无机材料学报 ›› 2026, Vol. 41 ›› Issue (1): 70-78.DOI: 10.15541/jim20250105 CSTR: 32189.14.10.15541/jim20250105
高源1,2( ), 魏波2(
), 魏波2( ), 金芳军1, 吕喆2, 凌意瀚1(
), 金芳军1, 吕喆2, 凌意瀚1( )
)
收稿日期:2025-03-11
修回日期:2025-05-05
出版日期:2026-01-20
网络出版日期:2025-05-22
通讯作者:
魏 波, 教授. E-mail: bowei@hit.edu.cn;作者简介:高 源(1996-), 男, 博士后. E-mail: tbh371@cumt.edu.cn
基金资助:
GAO Yuan1,2( ), WEI Bo2(
), WEI Bo2( ), JIN Fangjun1, LÜ Zhe2, LING Yihan1(
), JIN Fangjun1, LÜ Zhe2, LING Yihan1( )
)
Received:2025-03-11
Revised:2025-05-05
Published:2026-01-20
Online:2025-05-22
Contact:
WEI Bo, professor. E-mail: bowei@hit.edu.cn;About author:GAO Yuan (1996-), male, post doctor. E-mail: tbh371@cumt.edu.cn
Supported by:摘要: 铬中毒现象是制约固体氧化物燃料电池(SOFCs)阴极实际应用的重要因素。尤其是富碱土元素的钙钛矿氧化物阴极在高温下易发生离子偏析和杂质中毒, 进而导致阴极性能显著降低。为提高阴极耐铬能力, 本研究采取Ag掺杂策略, 调控阴极材料SrCo0.9Ta0.1O3-δ(SCT)的酸性位点, 并系统探究材料的电导率、催化活性和表面微观形貌及组分。