无机材料学报 ›› 2024, Vol. 39 ›› Issue (3): 337-344.DOI: 10.15541/jim20230428 CSTR: 32189.14.10.15541/jim20230428
所属专题: 【能源环境】燃料电池(202506)
• 研究快报 • 上一篇
陈正鹏1( ), 金芳军2,3(
), 金芳军2,3( ), 李明飞1, 董江波1, 许仁辞1, 徐韩昭4, 熊凯5, 饶睦敏1, 陈创庭1, 李晓伟2, 凌意瀚2(
), 李明飞1, 董江波1, 许仁辞1, 徐韩昭4, 熊凯5, 饶睦敏1, 陈创庭1, 李晓伟2, 凌意瀚2( )
)
收稿日期:2023-09-20
修回日期:2023-11-16
出版日期:2024-03-20
网络出版日期:2023-11-28
通讯作者:
凌意瀚, 教授. E-mail: lyhyy@cumt.edu.cn;作者简介:陈正鹏(1991-),男,硕士. E-mail: chenzhengpeng@geg.com.cn
CHEN Zhengpeng1( ), JIN Fangjun2,3(
), JIN Fangjun2,3( ), LI Mingfei1, DONG Jiangbo1, XU Renci1, XU Hanzhao4, XIONG Kai5, RAO Muming1, CHEN Chuangting1, LI Xiaowei2, LING Yihan2(
), LI Mingfei1, DONG Jiangbo1, XU Renci1, XU Hanzhao4, XIONG Kai5, RAO Muming1, CHEN Chuangting1, LI Xiaowei2, LING Yihan2( )
)
Received:2023-09-20
Revised:2023-11-16
Published:2024-03-20
Online:2023-11-28
Contact:
LING Yihan, professor. E-mail: lyhyy@cumt.edu.cn;About author:CHEN Zhengpeng (1991-), male, Master. E-mail: chenzhengpeng@geg.com.cn
Supported by:摘要:
随着操作温度降低, 中温固体氧化物燃料电池(IT-SOFCs)需要更高催化活性的阴极材料来提升电池性能。为此, 本研究采用溶胶-凝胶法合成了双钙钛矿Sr2CoFeO5+δ (SCF)阴极材料, 并探讨了SCF阴极与摩尔分数20% Sm2O3掺杂的CeO2(SDC)进行不同比例的复合对电极性能的影响, 优化了电极的化学膨胀和面积比电阻(ASR),进而提升了SOFC单电池的电化学性能。结果表明, SCF作为SOFC阴极, 经950 ℃退火10 h后与普通电解质具有良好的化学相容性; 其中, SCF与SDC按照质量比1 : 1复合的样品可以将纯SCF样品的平均热膨胀系数(TEC)从2.44×10−5 K−1显著降到15.4×10−5 K−1。此外, SCF-xSDC(x=20, 30, 40, 50, x为SDC的质量分数)复合阴极的ASR在800 ℃下分别低至0.036、0.034、0.028和0.092 Ω·cm2, SCF-40SDC在所有温度范围内都表现出更小的ASR。复合SDC可以优化SCF的三相界面且进一步提高SCF阴极的催化活性, 以0.3mm厚La0.9Sr0.1Ga0.8Mg0.2O3-δ(LSGM)为电解质的SCF-40SDC单电池(757 mW·cm−2)比SCF单电池(684 mW·cm−2)的最大功率密度更优, 且超过目前大部分的文献报道。本研究制备的SCF−40SDC是一种性能优异的复合阴极材料, 有望应用于中温固体氧化物燃料电池。
中图分类号:
陈正鹏, 金芳军, 李明飞, 董江波, 许仁辞, 徐韩昭, 熊凯, 饶睦敏, 陈创庭, 李晓伟, 凌意瀚. 双钙钛矿Sr2CoFeO5+δ阴极材料的制备及其中温固体氧化物燃料电池性能研究[J]. 无机材料学报, 2024, 39(3): 337-344.
