
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (3): 299-305.DOI: 10.15541/jim20230312
Special Issue: 【能源环境】储能电池(202506); 【能源环境】锂离子电池(202412)
• RESEARCH ARTICLE • Previous Articles Next Articles
CHENG Jie( ), ZHOU Yue, LUO Xintao, GAO Meiting, LUO Sifei, CAI Danmin, WU Xueyin, ZHU Licai, YUAN Zhongzhi(
), ZHOU Yue, LUO Xintao, GAO Meiting, LUO Sifei, CAI Danmin, WU Xueyin, ZHU Licai, YUAN Zhongzhi( )
)
Received:2023-07-11
Revised:2023-09-01
Published:2024-03-20
Online:2023-09-12
Contact:
YUAN Zhongzhi, professor. E-mail: yuanzz@scnu.edu.cnAbout author:CHENG Jie (1998-), female, Master candidate. E-mail: 15007936259@163.com
CLC Number:
CHENG Jie, ZHOU Yue, LUO Xintao, GAO Meiting, LUO Sifei, CAI Danmin, WU Xueyin, ZHU Licai, YUAN Zhongzhi. Construction and Electrochemical Properties of Yolk-shell Structured FeF3·0.33H2O@N-doped Graphene Nanoboxes[J]. Journal of Inorganic Materials, 2024, 39(3): 299-305.
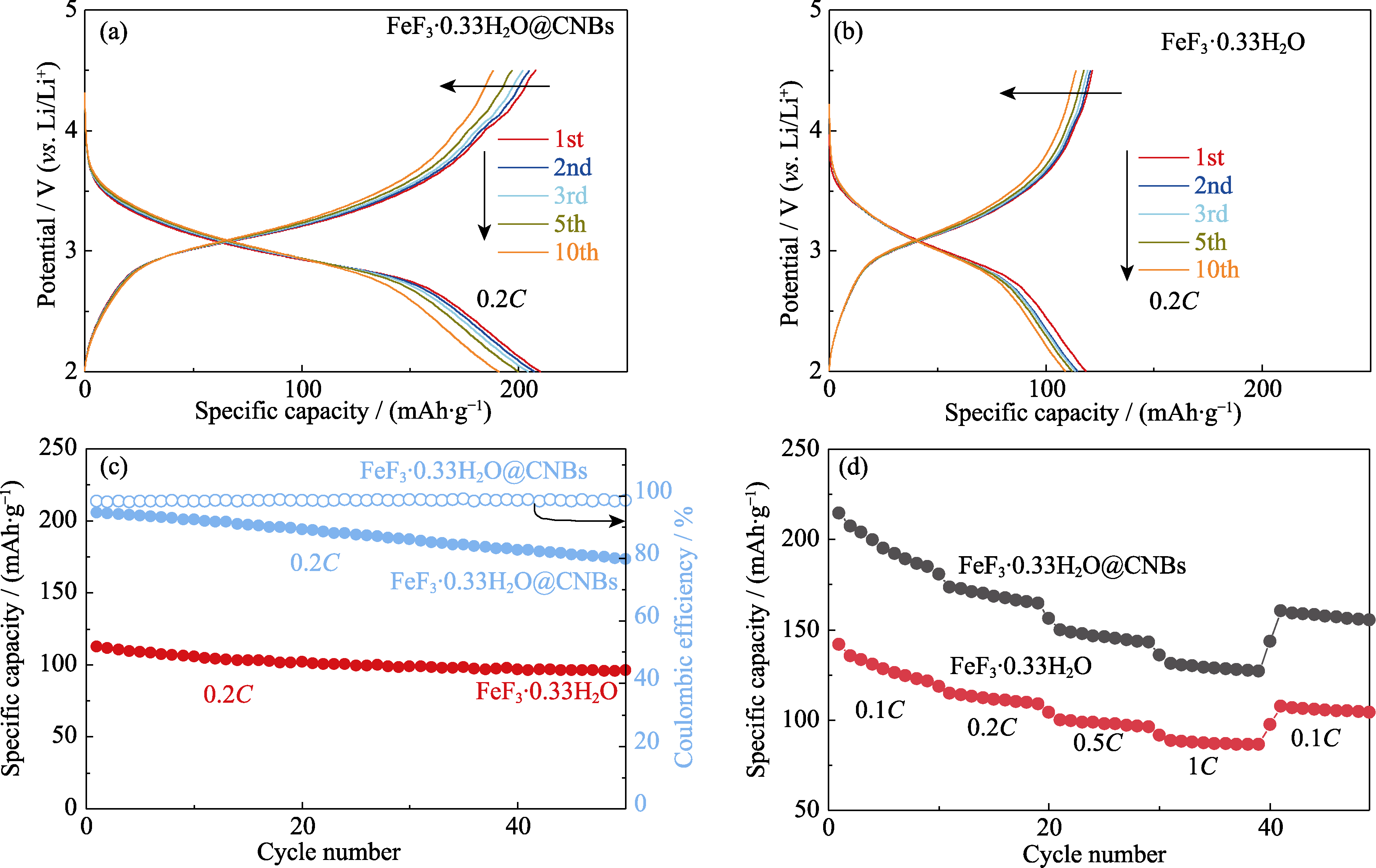
Fig. 7 Electrochemical performances of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O as cathodes of lithium ion cell (a) Voltage-capacity curves of FeF3·0.33H2O@CNBs at 0.2C; (b) Voltage-capacity curves of bare FeF3·0.33H2O at 0.2C; (c) Cycling and (d) rate performances of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O. Colorful figures are available on website
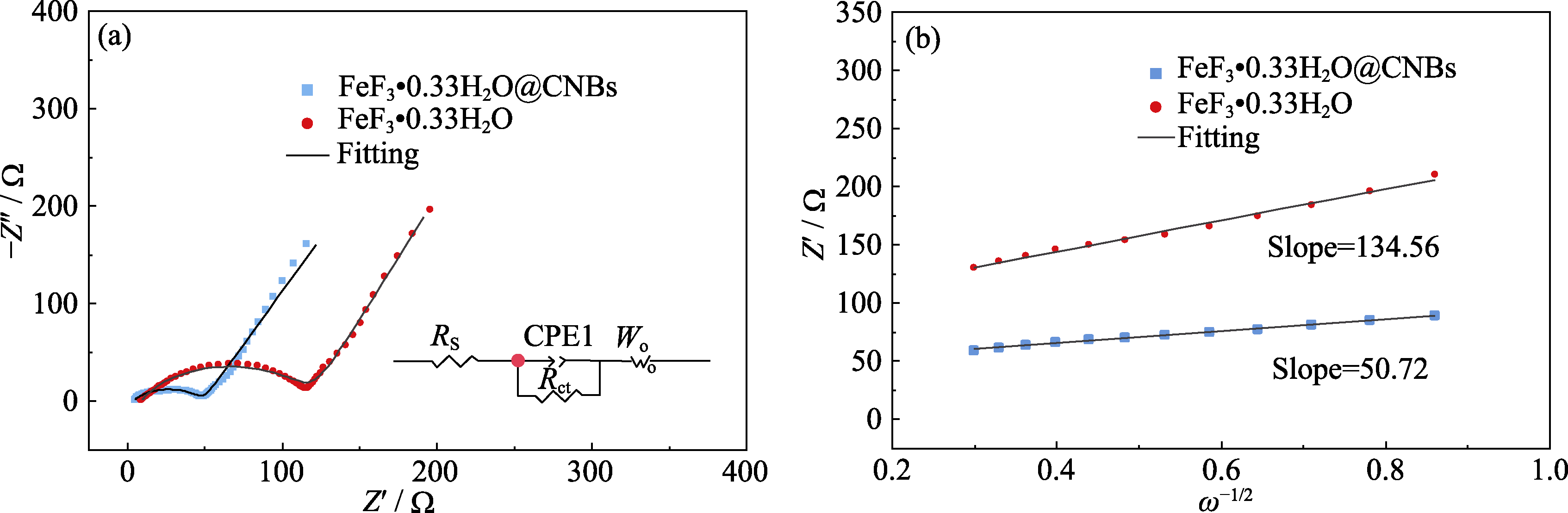
Fig. 8 Electrochemical impedance spectra of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O (a) Nyquist plots; (b) Relationship between Z′ and ω−1/2 in low-frequency region
| Material | Particle size | Voltage range/V | Discharge density | Initial discharge capacity/(mAh·g-1) | (Reversible capacity/ (mAh·g-1))/cycle number | Ref. |
