterns of NCM811 cycled between 3.0-4.4 V at a rate of C/100 for two cycles; (b) Cell parameters c and a as functions of specific capacity and cell potential; (c) XRD refinement patterns, corresponding cell parameters and Li/Ni occupation information of fresh NCM811 electrode and the recovered electrodes that cycled 200 times to 4.1, 4.2, 4.3 and 4.4 V, respectively. 1 Å=0.1 nm; Ch: Charged; DisCh: Discharged. Colorful figures are available on website

Fig. 9 In-situ XRD characterization of Li1.2Ni0.13Co0.13Mn0.54O2 during charge-discharge process (© 2021, Springer Nature)[54] (a) Charge-discharge curve and corresponding contour plot of XRD pattern during the 201st cycle with the colour red to blue representing the decreasing peak intensity; (b, c) Lattice parameters (c and V) as functions of potential during the 1st and 201st cycles after the electrode activated at a rate of 0.1C. 1 Å=0.1 nm. Colorful figures are available on website
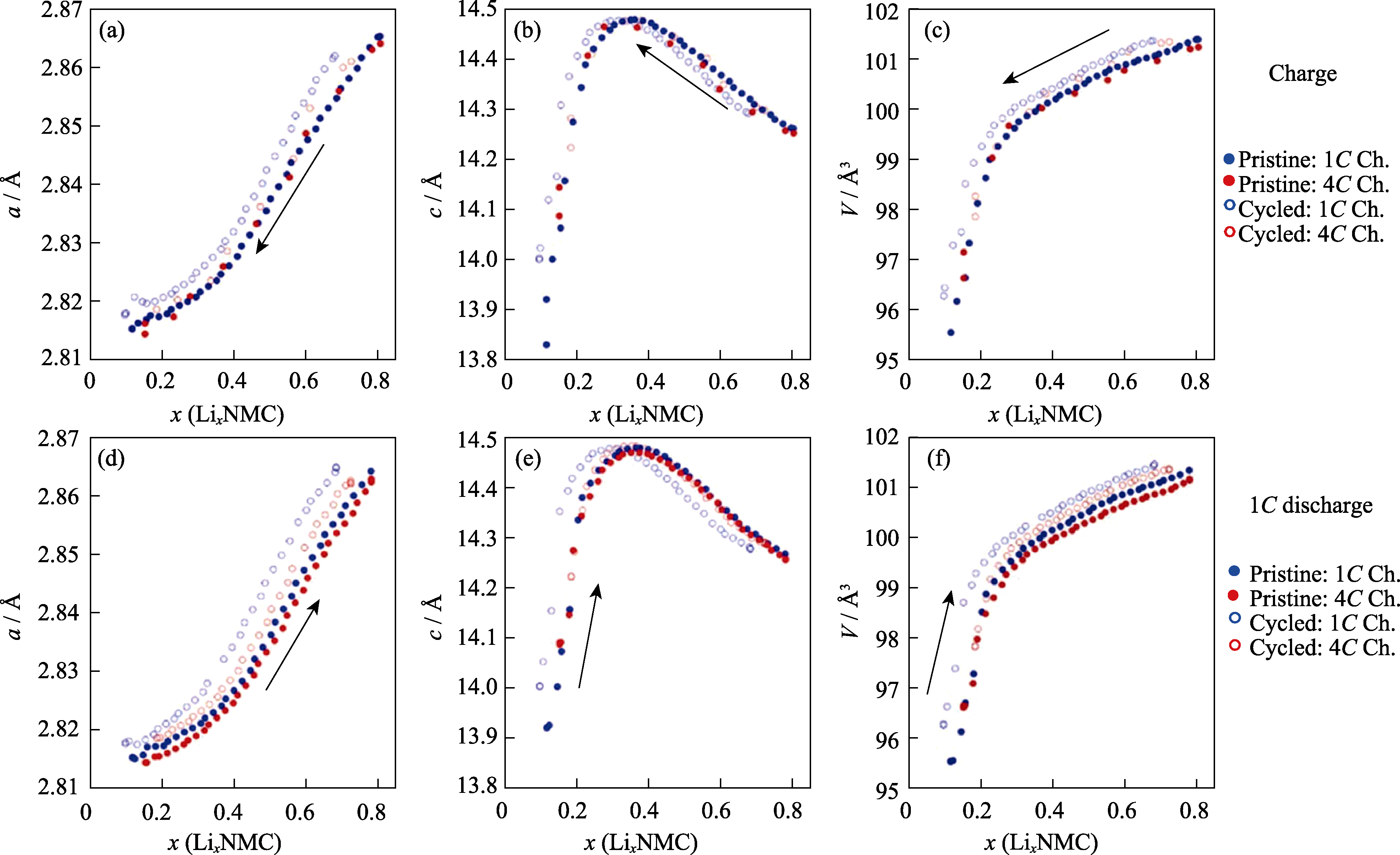
Fig. 10 In-situ XRD Rietveld refinement results of LiNi0.8Co0.1Mn0.1O2 cathode materials during charge and discharge process (© 2022, ECS)[57] (a, d) Lattice parameters a; (b, e) Lattice parameters c; (c, f) Unit cell volumes. 1 Å=0.1 nm. Colorful figures are available on website
| Sample | Doped atomic radius/nm | Displace ion radius/nm | Lattice constant/nm | Lattice volume/nm3 | Interatomic distance/nm | Reliability factor |
|---|---|---|---|---|---|---|
| LFP | rFe = 0.172 | rFe2+ = 0.074 | a=1.031634 b=0.600129 c=0.469139 | 0.29045 | Li-O1:0.21664 Li-O2:0.20901 Li-O3:0.21651 Li-O:0.214050 | Rwp = 7.72% Rp =5.63% χ2 = 2.794 |
| LFMgP | rMg = 0.172 | rMg2+ = 0.065 | a=1.031583 b=0.600035 c=0.469090 | 0.29036 | Li-O1:0.21712 Li-O2:0.21041 Li-O3:0.21665 Li-O:0.214720 | Rwp = 9.32% Rp = 6.79% χ2 = 2.878 |
| LFAlP | rAl = 0.182 | rAl3+ = 0.050 | a=1.032204 b=0.600358 c=0.469072 | 0.29068 | Li-O1:0.21670 Li-O2:0.21001 Li-O3:0.21747 Li-O:0.214730 | Rwp = 9.14% Rp = 6.60% χ2 = 2.989 |