CHEN Zhengpeng, JIN Fangjun, LI Mingfei, DONG Jiangbo, XU Renci, XU Hanzhao, XIONG Kai, RAO Muming, CHEN Chuangting, LI Xiaowei, LING Yihan. Double Perovskite Sr2CoFeO5+δ: Preparation and Performance as Cathode Material for Intermediate-temperature Solid Oxide Fuel Cells[J]. Journal of Inorganic Materials, 2024, 39(3): 337-344.
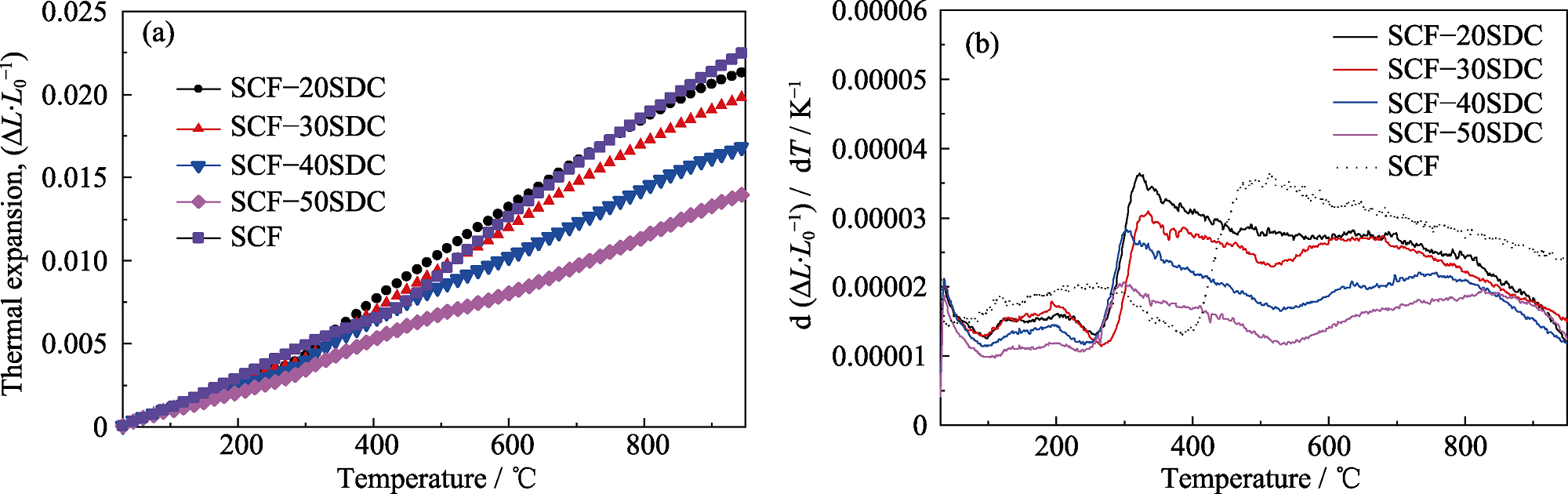
Fig. 4 (a) Thermal expansion behaviors and (b) thermal expansion coefficient curves of SCF-xSDC composite cathodes in the temperature range of 30-950 ℃ Colorful figures are available on website
| Sample | Average TEC/(×10−6, K−1) | |
|---|---|---|
| SCF | 17.4 (30−400 ℃) | 28.8 (400−1000 ℃) |
| SCF−20SDC | 15.6 (30−300 ℃) | 26.3 (300−950 ℃) |
| SCF−30SDC | 15.0 (30−300 ℃) | 24.3 (300−950 ℃) |
| SCF−40SDC | 14.5 (30−300 ℃) | 19.8 (300−950 ℃) |
| SCF−50SDC | 12.5 (30−300 ℃) | 16.3 (300−950 ℃) |
Table 1 TEC of SCF-xSDC composite cathodes
| Sample | Average TEC/(×10−6, K−1) | |
|---|---|---|
| SCF | 17.4 (30−400 ℃) | 28.8 (400−1000 ℃) |
| SCF−20SDC | 15.6 (30−300 ℃) | 26.3 (300−950 ℃) |
| SCF−30SDC | 15.0 (30−300 ℃) | 24.3 (300−950 ℃) |
| SCF−40SDC | 14.5 (30−300 ℃) | 19.8 (300−950 ℃) |
| SCF−50SDC | 12.5 (30−300 ℃) | 16.3 (300−950 ℃) |
| Atom | Wyck. | S.O.F. | x/a | y/b | z/c | U/Å2 |
|---|---|---|---|---|---|---|
| Sr1 | 8c | 1 | 0.25 | 0.25 | 0.25 | 0.01083(1) |
| Co1 | 4b | 1 | 0.5 | 0.5 | 0.5 | 0.01311(1) |
| Fe1 | 4a | 1 | 0 | 0 | 0 | 0.0115(2) |
| O1 | 24e | 1 | 0.2496(5) | 0 | 0 | 0.00995(2) |
Table S1 Atomic occupancy information (atomic parameters) of XRD refinement
| Atom | Wyck. | S.O.F. | x/a | y/b | z/c | U/Å2 |
|---|---|---|---|---|---|---|
| Sr1 | 8c | 1 | 0.25 | 0.25 | 0.25 | 0.01083(1) |
| Co1 | 4b | 1 | 0.5 | 0.5 | 0.5 | 0.01311(1) |
| Fe1 | 4a | 1 | 0 | 0 | 0 | 0.0115(2) |
| O1 | 24e | 1 | 0.2496(5) | 0 | 0 | 0.00995(2) |