结果表明, Ag掺杂使材料的电导率提升, 且掺杂后的材料具有更大的氧表面交换系数, 有利于提高其阴极催化活性。700 ℃时, Sr0.9Ag0.1Co0.9Ta0.1O3-δ(SACT10)阴极的极化电阻为0.0176 Ω·cm2, 明显低于SCT阴极(0.0366 Ω·cm2)。此外, 由于掺入Ag, SACT10材料中Co的平均价态升高, 使其相对酸度提高, 增强了材料的耐铬能力。在含铬气氛中运行22 h后, SACT10阴极的极化电阻为0.205 Ω·cm2, 明显低于SCT阴极(0.964 Ω·cm2), 这是因为SACT10阴极表面观察到更少的惰性二次相。以上结果证实在材料中掺杂Ag可以有效增加酸性位点, 提高活性, 增强耐铬能力。制备的SACT10有望成为具有应用前景的中温SOFCs阴极材料。
中图分类号:
高源, 魏波, 金芳军, 吕喆, 凌意瀚. Ag掺杂调控中温固体氧化物燃料电池阴极酸性位点增强耐铬能力[J]. 无机材料学报, 2026, 41(1): 70-78.
GAO Yuan, WEI Bo, JIN Fangjun, LÜ Zhe, LING Yihan. Ag Doping Modulating Cathode Acidic Sites to Enhance Chromium Resistance for Intermediate Temperature Solid Oxide Fuel Cells[J]. Journal of Inorganic Materials, 2026, 41(1): 70-78.
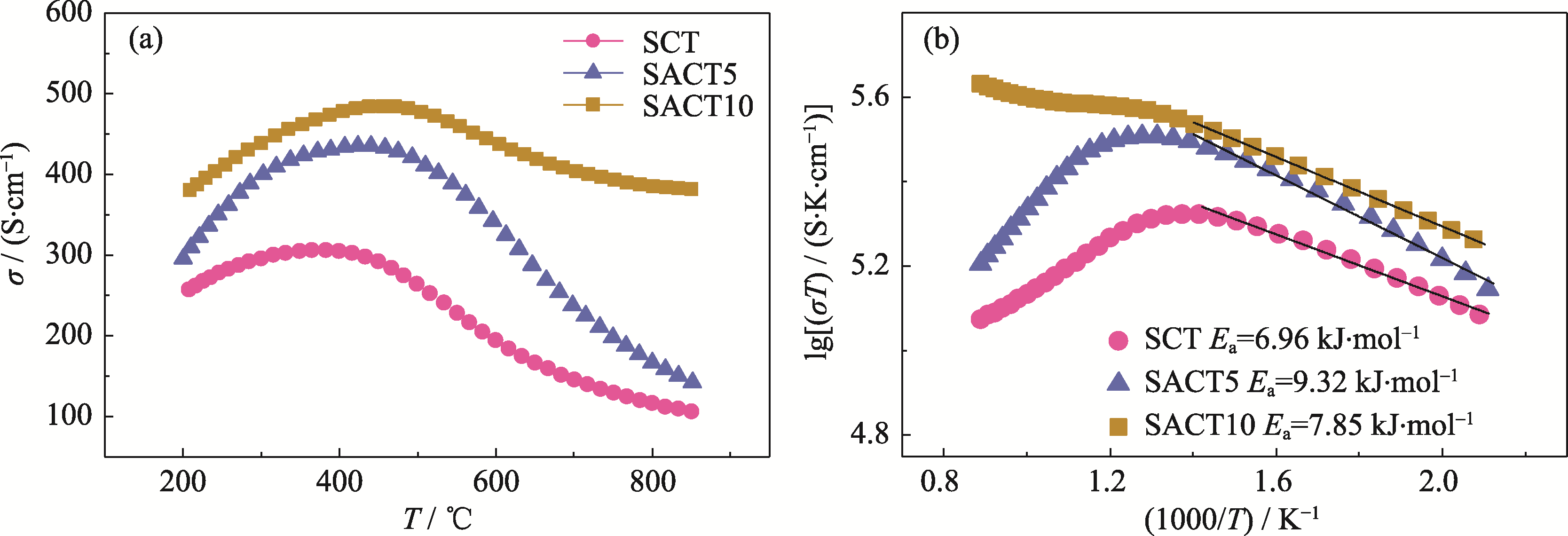
图2 SCT、SACT5及SACT10材料在空气中的(a)电导率及(b)相应的阿伦尼乌斯曲线
Fig. 2 (a) Electrical conductivities of SCT, SACT5 and SACT10 samples in air and (b) their corresponding Arrhenius plots