| LFNiP | rNi = 0.162 | rNi2+ = 0.072 | a=1.031083 b=0.599820 c=0.468923 | 0.29001 | Li-O1:0.21734 Li-O2:0.20863 Li-O3:0.21586 Li-O:0.213950 | Rwp = 8.26% Rp = 6.12% χ2= 2.929 |
| LFVP | rV = 0.192 | rV3+ = 0.074 | a=1.032223 b=0.600494 c=0.469485 | 0.291 | Li-O1:0.21864 Li-O2:0.21074 Li-O3:0.21794 Li-O:0.215770 | Rwp = 9.86% Rp = 7.15% χ2= 2.426 |
Table 2 Structure refinement result of LiFePO4 and LiFe0.95M0.05PO4 (M=Mg2+, Ni2+, Al3+, V3+) (© 2010, EC)[62]
| Sample | Doped atomic radius/nm | Displace ion radius/nm | Lattice constant/nm | Lattice volume/nm3 | Interatomic distance/nm | Reliability factor |
|---|---|---|---|---|---|---|
| LFP | rFe = 0.172 | rFe2+ = 0.074 | a=1.031634 b=0.600129 c=0.469139 | 0.29045 | Li-O1:0.21664 Li-O2:0.20901 Li-O3:0.21651 Li-O:0.214050 | Rwp = 7.72% Rp =5.63% χ2 = 2.794 |
| LFMgP | rMg = 0.172 | rMg2+ = 0.065 | a=1.031583 b=0.600035 c=0.469090 | 0.29036 | Li-O1:0.21712 Li-O2:0.21041 Li-O3:0.21665 Li-O:0.214720 | Rwp = 9.32% Rp = 6.79% χ2 = 2.878 |
| LFAlP | rAl = 0.182 | rAl3+ = 0.050 | a=1.032204 b=0.600358 c=0.469072 | 0.29068 | Li-O1:0.21670 Li-O2:0.21001 Li-O3:0.21747 Li-O:0.214730 | Rwp = 9.14% Rp = 6.60% χ2 = 2.989 |
| LFNiP | rNi = 0.162 | rNi2+ = 0.072 | a=1.031083 b=0.599820 c=0.468923 | 0.29001 | Li-O1:0.21734 Li-O2:0.20863 Li-O3:0.21586 Li-O:0.213950 | Rwp = 8.26% Rp = 6.12% χ2= 2.929 |
| LFVP | rV = 0.192 | rV3+ = 0.074 | a=1.032223 b=0.600494 c=0.469485 | 0.291 | Li-O1:0.21864 Li-O2:0.21074 Li-O3:0.21794 Li-O:0.215770 | Rwp = 9.86% Rp = 7.15% χ2= 2.426 |

Fig. 11 XRD Rietveld refinement results of LiFePO4 before and after modification (© 2021, RSC)[63] (a, b) XRD Rietveld refinement patterns of (a) LFP/C and (b) LFP/C-YF-2; (c, d) Schematic diagrams of change in Li-O bond length of (c) LFP/C and (d) LFP/C-YF-2. 1 Å=0.1 nm. Colorful figures are available on website
| Sample | a/nm | b/nm | c/nm | V/nm3 |
|---|---|---|---|---|
| LFP/C | 1.03229 | 0.60061 | 0.46941 | 0.29104 |
| LFP/C-YF-1 | 1.03054 | 0.59985 | 0.46903 | 0.28994 |