|---|---|---|---|---|---|---|
| FeF3·0.33H2O@CNBs | 250 nm | 2.0-4.5 | 0.2C | 208 | 173.4/50 | This work |
| FeF3·0.33H2O/C | 1-1.7 µm | 2.0-4.5 | 1C | 187.1 | 172.3/50 | [ |
| FeF3·0.33H2O@CNHs | 80-100 nm | 1.5-4.5 | 1C | 155 | 154/50 | [ |
| FeF3·0.33H2O/MWCNTs | 30 nm | 2.0-4.3 | 0.1C | 186 | 116/50 | [ |
| FeF3·0.33H2O@rGO | 400 nm | 2.0-4.5 | 0.1C | 205 | 183.8/60 | [ |
| FeF3·0.33H2O/C | 1-5 µm | 1.5-4.5 | 1C | 276.4 | 193.5/50 | [ |
| Co/Ni dual-doped FeF3·0.33H2O | 200 nm | 1.5-4.5 | 5C | 200.1 | 177.8/400 | [ |
| FeF3·0.33H2O/rGO | 150 nm | 1.8-4.5 | 0.5C | 208.3 | 133.1/100 | [ |
Table S1 Performance comparison of FeF3·0.33H2O based cathode materials of lithium ion battery in this work and literature
| Material | Particle size | Voltage range/V | Discharge density | Initial discharge capacity/(mAh·g-1) | (Reversible capacity/ (mAh·g-1))/cycle number | Ref. |
|---|---|---|---|---|---|---|
| FeF3·0.33H2O@CNBs | 250 nm | 2.0-4.5 | 0.2C | 208 | 173.4/50 | This work |
| FeF3·0.33H2O/C | 1-1.7 µm | 2.0-4.5 | 1C | 187.1 | 172.3/50 | [ |
| FeF3·0.33H2O@CNHs | 80-100 nm | 1.5-4.5 | 1C | 155 | 154/50 | [ |
| FeF3·0.33H2O/MWCNTs | 30 nm | 2.0-4.3 | 0.1C | 186 | 116/50 | [ |
| FeF3·0.33H2O@rGO | 400 nm | 2.0-4.5 | 0.1C | 205 | 183.8/60 | [ |
| FeF3·0.33H2O/C | 1-5 µm | 1.5-4.5 | 1C | 276.4 | 193.5/50 | [ |
| Co/Ni dual-doped FeF3·0.33H2O | 200 nm | 1.5-4.5 | 5C | 200.1 | 177.8/400 | [ |
| FeF3·0.33H2O/rGO | 150 nm | 1.8-4.5 | 0.5C | 208.3 | 133.1/100 | [ |
| Sample | Rs/Ω | Rct/Ω | DLi+ /(m2·s-1) |
|---|---|---|---|
| FeF3·0.33H2O@CNBs | 3.39 | 46.0 | 1.84×10-14 |
| FeF3·0.33H2O | 8.78 | 112.6 | 2.74×10-15 |
Table S2 Li-ion diffusion coefficients of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O