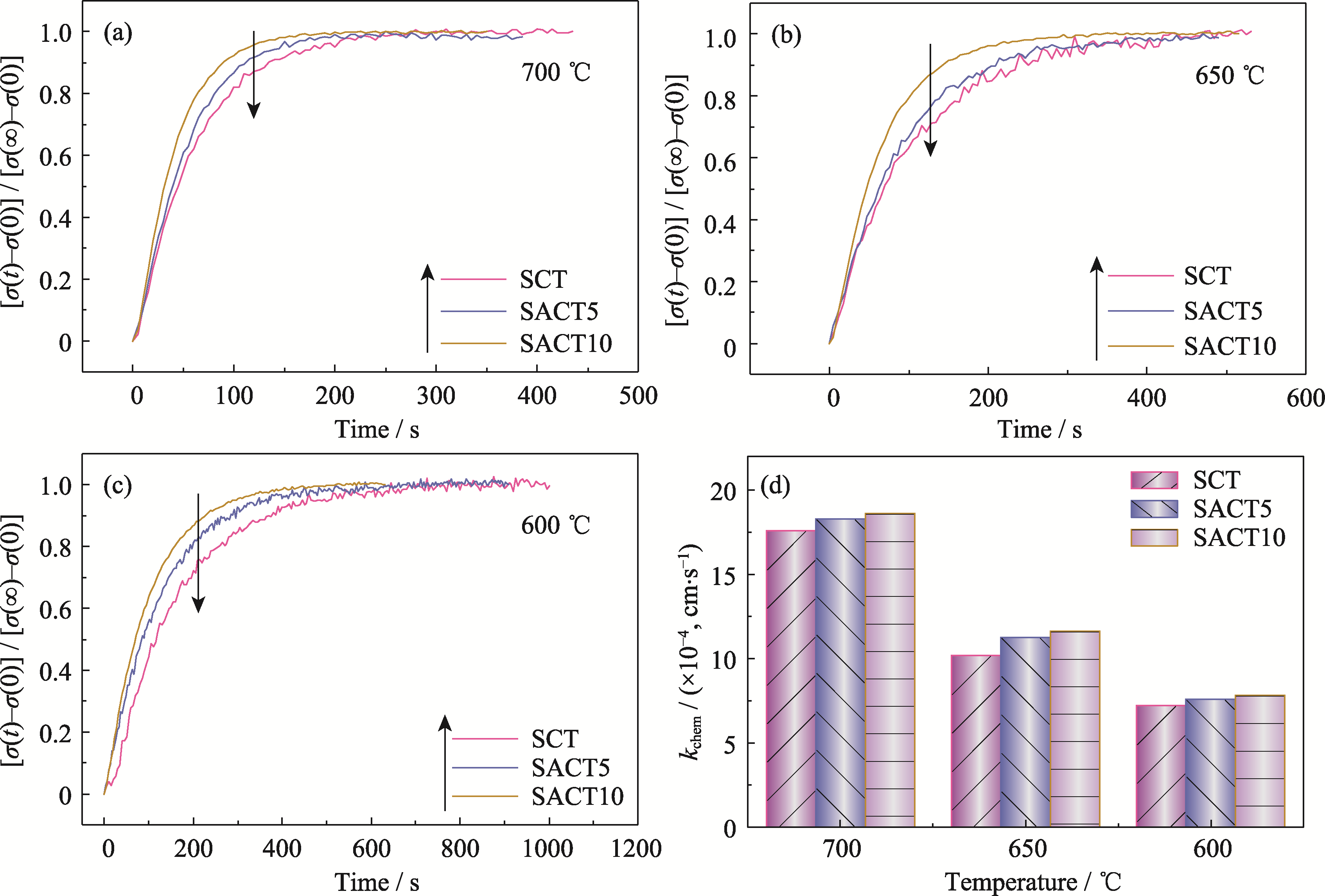
图3 SCT、SACT5和SACT10材料在(a) 700, (b) 650和(c) 600 ℃的ECR曲线以及(d) kchem
Fig. 3 ECR curves at (a) 700, (b) 650 and (c) 600 ℃ of SCT, SACT5 and SACT10, and (d) their kchem
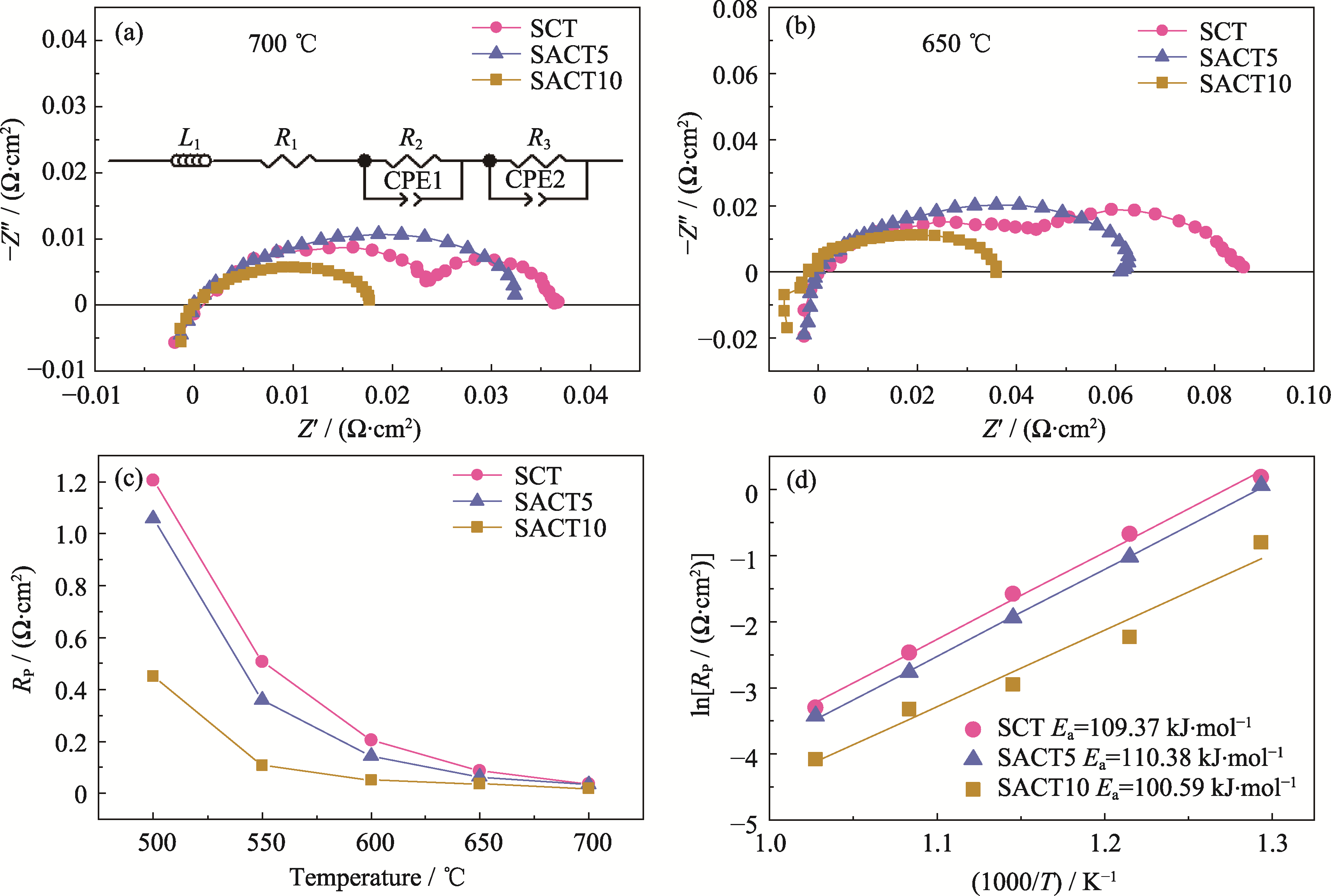
图4 SCT、SACT5和SACT10阴极在(a) 700和(b) 650 ℃的EIS谱图、(c) Rp和(d)相应的阿伦尼乌斯曲线
Fig. 4 EIS spectra of SCT, SACT5 and SACT10 cathodes at (a) 700 and (b) 650 ℃, and their (c) Rp and (d) corresponding Arrhenius plots Inset in (a): equivalent circuit
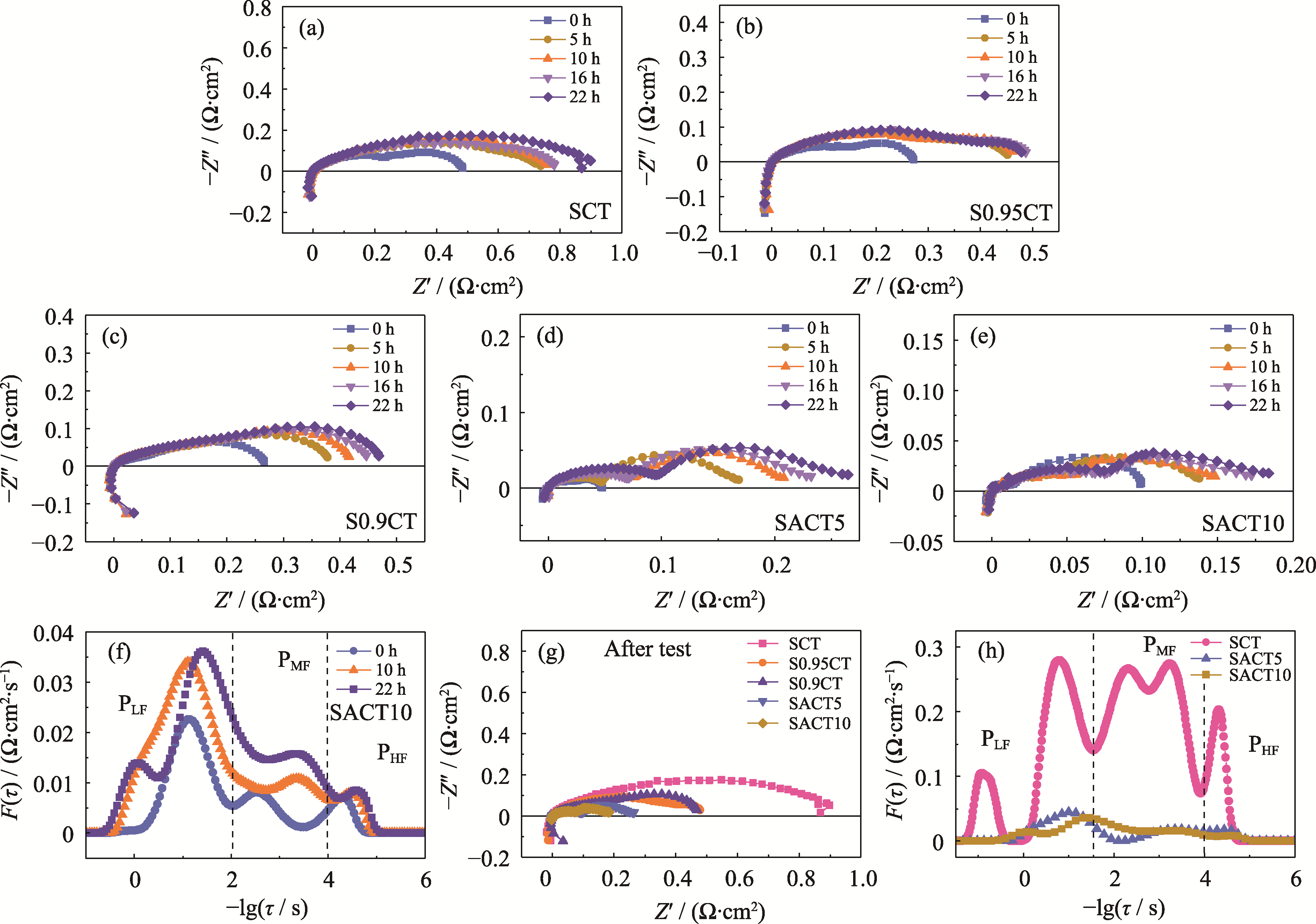
图5 (a~e)不同阴极在700 ℃含铬气氛下运行不同时间的EIS谱图; (f) SACT10阴极EIS谱图相应的DRT分析; (g, h)运行后不同阴极的(g) EIS谱图和(h)相应的DRT分析
Fig. 5 (a-e) EIS spectra of different cathodes at 700 ℃ under Cr-containing atmosphere for different operating times; (f) DRT analysis of the corresponding EIS spectra of SACT10 