| LFP/C-YF-2 | 1.03082 | 0.59977 | 0.46874 | 0.28980 |
| LFP/C-YF-3 | 1.03069 | 0.59989 | 0.46892 | 0.28994 |
Table 3 Cell parameters of LiFePO4 before and after modification by XRD refinement (© 2021, RSC)[63]
| Sample | a/nm | b/nm | c/nm | V/nm3 |
|---|---|---|---|---|
| LFP/C | 1.03229 | 0.60061 | 0.46941 | 0.29104 |
| LFP/C-YF-1 | 1.03054 | 0.59985 | 0.46903 | 0.28994 |
| LFP/C-YF-2 | 1.03082 | 0.59977 | 0.46874 | 0.28980 |
| LFP/C-YF-3 | 1.03069 | 0.59989 | 0.46892 | 0.28994 |
| Atom | Site | x | y | z | Occupancy | Uiso |
|---|---|---|---|---|---|---|
| Lia | 3a | 0 | 0 | 0 | 1.000 | 0.014(6) |
| Coa | 3b | 0 | 0 | 0.50000 | 1.000 | 0.023(8) |
| Oa | 6c | 0 | 0 | 0.2300(6) | 1.000 | 0.049(1) |
| Lib | 3a | 0 | 0 | 0 | 0.98(1) | 0.020(1) |
| Mgb | 3a | 0 | 0 | 0 | 0.01(9) | 0.020(1) |
| Cob | 3b | 0 | 0 | 0.50000 | 0.99(7) | 0.001(2) |
| Alb | 3b | 0 | 0 | 0.50000 | 0.002(0) | 0.001(2) |
| Tib | 3b | 0 | 0 | 0.50000 | 0.001(0) | 0.001(2) |
| Ob | 6c | 0 | 0 | 0.2476(3) | 1.000 | 0.068(5) |
Table 4 XRD refinement result of Al, Ti, Mg co-doped LiCoO2 and bare LiCoO2 (© 2019, Wiley-VCH)[64]
| Atom | Site | x | y | z | Occupancy | Uiso |
|---|---|---|---|---|---|---|
| Lia | 3a | 0 | 0 | 0 | 1.000 | 0.014(6) |
| Coa | 3b | 0 | 0 | 0.50000 | 1.000 | 0.023(8) |
| Oa | 6c | 0 | 0 | 0.2300(6) | 1.000 | 0.049(1) |
| Lib | 3a | 0 | 0 | 0 | 0.98(1) | 0.020(1) |
| Mgb | 3a | 0 | 0 | 0 | 0.01(9) | 0.020(1) |
| Cob | 3b | 0 | 0 | 0.50000 | 0.99(7) | 0.001(2) |
| Alb | 3b | 0 | 0 | 0.50000 | 0.002(0) | 0.001(2) |
| Tib | 3b | 0 | 0 | 0.50000 | 0.001(0) | 0.001(2) |
| Ob | 6c | 0 | 0 | 0.2476(3) | 1.000 | 0.068(5) |
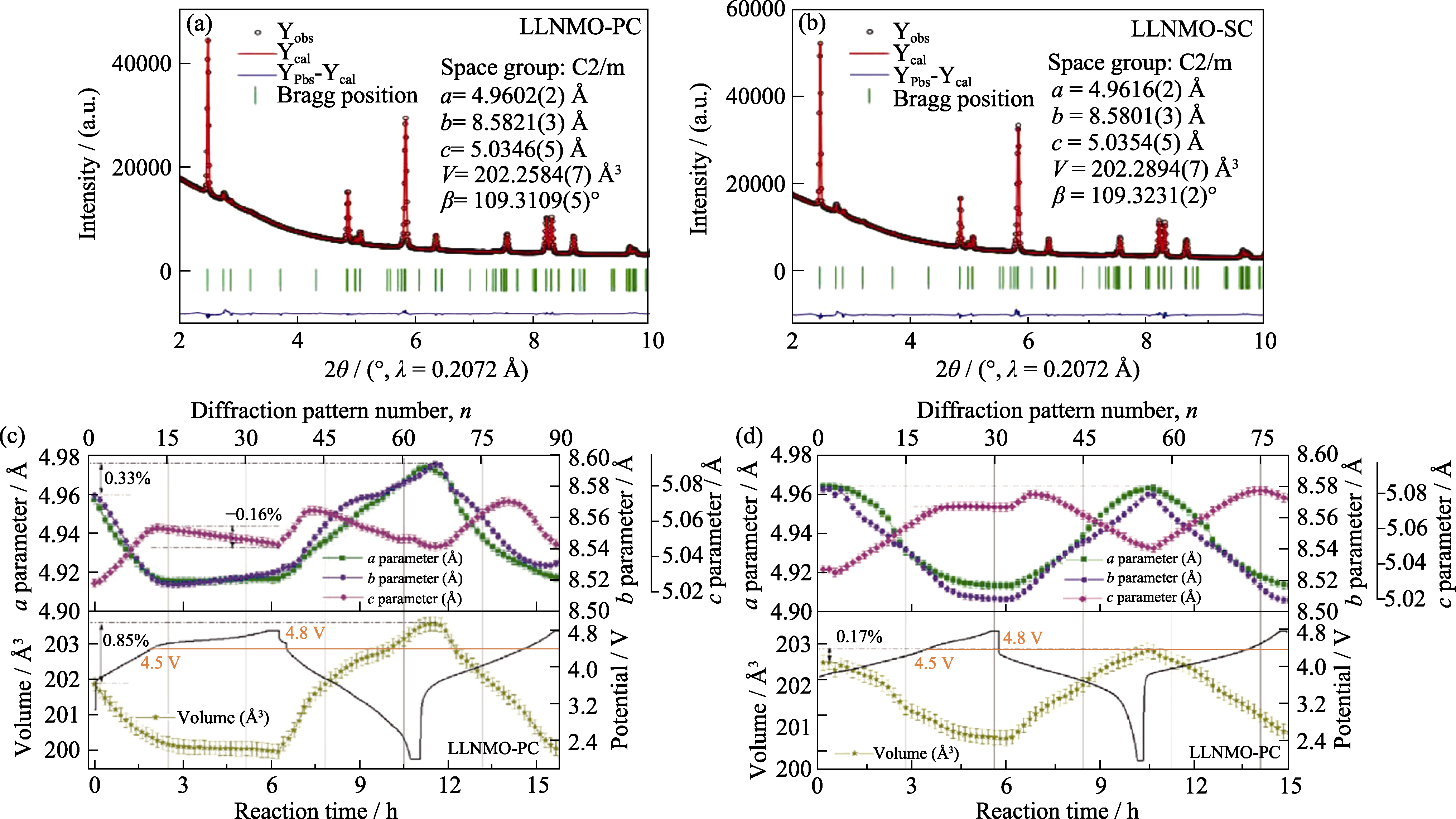
Fig. 12 XRD Rietveld refinement results of Li(Li0.2Ni0.2Mn0.6)O2 cathode materials (© 2022, Wiley-VCH)[66] (a, b) Experimental XRD patterns and Rietveld refinement results of (a) LLNMO-PC and (b) LLNMO-SC; (c, d) Changes of lattice parameters (a, b, c, and V) for (c) LLNMO-PC and (d) LLNMO-SC electrodes during charge and discharge 1 Å=0.1 nm. Colorful figures are available on website