| Cathode | Electrolyte | T/℃ | Power density/(mW·cm-2) | Ref. |
|---|---|---|---|---|
| YBaCo2/3Fe2/3Cu2/3O5+δ | LSGM | 800 | 543 | [ |
| SrCo0.7Fe0.2Ta0.1O3−δ | LSGM | 800 | 652.9 | [ |
| PrBaCo2/3Fe2/3Cu2/3O5+δ | GDC | 800 | 659 | [ |
| Pr1.9Ca0.1BaCoFeO5+δ | LSGM | 800 | 728 | [ |
| SCF−40SDC | LSGM | 800 | 757 | This work |
Table S2 Electrochemical performance for cathode materials using hydrogen fuels
| Cathode | Electrolyte | T/℃ | Power density/(mW·cm-2) | Ref. |
|---|---|---|---|---|
| YBaCo2/3Fe2/3Cu2/3O5+δ | LSGM | 800 | 543 | [ |
| SrCo0.7Fe0.2Ta0.1O3−δ | LSGM | 800 | 652.9 | [ |
| PrBaCo2/3Fe2/3Cu2/3O5+δ | GDC | 800 | 659 | [ |
| Pr1.9Ca0.1BaCoFeO5+δ | LSGM | 800 | 728 | [ |
| SCF−40SDC | LSGM | 800 | 757 | This work |
| [1] |
GUO T M, DONG J B, CHEN Z P, et al. Enhanced compatibility and activity of high-entropy double perovskite cathode material for IT-SOFC. Journal of Inorganic Materials, 2023, 38(6): 693.
DOI |
| [2] | HAN X, LING Y H, YANG Y, et al. Utilizing high entropy effects for developing chromium-tolerance cobalt-free cathode for solid oxide fuel cells. Advanced Functional Materials, 2023, 33(43): 202304728. |
| [3] |
TAI L W, NASRALLAH M M, ANDERSON H U, et al. Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 1. The system La0.8Sr0.2Co1-yFeyO3. Solid State Ionics, 1995, 76(3/4): 259.
DOI URL |
| [4] |
TAI L W, NASRALLAH M M, ANDERSON H U, et al. Structure and electrical properties of La1-xSrxCo1-yFeyO3. Part 2. The system La1-xSrxCo0.2Fe0.8O3. Solid State Ionics, 1995, 76(3/4): 273.
DOI URL |
| [5] |
SHAO Z, HAILE S M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature, 2004, 431(7005): 170.
DOI |
| [6] |
WEI B, LU Z, LI S Y, et al. Thermal and electrical properties of new cathode material Ba0.5Sr0.5Co0.8Fe0.2O3-δ for solid oxide fuel cells. Electrochemical and Solid State Letters, 2005, 8(8): A428.