cathode; (g) EIS spectra for different cathodes after operating, and (h) their corresponding DRT analysis (a) SCT; (b) S0.95CT; (c) S0.9CT; (d) SACT5; (e) SACT10
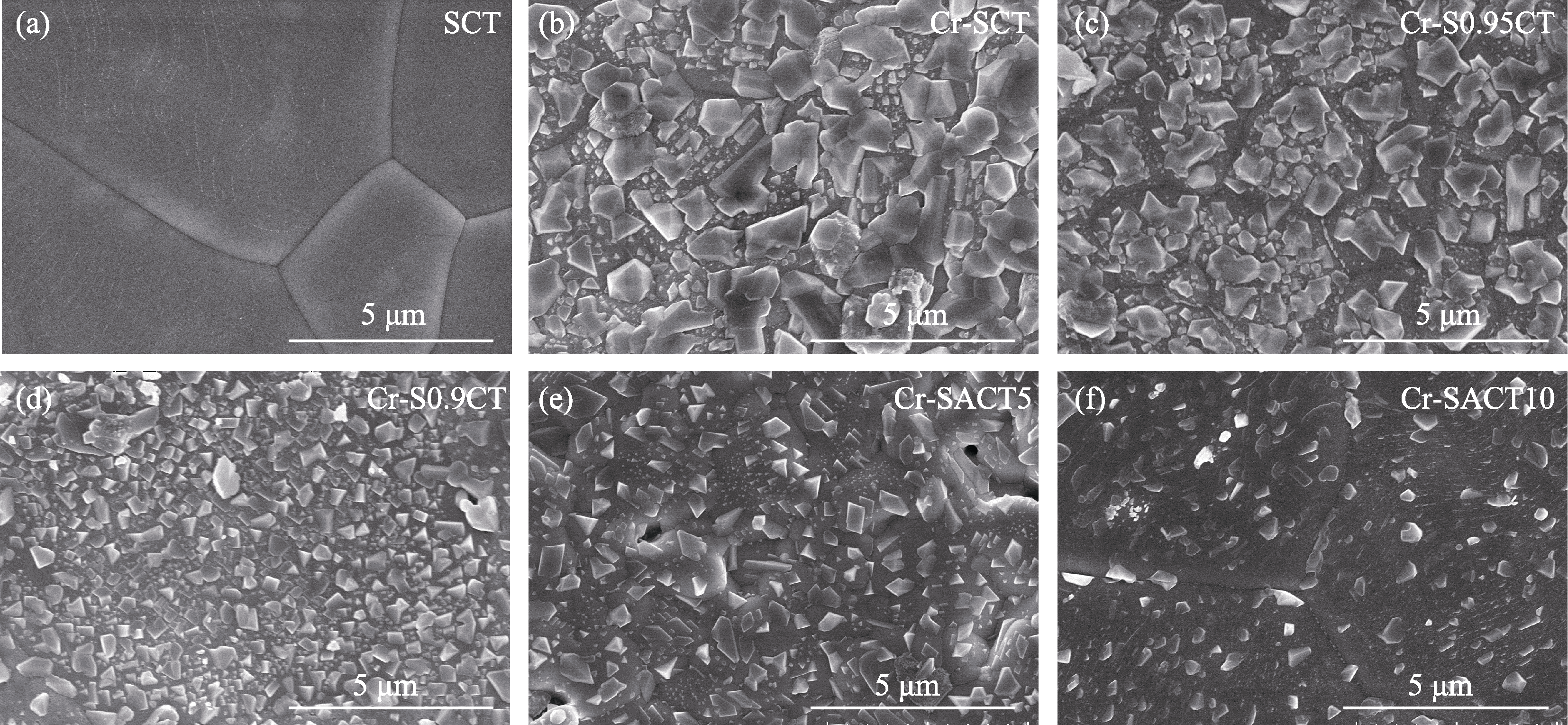
图6 700 ℃含铬气氛中处理22 h前后(a) SCT、(b) Cr-SCT、(c) Cr-S0.95CT、(d) Cr-S0.9CT、(e) Cr-SACT5和(f) Cr-SACT10致密样品片的SEM照片
Fig. 6 SEM images of (a) SCT, (b) Cr-SCT, (c) Cr-S0.95CT, (d) Cr-S0.9CT, (e) Cr-SACT5, and (f) Cr-SACT10 dense pellets before and after treatment at 700 ℃ under Cr-containing atmosphere for 22 h
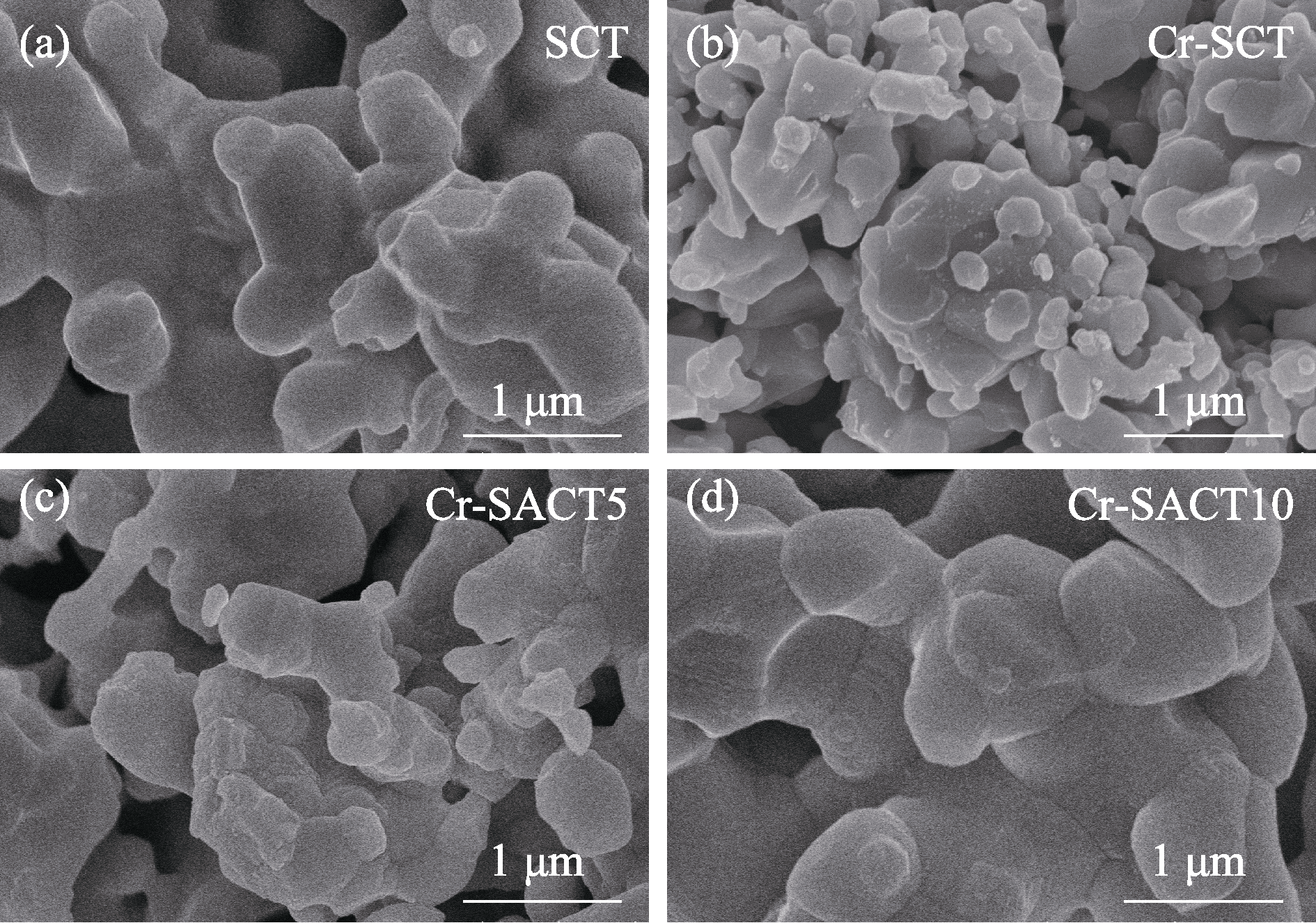
图7 700 ℃含铬气氛中运行22 h前后(a) SCT、(b) Cr-SCT、(c) Cr-SACT5和(d) Cr-SACT10阴极的SEM照片
Fig. 7 SEM images of (a) SCT, (b) Cr-SCT, (c) Cr-SACT5, and (d) Cr-SACT10 cathodes before and after operating at 700 ℃ under Cr-containing atmosphere for 22 h
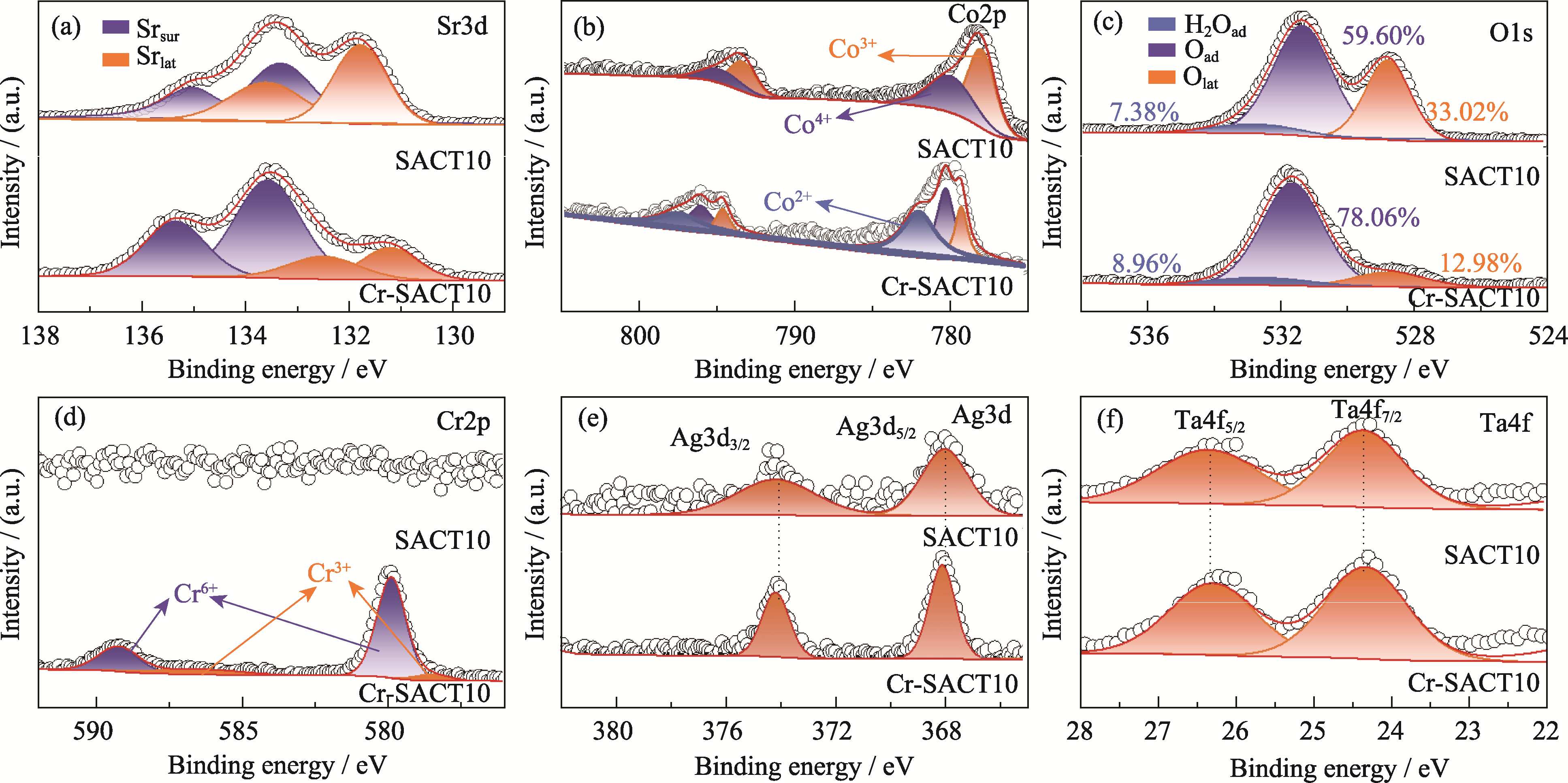
图8 700 ℃含铬气氛处理前后SACT10样品的XPS谱图