| Formula | Calculated | Experimental | ||||
|---|---|---|---|---|---|---|
| a/nm | b/nm | c/nm | V/nm3 | a/nm | V/nm3 | |
| Li8Mn16O32 | 0.886205 | 0.886205 | 0.886205 | 0.695990 | - | - |
| Li8Mn15AlO32 | 0.826725 | 0.826725 | 0.826725 | 0.567617 | 0.82507 | 0.561658 |
| Li8Mn14Al2O32 | 0.831493 | 0.831493 | 0.799071 | 0.552416 | 0.82466 | 0.560821 |
| Li8Mn13Al3O32 | 0.814375 | 0.826337 | 0.820583 | 0.551780 | 0.82110 | 0.553590 |
Table 5 XRD structure refinement result of Al doped LiMn2O4 (© 2019, Elsevier Ltd.)[69]
| Formula | Calculated | Experimental | ||||
|---|---|---|---|---|---|---|
| a/nm | b/nm | c/nm | V/nm3 | a/nm | V/nm3 | |
| Li8Mn16O32 | 0.886205 | 0.886205 | 0.886205 | 0.695990 | - | - |
| Li8Mn15AlO32 | 0.826725 | 0.826725 | 0.826725 | 0.567617 | 0.82507 | 0.561658 |
| Li8Mn14Al2O32 | 0.831493 | 0.831493 | 0.799071 | 0.552416 | 0.82466 | 0.560821 |
| Li8Mn13Al3O32 | 0.814375 | 0.826337 | 0.820583 | 0.551780 | 0.82110 | 0.553590 |

Fig. 13 Refined XRD patterns of multiphase materials (a) Li1.25Co0.25Mn0.50O2 (© 2018, Wiley-VCH)[70]; (b) LiMnPO4·Li3V2(PO4)3/C (© 2016, American Chemical Society)[71]
| [1] | 郑琼, 江丽霞, 徐玉杰, 等. 碳达峰、碳中和背景下储能技术研究进展与发展建议. 中国科学院院刊, 2022, 37(4):529. |
| [2] |
TARASCON J, ARMAND M. Issues and challenges facing rechargeable lithium batteries. Nature, 2001, 414(6861):359.
DOI URL |
| [3] |
ZENG H, HANG F. Energy materials in new era. Journal of Inorganic Materials, 2022, 37(2):113.
DOI |
| [4] |
ARMAND M, TARASCON J M. Building better batteries. Nature, 2008, 451(7179):652.
DOI |
| [5] |
XIANG J, WEI Y, ZHONG Y, et al. Building practical high-voltage cathode materials for lithium-ion batteries. Advanced Materials, 2022, 34(52): 2200912.
DOI URL |
| [6] |
WANG L, QIU J, WANG X, et al. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience, 2022, 2(2):125.
DOI URL |
| [7] |
ASSAT G, TARASCON J M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nature Energy, 2018, 3(5):373.
DOI |
| [8] |