| Sample | Rs/Ω | Rct/Ω | DLi+ /(m2·s-1) |
|---|---|---|---|
| FeF3·0.33H2O@CNBs | 3.39 | 46.0 | 1.84×10-14 |
| FeF3·0.33H2O | 8.78 | 112.6 | 2.74×10-15 |
| [1] |
DUFFNER F, KRONEMEYER N, TUBKE J, et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nature Energy, 2021, 6(2): 123.
DOI |
| [2] |
MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry. Nature Communications, 2020, 11: 1550.
DOI PMID |
| [3] | SUN L D, LI Y, FENG W. Metal fluoride cathode materials for lithium rechargeable batteries: focus on iron fluorides. Small Methods, 2023, 7: 202201152. |
| [4] |
LIU L, GUO H, ZHOU M, et al. A comparison among FeF3·3H2O, FeF3·0.33H2O and FeF3 cathode materials for lithium ion batteries: structural, electrochemical, and mechanism studies. Journal of Power Sources, 2013, 238: 501.
DOI URL |
| [5] | XIAO A W, LEE H J, CAPONE I, et al. Understanding the conversion mechanism and performance of monodisperse FeF2 nanocrystal cathodes. Nature Materials, 2020, 644(2): 644. |
| [6] |
LI C, GU L, TSUKIMOTO S, et al. Low-temperature ionic-liquid- based synthesis of nanostructured iron-based fluoride cathodes for lithium batteries. Advanced Materials, 2010, 22(33): 3650.
DOI URL |
| [7] |
TAN J, LIU J, HU H, et al. Iron fluoride with excellent cycle performance synthesized by solvothermal method as cathodes for lithium ion batteries. Journal of Power Sources, 2014, 251: 75.
DOI URL |
| [8] |
BADWAY F, COSANDEY F, PEREIRA N, et al. Carbon metal fluoride nanocomposites: high-capacity reversible metal fluoride conversion materials as rechargeable positive electrodes for Li batteries. Journal of The Electrochemical Society, 2003, 150(10): A1318.