Fig. 8 XPS spectra of SACT10 before and after treatment under Cr-containing atmosphere at 700 ℃ (a) Sr3d; (b) Co2p; (c) O1s; (d) Cr2p; (e) Ag3d; (f) Ta4f. Colorful figures are available on website
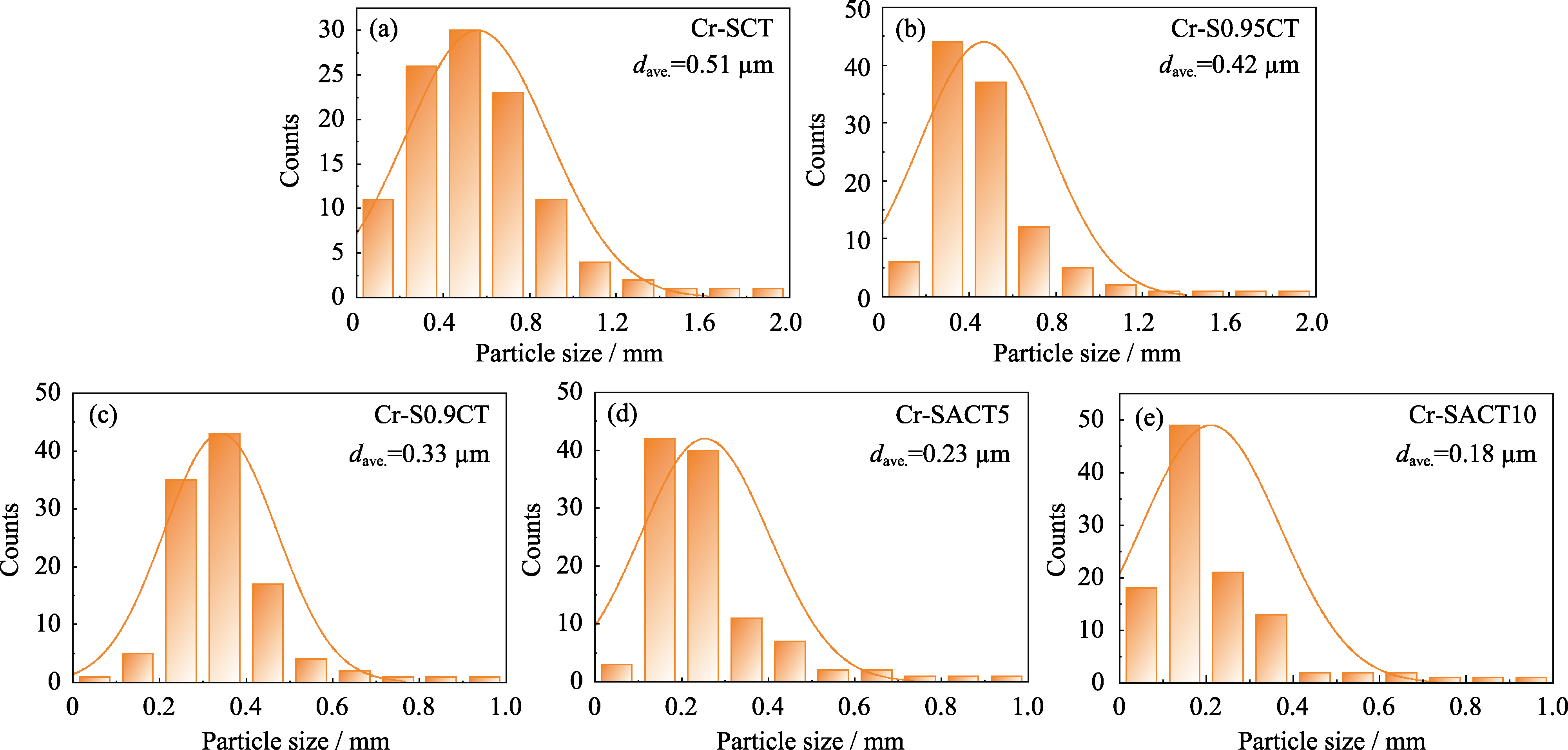
图S4 700 ℃含铬气氛下处理22 h前后致密样品片(a) Cr-SCT、(b) Cr-S0.95CT、(c) Cr-S0.9CT、(d) Cr-SACT5和(e) Cr-SACT10表面二次相的粒径分布图
Fig. S4 Particle size distributions of the secondary phases on the surface of dense pellets (a) Cr-SCT, (b) Cr-S0.95CT, (c) Cr-S0.9CT, (d) Cr-SACT5, and (e) Cr-SACT10 before and after treatment at 700 ℃ under Cr-containing atmosphere for 22 h
| [1] |
FU M, GAO Y, ZHANG M, et al. Vanadium-assisted surface engineering of heterostructured cathode for enhanced protonic ceramic fuel cell performance. Chemical Engineering Journal, 2025, 505: 159722.
DOI URL |
| [2] |
YE Z, ZOU G, WU Q, et al. Preparation and performances of tubular cone-shaped anode-supported segmented-in-series direct carbon solid oxide fuel cell. Journal of Inorganic Materials, 2024, 39(7): 819.
DOI URL |
| [3] |
ISHFAQ H A, KHAN M Z, SHIRKE Y M, et al. A heuristic approach to boost the performance and Cr poisoning tolerance of solid oxide fuel cell cathode by robust multi-doped ceria coating. Applied Catalysis B: Environmental, 2023, 323: 122178.
DOI URL |
| [4] |
GAO Y, HUANG X, WANG Z, et al. Cr deposition and poisoning on SrCo0.9Ta0.1O3-δ cathode of solid oxide fuel cells. International Journal of Hydrogen Energy, 2023, 48(6): 2341.
DOI URL |
| [5] |
ZHOU Y, LÜ Z, ZHANG R, et al. Mechanism of chromium poisoning on La0.5Sr0.5Co0.25Fe0.75O3 cathode and enhancing chromium tolerance using a novel anion doping strategy. International Journal of Hydrogen Energy, 2024, 85: 114.