CHEN Z, ZHANG W, YANG Z. A review on cathode materials for advanced lithium ion batteries: microstructure designs and performance regulations. Nanotechnology, 2020, 31(1):012001.
DOI URL |
| [9] |
SINGH J P, PAIDI A K, CHAE K H, et al. Synchrotron radiation based X-ray techniques for analysis of cathodes in Li rechargeable batteries. RSC Advance, 2022, 12(31):20360.
DOI URL |
| [10] |
RIETVELD H. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallographica, 1967, 22(1):151.
DOI URL |
| [11] | BRAGG W L. The structure of some crystals as indicated by their diffraction of X-rays. Proceedings of the Royal Society of London A, 1913, 89(610):248. |
| [12] | CULLITY B D. Elements of X-ray diffraction. Boston: Addison- Wesley Publishing Company, 1978:102. |
| [13] | HULBERT S L, WILLIAMS G P. 1. Synchrotron Radiation Sources. In: SAMSON J A R, EDERER D L. Experimental Methods in the Physical Sciences. Salt Lake City: Academic Press, 1998:1-25. |
| [14] |
MORCRETTE M, CHABRE Y, VAUGHAN G, et al. In situ X-ray diffraction techniques as a powerful tool to study battery electrode materials. Electrochemica Acta, 2002, 47(19):3137.
DOI URL |
| [15] |
HUANG N, DENG H, LIU B, et al. Features and futures of X-ray free-electron lasers. The Innovation, 2021, 2(2):100097.
DOI URL |
| [16] | ISHIKAWA T. Accelerator-based X-ray sources: synchrotron radiation, X-ray free electron lasers and beyond. Philosophical Transactions of the Royal Society A, 2019, 377(2147):20180231. |
| [17] | WELZEL U, MITTEMEIJER E J. Laboratory Instrumentation for X-Ray Powder Diffraction:Developments and Examples// MITTEMEIJER E J, WELZEL U. Modern Diffraction Methods. San Francisco: John Wiley and Sons, 2012:359-398. |
| [18] |
DAHN J R, HAERING R R. Anomalous Bragg peak widths in LixTiS2. Solid State Communications, 1981, 40(3):245.
DOI URL |
| [19] |
XIA M, LIU T, PENG N, et al. Lab-scale in situ X-ray diffraction technique for different battery systems: designs, applications, and perspectives. Small Methods, 2019, 3(7):1900119.