DOI URL |
| [7] |
ZHANG K, GE L, RAN R, et al. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Materialia, 2008, 56(17): 4876.
DOI URL |
| [8] |
JIN F J, SHEN Y, WANG R, et al. Double-perovskite PrBaCo2/3Fe2/3Cu2/3O5+δ as cathode material for intermediate-temperature solid-oxide fuel cells. Journal of Power Sources, 2013, 234: 244.
DOI URL |
| [9] |
LI J, SUN N, LIU X, et al. Investigation on Nd1-xCaxBaCo2O5+δ double perovskite as new oxygen electrode materials for reversible solid oxide cells. Journal of Alloys and Compounds, 2022, 913: 165245.
DOI URL |
| [10] |
JIN F, XU H, LONG W, et al. Characterization and evaluation of double perovskites LnBaCoFeO5+δ (Ln=Pr and Nd) as intermediate- temperature solid oxide fuel cell cathodes. Journal of Power Sources, 2013, 243: 10.
DOI URL |
| [11] |
LIU X, JIN F, SUN N, et al. Nd3+-deficiency double perovskite Nd1-xBaCo2O5+s and performance optimization as cathode materials for intermediate-temperature solid oxide fuel cells. Ceramics International, 2021, 47(23): 33886.
DOI URL |
| [12] |
BEZDICKA P, FOURNES L, WATTIAUX A, et al. Mössbauer characteristics of the Sr2CoFeO6 perovskite obtained by electrochemical oxidation. Solid State Communications, 1994, 91(7): 501.
DOI URL |
| [13] |
MARTÍNEZ-LOPE MARÍA J, ALONSO JOSÉ A, CASAIS MARÍA T, et al. Preparation, crystal and magnetic structure of the double perovskites Ba2CoBO6 (B=Mo, W). European Journal of Inorganic Chemistry, 2002, 2002(9): 2463.
DOI URL |
| [14] |
YOSHII K. Magnetic transition in the perovskite Ba2CoNbO6-δ. Journal of Solid State Chemistry, 2000, 151(2): 294.
DOI URL |
| [15] |
COX D E, SHIRANE G, FRAZER B C. Neutron-diffraction study of antiferromagnetic Ba2CoWO6 and Ba2NiWO6. Journal of Applied Physics, 1967, 38(3): 1459.
DOI URL |
| [16] | RAMMEH N, EHRENBERG H, FUESS H, et al. Structure and magnetic properties of the double-perovskites Ba2(B,Re)2O6 (B=Fe, Mn, Co and Ni). Physica Status Solidi (c), 2006, 3(9): 3225. |
| [17] |
XIAO G L, LIU Q A, ZHAO F, et al. Sr2Fe1.5Mo0.5O6 as cathodes for intermediate-temperature solid oxide fuel cells with La0.8Sr0.2Ga0.87Mg0.13O3 electrolyte. Journal of Electrochem Society, 2011, 158: B455.
DOI URL |
| [18] |
MENG J L, LIU X J, HAN L, et al. Improved electrochemical performance by doping cathode materials Sr2Fe1.5Mo0.5-xTaxO6-δ (0≤x≤0.15) for solid state fuel cell. Journal of Power Sources, 2014, 247: 845.
DOI URL |
| [19] |
DENG Z Q, SMIT J P, NIU H J, et al. B cation ordered double perovskite Ba2CoMo0.5Nb0.5O6-δ as a potential SOFC cathode. Chemistry of Materials, 2009, 21(21): 5154.
DOI URL |
| [20] |
PRADHEESH R, NAIR H S, KUMAR C M N, et al. Observation of spin glass state in weakly ferromagnetic Sr2FeCoO6 double perovskite. Journal of Applied Physics, 2012, 111(5): 053905.
DOI URL |
| [21] |
PRADHEESH R, NAIR HS, SANKARANARAYANAN V, et al. Large magnetoresistance and Jahn-Teller effect in Sr2FeCoO6. The European Physical Journal B, 2012, 85: 260.