DOI URL |
| [6] |
ZHENG T, LI Z, WANG D, et al. Enhanced anti-chromium poisoning ability of high entropy La0.2Nd0.2Sm0.2Sr0.2Ba0.2Co0.2Fe0.8O3-δ cathodes for solid oxide fuel cells. Journal of Alloys and Compounds, 2024, 982: 173753.
DOI URL |
| [7] |
HAO H, ZHANG Y, WANG Z, et al. Hydrogen spillover in superwetting Ni/NiMoN Mott-Schottky heterostructures for boosting ampere-level hydrogen evolution. Applied Physics Letters, 2025, 126: 113901.
DOI URL |
| [8] |
XIA B, ZHANG H, YAO C, et al. Enhancing ORR activity and CO2 tolerance of Pr0.4Sr0.6Co0.2Fe0.8O3-δ-based SOFC cathode through synergistic doping and surface modification. Applied Surface Science, 2024, 649: 159143.
DOI URL |
| [9] |
BAI J, ZHOU D, NIU L, et al. Preparation of high-performance multiphase heterostructures IT-SOFC cathode materials by Pr-induced in situ assembly. Applied Catalysis B: Environment and Energy, 2024, 355: 124174.
DOI URL |
| [10] |
ZHANG X, LIU B, YANG Y, et al. Advances in component and operation optimization of solid oxide electrolysis cell. Chinese Chemical Letters, 2023, 34(5): 108035.
DOI |
| [11] |
LI J, HOU J, LU Y, et al. Ca-containing Ba0.95Ca0.05Co0.4Fe0.4Zr0.1Y0.1O3-δ cathode with high CO2-poisoning tolerance for proton-conducting solid oxide fuel cells. Journal of Power Sources, 2020, 453: 227909.
DOI URL |
| [12] |
CHEN Z, MA B, DANG C, et al. Entropy engineering strategies for optimizing solid oxide cell air electrode performance: a review. Journal of Alloys and Compounds, 2025, 1010: 177585.
DOI URL |
| [13] |
ZHANG X, JIN Y, JIANG Y, et al. Enhancing chromium poisoning tolerance of La0.8Sr0.2Co0.2Fe0.8O3-δ cathode by Ce0.8Gd0.2O1.9-δ coating. Journal of Power Sources, 2022, 547: 231996.
DOI URL |
| [14] |
YUAN M, WANG Z, GAO J, et al. Turning bad into good: a medium-entropy double perovskite oxide with beneficial surface reconstruction for active and robust cathode of solid oxide fuel cells. Journal of Colloid and Interface Science, 2024, 672: 787.
DOI PMID |
| [15] |
SHEN L, DU Z, ZHANG Y, et al. Medium-entropy perovskites Sr(FeαTiβCoγMnζ)O3-δ as promising cathodes for intermediate temperature solid oxide fuel cell. Applied Catalysis B: Environmental, 2021, 295: 120264.
DOI URL |
| [16] |
GAO Y, LING Y, WANG X, et al. Sr-deficient medium-entropy Sr1-xCo0.5Fe0.2Ti0.1Ta0.1Nb0.1O3-δ cathodes with high Cr tolerance for solid oxide fuel cells. Chemical Engineering Journal, 2024, 479: 147665.
DOI URL |
| [17] |
GAO L, LI Q, SUN L, et al. A novel family of Nb-doped Bi0.5Sr0.5FeO3-δ perovskite as cathode material for intermediate- temperature solid oxide fuel cells. Journal of Power Sources, 2017, 371: 86.
DOI URL |
| [18] |
GAO Y, HUANG X, YUAN M, et al. A SrCo0.9Ta0.1O3-δ derived medium-entropy cathode with superior CO2 poisoning tolerance for solid oxide fuel cells. Journal of Power Sources, 2022, 540: 231661.
DOI URL |
| [19] |
CHEN Z P, JIN F J, LI M F, et al. Double perovskite Sr2CoFeO5+δ: preparation and performance as cathode material for intermediate- temperature solid oxide fuel cells. Journal of Inorganic Materials, 2024, 39(3): 337.
DOI URL |
| [20] | KIM D, PARK J W, YUN B, et al. Correlation of time-dependent oxygen surface exchange kinetics with surface chemistry of La0.6Sr0.4Co0.2Fe0.8O3-δ catalysts. ACS Applied Materials & Interfaces, 2019, 11(35): 31786. |
| [21] |
CHEN D, CHEN C, BAIYEE Z M, et al. Nonstoichiometric oxides as low-cost and highly-efficient oxygen reduction/evolution catalysts for low-temperature electrochemical devices. Chemical Reviews, 2015, 115(18): 9869.
DOI PMID |
| [22] |
LIU X, ZHANG L, ZHENG Y, et al. Uncovering the effect of lattice strain and oxygen deficiency on electrocatalytic activity of perovskite cobaltite thin films. Advanced Science, 2019, 6(6): 1801898.
DOI URL |
| [23] |
XU C, SUN W, REN R, et al. A highly active and carbon-tolerant anode decorated with in situ grown cobalt nano-catalyst for intermediate-temperature solid oxide fuel cells. Applied Catalysis B: Environmental, 2021, 282: 119553.
DOI URL |
| [24] |
YAO C, YANG J, ZHANG H, et al. Evaluation of A-site Ba-deficient PrBa0.5-xSr0.5Co2O5+δ (x = 0, 0.04 and 0.08) as cathode materials for solid oxide fuel cells. Journal of Alloys and Compounds, 2021, 883: 160759.
DOI URL |
| [25] |
WANG G, ZHANG Y, HAN M. Densification of Ce0.9Gd0.1O2-δ interlayer to improve the stability of La0.6Sr0.4Co0.2Fe0.8O3-δ/ Ce0.9Gd0.1O2-δ interface and SOFC. Journal of Electroanalytical Chemistry, 2020, 857: 113591.
DOI URL |
| [26] |
WANG R, SUN Z, LU Y, et al. Comparison of chromium poisoning between lanthanum strontium manganite and lanthanum strontium ferrite composite cathodes in solid oxide fuel cells. Journal of Power Sources, 2020, 476: 228743.