DOI URL |
| [20] |
LLEWELLYN A V, MATRUGLIO A, BRETT D J L, et al. Using in-situ laboratory and synchrotron-based X-ray diffraction for lithium-ion batteries characterization: a review on recent developments. Condensed Matter, 2020, 5(4):75.
DOI URL |
| [21] |
MUHAMMAD S, LEE S, KIM H, et al. Deciphering the thermal behavior of lithium rich cathode material by in situ X-ray diffraction technique. Journal of Power Sources, 2015, 285:156.
DOI URL |
| [22] |
MCCUSKER L B, VON DREELE R B, COX D E, et al. Rietveld refinement guidelines. Journal of Applied Crystallography, 1999, 32(1):36.
DOI URL |
| [23] | YOUNG R A. The Rietveld method. New York: Oxford University Press, 1993: 1. |
| [24] |
THOMAS M G S R, BRUCE P G, GOODENOUGH J B. AC impedance analysis of polycrystalline insertion electrodes: application to Li1-xCoO2. Journal of The Electrochemical Society, 1985, 132(7):1521.
DOI |
| [25] | ASTUTI F, MAGHFIROHTUZZOIMAH V L, INTIFADHAH S H, et al. Local structure and electronic structure of LiFePO4 as a cathode for lithium-ion batteries. Journal of Physics: Conference Series, 2021, 1951(1): 012007. |
| [26] |
YU F, WANG Y, GUO C, et al. Spinel LiMn2O4 cathode materials in wide voltage window: single-crystalline versus polycrystalline. Crystals, 2022, 12(3):317.
DOI URL |
| [27] |
LÜ Y, HUANG S, ZHAO Y, et al. A review of nickel-rich layered oxide cathodes: synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects. Applied Energy, 2022, 305:117849.
DOI URL |
| [28] |
NIE L, CHEN S, LIU W. Challenges and strategies of lithium-rich layered oxides for Li-ion batteries. Nano Research, 2023, 16:391.
DOI |
| [29] |
PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. Journal of The Electrochemical Society, 1997, 144(4):1188.
DOI |
| [30] | RADZI Z I, ARIFIN K H, KUFIAN M Z, et al. Review of spinel LiMn2O4 cathode materials under high cut-off voltage in lithium-ion batteries: challenges and strategies. Journal of Electroanalytical Chemistry, 2022, 920: 116623. |
| [31] |
THACKERAY M M.Exploiting the spinel structure for Li-ion battery applications: a tribute to John B. Goodenough. Advanced Energy Materials, 2021, 11(2):2001117.
DOI URL |
| [32] |
GRENIER A, REEVES P J, LIU H, et al. Intrinsic kinetic limitations in substituted lithium-layered transition-metal oxide electrodes. Journal of the American Chemical Society, 2020, 142(15):7001.
DOI PMID |
| [33] |
LIU J, WANG J, NI Y, et al. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Materials Today, 2021, 43:132.
DOI URL |
| [34] |
YOON C S, PARK K J, KIM U H, et al. High-energy Ni-rich Li[NixCoyMn1-x-y]O2 cathodes via compositional partitioning for next-generation electric vehicles. Chemistry of Materials, 2017, 29(24):10436.
DOI URL |
| [35] | 朱先军, 詹晖, 周运鸿. LiNi0.85Co0.15O2合成和结构与电化学性能关系. 化学学报, 2002, 10(60):1742. |
| [36] |
NI L, GUO R, FANG S, et al. Crack-free single-crystalline Co-free Ni-rich LiNi0.95Mn0.05O2 layered cathode. eScience, 2022, 2(1):116.
DOI URL |
| [37] |
HUA W, YANG X, CASATI N P M, et al. Probing thermally- induced structural evolution during the synthesis of layered Li-, Na-, or K-containing 3D transition-metal oxides. eScience, 2022, 2(2):183.
DOI URL |
| [38] |
ZHAO S, YAN K, ZHANG J, et al. Reaction mechanisms of layered lithium-rich cathode materials for high-energy lithium-ion batteries. Angewandte Chemie International Edition, 2021, 60(5): 2208.
DOI URL |
| [39] | YE Z, QIU L, YANG W, et al. Nickel-rich layered cathode materials for lithium-ion batteries. Chemistry, 2021, 27(13):4249. |
| [40] |
ZHENG J, YE Y, LIU T, et al. Ni/Li disordering in layered transition metal oxide: electrochemical impact, origin, and control. Accounts of Chemical Research, 2019, 52(8): 2201.