DOI URL |
| [9] |
ZENG C, CHEN F, YE Q, et al. Facile preparation of hierarchical micro-nano FeF3·0.33H2O by a one-pot method with dual surfactants. Nanotechnology, 2021, 32(15): 155402.
DOI |
| [10] |
LIN J, CHEN S, ZHU L, et al. Soft-template fabrication of hierarchical nanoparticle iron fluoride as high-capacity cathode materials for Li-ion batteries. Electrochimica Acta, 2020, 364: 137293.
DOI URL |
| [11] |
SHI Q, ZHOU Y, CHENG J, et al. Turning carbon black into hollow carbon nanospheres to encapsulate Fe2O3 as high-performance lithium-ion batteries anode. Microporous and Mesoporous Materials, 2022, 332: 111681.
DOI URL |
| [12] |
CHEN S, LIN J, SHI Q, et al. Nanoscale iron fluoride supported by three-dimensional porous graphene as long-life cathodes for lithium-ion batteries. Journal of The Electrochemical Society, 2020, 167: 080506.
DOI URL |
| [13] | ZHOU Y, CHENG J, WU X, et al. Octahedral FeF3·0.33H2O nanocrystalline fixed on carbon fibers as the cathode of lithium-ion battery based on the “gravel and glue” strategy. Elecchimica Acta, 2022, 435: 141363. |
| [14] |
WANG Y, XIE K, ZHU Y, et al. Prussian blue microcubes-derived FeF3 cathodes for high-energy and ultra-stable lithium and lithium- ion batteries. Journal of Power Sources, 2023, 577: 233234.
DOI URL |
| [15] |
ZHANG L, YU L, LI O L, et al. FeF3·0.33H2O@carbon nanosheets with honeycomb architectures for high-capacity lithium-ion cathode storage by enhanced pseudocapacitance. Journal of Materials Chemistry A, 2021, 9(30): 16370.
DOI URL |
| [16] |
CHEN S, SHI Q, LIN J, et al. Growth behavior and influence factors of three-dimensional hierarchical flower-like FeF3·0.33H2O. CrystEngComm, 2020, 22(33): 5550.
DOI URL |
| [17] |
ZHOU H, SUN H, WANG T, et al. Low temperature nanotailoring of hydrated compound by alcohols: FeF3·3H2O as an example. preparation of nanosized FeF3·0.33H2O cathode material for Li-ion batteries. Inorganic Chemistry, 2019, 58: 6765.
DOI URL |
| [18] | MURATA Y, MINAMI R, TAKADA S, et al. A fundamental study on carbon composites of FeF3·0.33H2O as open-framework cathode materials for calcium-ion batteries. AIP Conference Proceedings, 2017, 1807: 020005. |
| [19] |
PACHFULE P, SHINDE D, MAJUMDER M, et al. Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework. Nature Chemistry, 2016, 8(7): 718.
DOI PMID |
| [20] |
WANG J, HU Q, HU W, et al. Preparation of hollow core-shell Fe3O4/nitrogen-doped carbon nanocomposites for lithium-ion batteries. Molecules, 2022, 27(2): 396.
DOI URL |
| [21] |
YANG F, GAO H, HAO J, et al. Yolk-shell structured FeP@C nanoboxes as advanced anode materials for rechargeable lithium-/potassium-ion batteries. Advanced Functional Materials, 2019, 29(16): 1808291.
DOI URL |
| [22] |
LIAN P, ZHU X, LIANG S, et al. High reversible capacity of SnO2/graphene nanocomposite as an anode material for lithium-ion batteries. Electrochimica Acta, 2011, 56(12): 4532.
DOI URL |
| [23] |
MANTIA F L, HUGGINS R A, CUI Y. Oxidation processes on conducting carbon additives for lithium-ion batteries. Journal of Applied Electrochemistry, 2013, 43: 1.
DOI URL |
| [24] |
LI J, FU L, ZHU J, et al. Improved electrochemical performance of FeF3 by inlaying in a nitrogen-doped carbon matrix. ChemElectroChem, 2019, 6(20): 5203.