DOI URL |
| [22] |
PRADHEESH R, NAIR HS, SANKARANARAYANAN V, et al. Exchange bias and memory effect in double perovskite Sr2FeCoO6. Applied Physics Letters, 2012, 101(14): 142401.
DOI URL |
| [23] |
CONG LG, HE TM, JI YA, et al. Synthesis and characterization of IT-electrolyte with perovskite structure La0.8Sr0.2Ga0.85Mg0.15O3-δ by glycine-nitrate combustion method. Journal of Alloys and Compounds. 2003, 348(1/2): 325.
DOI URL |
| [24] |
WANG Y, JIN F, HAO X, et al. B-site-ordered Co-based double perovskites Sr2Co1-xNbxFeO5+δ as active and stable cathodes for intermediate-temperature solid oxide fuel cells. Journal of Alloys and Compounds, 2020, 829: 154470.
DOI URL |
| [25] |
WU H, QIAN Y, TAN W, et al. The theoretical search for half- metallic material: the non-stoichiometric perovskite oxide Sr2FeCoO6-δ. Applied Physics Letters, 2011, 99(12): 123116.
DOI URL |
| [26] |
SHAO Z, XIONG G, TONG J, et al. Ba effect in doped Sr(Co0.8Fe0.2)O3-δ on the phase structure and oxygen permeation properties of the dense ceramic membranes. Separation and Purification Technology, 2001, 25(1/2/3): 419.
DOI URL |
| [27] |
SHAO Z, YANG W, CONG Y, et al. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3-δ oxygen membrane. Journal of Membrane Science, 2000, 172(1/2): 177.
DOI URL |
| [28] |
LIU X, JIN F, LIU X, et al. Effect of calcium doping on Sm1-xCaxBaCo2O5+δ cathode materials for intermediate-temperature solid oxide fuel cells. Electrochim Acta, 2021, 390: 138830.
DOI URL |
| [29] |
LING Y H, GUO T M, GUO Y Y, et al. New two-layer Ruddlesden-Popper cathode materials for protonic ceramics fuel cells. Journal of Advanced Ceramics, 2021, 10: 1052.
DOI |
| [30] |
JIN F, LIU J, NIU B, et al. Evaluation and performance optimization of double-perovskite LaSrCoTiO5+δ cathode for intermediate-temperature solid-oxide fuel cells. International Journal of Hydrogen Energy, 2016, 41(46): 21439.
DOI URL |
| [31] |
GHAFFARI M, SHANNON M, HUI H, et al. Preparation, surface state and band structure studies of SrTi(1-x)Fe(x)O(3-δ) (x=0-1) perovskite-type nano structure by X-ray and ultraviolet photoelectron spectroscopy. Surface Science, 2012, 606(5/6): 670.
DOI URL |
| [32] |
PIKALOVA EY, MARAGOU VI, DEMINA AN, et al. The effect of co-dopant addition on the properties of Ln0.2Ce0.8O2-δ (Ln=Gd, Sm, La) solid-state electrolyte. Journal of Power Sources, 2008, 181(2): 199
DOI URL |
| [33] |
JIN F, LIU J, SHEN Y, et al. Improved electrochemical performance and thermal expansion compatibility of LnBaCoFeO5+δ- Sm0.2Ce0.8O1.9 (LnPr and Nd) composite cathodes for IT-SOFCs. Journal of Alloys and Compounds, 2016, 685: 483.
DOI URL |
| [34] |
LIU B, SUNARSO J, ZHANG Y, et al. Highly oxygen non- stoichiometric BaSc0.25Co0.75O3-δ as a high-performance cathode for intermediate-temperature solid oxide fuel cells. ChemElectroChem, 2018, 5(5): 785.
DOI URL |
| [35] |
BU Y F, DING D, LAI S Y, et al. Evaluation of La0.4Ba0.6Fe0.8Zn0.2O3-δ+Sm0.2Ce0.8O1.9 as a potential cobalt-free composite cathode for intermediate temperature solid oxide fuel cells. Journal of Power Sources, 2015, 275: 808.