DOI PMID |
| [41] |
ZHAO J, ZHANG W, HUQ A, et al. In situ probing and synthetic control of cationic ordering in Ni-rich layered oxide cathodes. Advanced Energy Materials, 2017, 7(3):1601266.
DOI URL |
| [42] |
WEBER R, LI H, CHEN W, et al. In situ XRD studies during synthesis of single-crystal LiNiO2, LiNi0.975Mg0.025O2, and LiNi0.95Al0.05O2 cathode materials. Journal of the Electrochemical Society, 2020, 167(10):100501.
DOI |
| [43] |
WANG S, HUA W, MISSYUL A, et al. Kinetic control of long- range cationic ordering in the synthesis of layered Ni-rich oxides. Advanced Functional Materials, 2021, 31(19):2009949.
DOI URL |
| [44] |
ZHANG M J, DUAN Y, YIN C, et al. Ultrafast solid-liquid intercalation enabled by targeted microwave energy delivery. Science Advances, 2020, 6(51):eabd9472.
DOI URL |
| [45] |
LEE E J, CHEN Z, NOH H J, et al. Development of microstrain in aged lithium transition metal oxides. Nano Letters, 2014, 14(8):4873.
DOI URL |
| [46] |
XU C, MARKER K, LEE J, et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nature Materials, 2021, 20(1):84.
DOI |
| [47] |
PUSHNITSA K A, KIM A E, POPOVICH A A, et al. Structural transformation of LiNi0.8Co0.1Mn0.1O2 cathode material during cycling with overcharge investigated by in situ X-ray diffraction. Journal of Electronic Materials, 2019, 48(10):6694.
DOI |
| [48] |
DE BIASI L, SCHIELE A, ROCA-AYATS M, et al. Phase transformation behavior and stability of LiNiO2 cathode material for Li-ion batteries obtained from in situ gas analysis and operando X-ray diffraction. ChemSusChem, 2019, 12(10):2240.
DOI URL |
| [49] |
LI J, DOWNIE L E, MA L, et al. Study of the failure mechanisms of LiNi0.8Mn0.1Co0.1O2 cathode material for lithium ion batteries. Journal of the Electrochemical Society, 2015, 162(7):A1401.
DOI URL |
| [50] |
LIU J, WU Z, YU M, et al. Building homogenous Li2TiO3 coating layer on primary particles to stabilize Li-rich Mn-based cathode materials. Small, 2022, 18(10):2106337.
DOI URL |
| [51] |
LIU T, LIU J, LI L, et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature, 2022, 606(7913):305.
DOI |
| [52] |
ZHANG S, CHEN J, TANG T, et al. A novel strategy to significantly enhance the initial voltage and suppress voltage fading of a Li-and Mn-rich layered oxide cathode material for lithium-ion batteries. Journal of Materials Chemistry A, 2018, 6(8):3610.
DOI URL |
| [53] |
CHERNYAVSKY V, KIM A, KOSHTYAL Y, et al. Structural features of complete and partial activation of Li-rich cathodes studied by in-situ XRD. Electrochemica Acta, 2022, 414:140237.
DOI URL |
| [54] |
WANG L, LIU T, DAI A, et al. Reaction inhomogeneity coupling with metal rearrangement triggers electrochemical degradation in lithium-rich layered cathode. Nature Communications, 2021, 12(1):5370.
DOI PMID |
| [55] |
YANG Z, CHARALAMBOUS H, LIN Y, et al. Extreme fast charge aging: correlation between electrode scale and heterogeneous degradation in Ni-rich layered cathodes. Journal of Power Sources, 2022, 521:230961.
DOI URL |
| [56] | SU Y, CHEN G, CHEN L, et al. High-rate structure-gradient Ni- rich cathode material for lithium-ion batteries. ACS Applied Materials & Interfaces, 2019, 11(40):36697. |
| [57] |
QUILTY C D, WEST P J, WHEELER G P, et al. Elucidating cathode degradation mechanisms in LiNi0.8Mn0.1Co0.1O2 (NMC811)/ graphite cells under fast charge rates using operando synchrotron characterization. Journal of the Electrochemical Society, 2022, 169(2):020545.
DOI |
| [58] |
LÜ C, LI Z, REN X, et al. Revealing the degradation mechanism of Ni-rich cathode materials after ambient storage and related regeneration method. Journal of Materials Chemistry A, 2021, 9(7):3995.