DOI URL |
| [27] |
JIN F, LIU X, CHU X, et al. Effect of nonequivalent substitution of Pr3+/4+ with Ca2+ in PrBaCoFeO5+δ as cathodes for IT-SOFC. Journal of Materials Science, 2021, 56(2): 1147.
DOI |
| [28] |
YUAN M, WANG Z, GAO J, et al. Configuration entropy tailored beneficial surface segregation on double perovskite cathode with enhanced Cr-tolerance for SOFC. Ceramics International, 2024, 50(9): 15076.
DOI URL |
| [29] |
XIONG C, QIU P, ZHANG W, et al. Influence of practical operating temperature on the Cr poisoning for LSCF-GDC cathode. Ceramics International, 2022, 48(22): 33999.
DOI URL |
| [30] |
GAO M, KONYSHEVA E Y, YANG J. Tailoring kinetics of Cr-chemisorption over La0.6Sr0.4Fe0.8Co0.2O3 cathode material through its porosity variation. International Journal of Hydrogen Energy, 2022, 47(97): 41336.
DOI URL |
| [31] |
PAN Y, XU X, ZHONG Y, et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nature Communications, 2020, 11: 2002.
DOI PMID |
| [32] |
ZHAO Y, DONGFANG N, HUANG C, et al. Operando monitoring of the functional role of tetrahedral cobalt centers for the oxygen evolution reaction. Nature Communications, 2025, 16: 580.
DOI |
| [33] |
JEONG N C, LEE J S, TAE E L, et al. Acidity scale for metal oxides and Sanderson's electronegativities of lanthanide elements. Angewandte Chemie International Edition, 2008, 47(52): 10128.
DOI URL |
| [34] |
NICOLLET C, TOPARLI C, HARRINGTON G F, et al. Acidity of surface-infiltrated binary oxides as a sensitive descriptor of oxygen exchange kinetics in mixed conducting oxides. Nature Catalysis, 2020, 3(11): 913.
DOI |
| [35] |
XIAOKAITI P, YU T, YOSHIDA A, et al. Effects of cobalt and iron proportions in Pr0.4Sr0.6Co0.9-xFexNb0.1O3-δ electrode material for symmetric solid oxide fuel cells. Journal of Alloys and Compounds, 2020, 831: 154738.
DOI URL |
| [36] |
ZHANG W, ZHANG L, GUAN K, et al. Effective promotion of oxygen reduction activity by rare earth doping in simple perovskite cathodes for intermediate-temperature solid oxide fuel cells. Journal of Power Sources, 2020, 446: 227360.
DOI URL |
| [1] | 柴润宇, 张镇, 王孟龙, 夏长荣. 直接组装法制备氧化铈基金属支撑固体氧化物燃料电池[J]. 无机材料学报, 2025, 40(7): 765-771. |
| [2] | 渠吉发, 王旭, 张维轩, 张康喆, 熊永恒, 谭文轶. 掺杂改性NaYTiO4增强固体氧化物燃料电池阳极抗硫中毒性能[J]. 无机材料学报, 2025, 40(5): 489-496. |
| [3] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [4] | 刘弘明, 张金柯, 陈正鹏, 李明飞, 钱秀洋, 孙传骐, 熊凯, 饶睦敏, 陈创庭, 高源, 凌意瀚. B位高熵策略提高La0.7Sr0.3FeO3-δ基阴极性能[J]. 无机材料学报, 2025, 40(12): 1433-1442. |
| [5] | 杨恒强, 张馨月, 马义初, 周青军. 铁基钙钛矿La0.25M0.75FeO3-δ (M=Ba, Sr, Ca)的制备及其作为固体氧化物燃料电池阴极材料的性能研究[J]. 无机材料学报, 2025, 40(12): 1365-1372. |
| [6] | 王哲, 郝鸿儒, 吴宗辉, 徐玲玲, 吕喆, 魏波. 构型熵工程增强双钙钛矿型氧电极抗Cr中毒能力[J]. 无机材料学报, 2025, 40(12): 1341-1348. |
| [7] | 姜玥宏, 宋云峰, 张磊磊, 马季, 宋昭远, 龙文. 质子传导型固体氧化物燃料电池BaZr0.1Ce0.7Y0.1Yb0.1O3电解质的氟化研究[J]. 无机材料学报, 2025, 40(12): 1356-1364. |
| [8] | 薛子轩, 殷超凡, 姚跃超, 王彦敏, 孙跃跃, 刘峥嵘, 周玉存, 周峻, 吴锴. 泛氢燃料质子导体固体氧化物燃料电池研究进展[J]. 无机材料学报, 2025, 40(12): 1324-1340. |
| [9] | 刘通, 黄溯, 朱诗悦, 查方林, 胡学雷, 王瑶. 一锅法合成高温氢燃料电池用高效无钴复合阴极[J]. 无机材料学报, 2025, 40(12): 1349-1355. |
| [10] | 张婧慧, 陆晓彤, 毛海雁, 田亚州, 张山林. 烧结助剂对BaZr0.1Ce0.7Y0.2O3-δ电解质烧结行为及电导率的影响[J]. 无机材料学报, 2025, 40(1): 84-90. |
| [11] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [12] | 叶梓滨, 邹高昌, 吴琪雯, 颜晓敏, 周明扬, 刘江. 阳极支撑型锥管串接式直接碳固体氧化物燃料电池组的制备及性能[J]. 无机材料学报, 2024, 39(7): 819-827. |
| [13] | 张琨, 王宇, 朱腾龙, 孙凯华, 韩敏芳, 钟秦. LaNi0.6Fe0.4O3阴极接触材料导电特性调控及其对SOFC电化学性能的影响[J]. 无机材料学报, 2024, 39(4): 367-373. |
| [14] | 陈正鹏, 金芳军, 李明飞, 董江波, 许仁辞, 徐韩昭, 熊凯, 饶睦敏, 陈创庭, 李晓伟, 凌意瀚. 双钙钛矿Sr2CoFeO5+δ阴极材料的制备及其中温固体氧化物燃料电池性能研究[J]. 无机材料学报, 2024, 39(3): 337-344. |
| [15] | 薛顶喜, 伊炳尧, 李国君, 马帅, 刘克勤. 功能梯度阳极固体氧化物燃料电池热应力数值模拟研究[J]. 无机材料学报, 2024, 39(11): 1189-1196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||