DOI URL |
| [25] | QIU D, FU L, ZHAN C, et al. Seeding iron trifluoride nanoparticles on reduced graphite oxide for lithium-ion batteries with enhanced loading and stability. ACS Applied Materials & Interfaces, 2018, 10(35): 29505. |
| [1] | ZHU Zhijie, SHEN Mingyuan, WU Tao, LI Wencui. Inhibition of P2-O2 Phase Transition for P2-Na2/3Ni1/3Mn2/3O2 as Cathode of Sodium-ion Battery via Synergetic Substitution of Cu and Mg [J]. Journal of Inorganic Materials, 2025, 40(2): 184-195. |
| [2] | ZHOU Jingyu, LI Xingyu, ZHAO Xiaolin, WANG Youwei, SONG Erhong, LIU Jianjun. Rate and Cycling Performance of Ti and Cu Doped β-NaMnO2 as Cathode of Sodium-ion Battery [J]. Journal of Inorganic Materials, 2024, 39(12): 1404-1412. |
| [3] | HU Mengfei, HUANG Liping, LI He, ZHANG Guojun, WU Houzheng. Research Progress on Hard Carbon Anode for Li/Na-ion Batteries [J]. Journal of Inorganic Materials, 2024, 39(1): 32-44. |
| [4] | KONG Guoqiang, LENG Mingzhe, ZHOU Zhanrong, XIA Chi, SHEN Xiaofang. Sb Doped O3 Type Na0.9Ni0.5Mn0.3Ti0.2O2 Cathode Material for Na-ion Battery [J]. Journal of Inorganic Materials, 2023, 38(6): 656-662. |
| [5] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [6] | LI Tao, CAO Pengfei, HU Litao, XIA Yong, CHEN Yi, LIU Yuejun, SUN Aokui. NH4+ Assisted Interlayer-expansion of MoS2: Preparation and Its Zinc Storage Performance [J]. Journal of Inorganic Materials, 2023, 38(1): 79-86. |
| [7] | WANG Yang, FAN Guangxin, LIU Pei, YIN Jinpei, LIU Baozhong, ZHU Linjian, LUO Chengguo. Microscopic Mechanism of K+ Doping on Performance of Lithium Manganese Cathode for Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(9): 1023-1029. |
| [8] | LI Wenbo, HUANG Minsong, LI Yueming, LI Chilin. CoS2 as Cathode Material for Magnesium Batteries with Dual-salt Electrolytes [J]. Journal of Inorganic Materials, 2022, 37(2): 173-181. |
| [9] | ZHAN Jing,XU Changfan,LONG Yiyu,LI Qihou. Bi2Mn4O10: Preparation by Polyacrylamide Gel Method and Electrochemical Performance [J]. Journal of Inorganic Materials, 2020, 35(7): 827-833. |
| [10] | WANG Wu-Lian, ZHANG Jun, WANG Qiu-Shi, CHEN Liang, LIU Zhao-Ping. High-quality Fe4[Fe(CN)6]3 Nanocubes: Synthesis and Electrochemical Performance as Cathode Material for Aqueous Sodium-ion Battery [J]. Journal of Inorganic Materials, 2019, 34(12): 1301-1308. |
| [11] | WANG Jia-Hu, WANG Wen-Xin, DU Peng, HU Fang-Dong, JIANG Xiao-Lei, YANG Jian. Synthesis of Na3V2(PO4)2F3@V2O5-x as Cathode Material for Sodium-ion Battery [J]. Journal of Inorganic Materials, 2019, 34(10): 1097-1102. |
| [12] | LEE Sai-Xi, WANG Xue-Yin, GU Qing-Wen, XIA Yong-Gao, LIU Zhao-Ping, HE Jie. Tuning Electrochemical Performance through Non-stoichiometric Compositions in High-voltage Spinel Cathode Materials [J]. Journal of Inorganic Materials, 2018, 33(9): 993-1000. |
| [13] | LUO Ling-Hong, HU Jia-Xing, CHENG Liang, XU Xu, WU Ye-Fan, LIN You-Chen. Performance of the Composite Cathode Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2-δ for Medium-low Temperature Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2018, 33(4): 441-446. |
| [14] | CAI Jian-Xin, LI Zhi-Peng, LI Wei, ZHAO Peng-Fei, YANG Zhen-Yu, YU Ji. Synthesis and Electrochemical Performance of Fe2O3 Nanofibers as Anode Materials for LIBs [J]. Journal of Inorganic Materials, 2018, 33(3): 301-306. |
| [15] | LI Ling, LI Yun-Jiao, XU Bin, LU Wei-Sheng, SU Qian-Ye, CHEN Yong-Xiang, LI Lin. LiNixCoyMn1-x-yO2 Cathode Material Synthesized through Construction of E-pH Diagram and Its Electrochemical Performance [J]. Journal of Inorganic Materials, 2018, 33(3): 320-324. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||