DOI URL |
| [59] |
BLYR A, PASQUIER A D, AMATUCCI G, et al. Origin of self- discharge mechanism in LiMn2O4-based Li-ion cells: a chemical and electrochemical approach. Ionics, 1997, 3:321.
DOI URL |
| [60] | ZHANG C, SU J, WANG T, et al. Significant improvement on electrochemical performance of LiMn2O4 at elevated temperature by atomic layer deposition of TiO2 nanocoating. ACS Sustainable Chemistry & Engineering, 2018, 6(6):7890. |
| [61] |
TANG X, ZHOU J, BAI M, et al. Investigation of the self- discharge behaviors of the LiMn2O4 cathode at elevated temperatures: in situ X-ray diffraction analysis and a co-doping mitigation strategy. Journal of Materials Chemistry A, 2019, 7(21):13364.
DOI URL |
| [62] |
YANG M R, KE W H. The doping effect on the electrochemical properties of LiFe0.95M0.05PO4 (M=Mg2+, Ni2+, Al3+, or V3+ ) as cathode materials for lithium-ion cells. Journal of the Electrochemical Society, 2008, 155(10):A729.
DOI URL |
| [63] |
WANG H, LAI A, HUANG D, et al. Y-F co-doping behavior of LiFePO4/C nanocomposites for high-rate lithium-ion batteries. New Journal of Chemistry, 2021, 45(12):5695.
DOI URL |
| [64] |
WANG L, MA J, WANG C, et al. A novel bifunctional self- stabilized strategy enabling 4.6 V LiCoO2 with excellent long-term cyclability and high-rate capability. Advanced Science, 2019, 6(12): 1900355.
DOI URL |
| [65] |
GE M, WI S, LIU X, et al. Kinetic limitations in single-crystal high-nickel cathodes. Angewandte Chemie International Edition, 2021, 60(32):17350.
DOI URL |
| [66] |
YANG X, WANG S, HAN D, et al. Structural origin of suppressed voltage decay in single-crystalline Li-rich layered Li[Li0.2Ni0.2Mn0.6]O2 Cathodes. Small, 2022, 18(25):2201522.
DOI URL |
| [67] | TAN Z, LI Y, XI X, et al. Lattice engineering to refine particles and strengthen bonds of the LiNi0.9Co0.05Mn0.05O2 cathode toward efficient lithium ion storage. ACS Sustainable Chemistry & Engineering, 2022, 10(11):3532. |
| [68] |
ZHANG Y, LIU J, XU W, et al. Gradient doping Mg and Al to stabilize Ni-rich cathode materials for rechargeable lithium-ion batteries. Journal of Power Sources, 2022, 535:231445.
DOI URL |
| [69] |
CAI Z, MA Y, HUANG X, et al. High electrochemical stability Al-doped spinel LiMn2O4 cathode material for Li-ion batteries. Journal of Energy Storage, 2020, 27:101036.
DOI URL |
| [70] |
ZUO Y, LI B, JIANG N, et al. A high-capacity O2-type Li-rich cathode material with a single-layer Li2MnO3 superstructure. Advanced Materials, 2018, 30(16):1707255.
DOI URL |
| [71] | CAO X, PAN A, ZHANG Y, et al. Nanorod-nanoflake interconnected LiMnPO4·Li3V2(PO4)3/C composite for high-rate and long-life lithium-ion batteries. ACS Applied Materials & Interfaces, 2016, 8(41):27632. |
| [72] |
LU J, CAO B, HU B, et al. Heavy fluorination via ion exchange achieves high-performance Li-Mn-O-F layered cathode for Li-ion batteries. Small, 2022, 18(6):2103499.
DOI URL |
| [73] | HATSUI T, GRAAFSMA H. X-ray imaging detectors for synchrotron and XFEL sources. IUCrJ, 2015, 2(3):37 |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | TAN Bowen, GENG Shuanglong, ZHANG Kai, ZHENG Bailin. Composition-gradient Design of Silicon Electrodes to Mitigate Mechanochemical Coupling Degradation [J]. Journal of Inorganic Materials, 2025, 40(7): 772-780. |
| [3] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [4] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [5] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [6] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [7] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [11] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [12] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [13] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [14] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [15] | ZHU Zhijie, SHEN Mingyuan, WU Tao, LI Wencui. Inhibition of P2-O2 Phase Transition for P2-Na2/3Ni1/3Mn2/3O2 as Cathode of Sodium-ion Battery via Synergetic Substitution of Cu and Mg [J]. Journal of Inorganic Materials, 2025, 40(2): 184-195. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||