DOI URL |
| [36] |
LI S L, ZHANG L K, XIA T, et al. Synergistic effect study of EuBa0.98Co2O5+δ-Ce0.8Sm0.2O1.9 composite cathodes for intermediate- temperature solid oxide fuel cells. Journal of Alloys and Compounds, 2019, 771: 513.
DOI URL |
| [37] | STEELE BCH. Survey of materials selection for ceramic fuel cells II. cathodes and anodes. Solid State Ionics, 1996, 86: 1223. |
| [1] | 柴润宇, 张镇, 王孟龙, 夏长荣. 直接组装法制备氧化铈基金属支撑固体氧化物燃料电池[J]. 无机材料学报, 2025, 40(7): 765-771. |
| [2] | 渠吉发, 王旭, 张维轩, 张康喆, 熊永恒, 谭文轶. 掺杂改性NaYTiO4增强固体氧化物燃料电池阳极抗硫中毒性能[J]. 无机材料学报, 2025, 40(5): 489-496. |
| [3] | 万俊池, 杜路路, 张永上, 李琳, 刘建德, 张林森. Na4FexP4O12+x/C钠离子电池正极材料的结构演变及其电化学性能[J]. 无机材料学报, 2025, 40(5): 497-503. |
| [4] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [5] | 张婧慧, 陆晓彤, 毛海雁, 田亚州, 张山林. 烧结助剂对BaZr0.1Ce0.7Y0.2O3-δ电解质烧结行为及电导率的影响[J]. 无机材料学报, 2025, 40(1): 84-90. |
| [6] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [7] | 叶梓滨, 邹高昌, 吴琪雯, 颜晓敏, 周明扬, 刘江. 阳极支撑型锥管串接式直接碳固体氧化物燃料电池组的制备及性能[J]. 无机材料学报, 2024, 39(7): 819-827. |
| [8] | 张琨, 王宇, 朱腾龙, 孙凯华, 韩敏芳, 钟秦. LaNi0.6Fe0.4O3阴极接触材料导电特性调控及其对SOFC电化学性能的影响[J]. 无机材料学报, 2024, 39(4): 367-373. |
| [9] | 薛顶喜, 伊炳尧, 李国君, 马帅, 刘克勤. 功能梯度阳极固体氧化物燃料电池热应力数值模拟研究[J]. 无机材料学报, 2024, 39(11): 1189-1196. |
| [10] | 王马超, 唐扬敏, 邓明雪, 周真真, 刘小峰, 王家成, 刘茜. 共沉淀法制备Cs2Ag0.1Na0.9BiCl6:Tm3+双钙钛矿及其近红外发光性能[J]. 无机材料学报, 2023, 38(9): 1083-1088. |
| [11] | 张伦, 吕梅, 朱俊. Cs2AgBiBr6钙钛矿太阳能电池研究进展[J]. 无机材料学报, 2023, 38(9): 1044-1054. |
| [12] | 郭天民, 董江波, 陈正鹏, 饶睦敏, 李明飞, 李田, 凌意瀚. 中温固体氧化物燃料电池的高熵双钙钛矿阴极材料: 兼容性与活性研究[J]. 无机材料学报, 2023, 38(6): 693-700. |
| [13] | 杨颖康, 邵怡晴, 李柏良, 吕志伟, 王路路, 王亮君, 曹逊, 吴宇宁, 黄荣, 杨长. Cl掺杂对CuI薄膜发光性能增强研究[J]. 无机材料学报, 2023, 38(6): 687-692. |
| [14] | 樊帅, 金天, 张山林, 雒晓涛, 李成新, 李长久. Li2O烧结助剂对固体氧化物燃料电池LSGM电解质烧结特性及离子电导率的影响[J]. 无机材料学报, 2022, 37(10): 1087-1092. |
| [15] | 刘芳芳, 传秀云, 杨扬, 李爱军. 氮/硫共掺杂对纤水镁石模板碳纳米管电化学性能的影响[J]. 无机材料学报, 2021, 36(7): 711-717. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||