无机材料学报 ›› 2025, Vol. 40 ›› Issue (12): 1433-1442.DOI: 10.15541/jim20240410 CSTR: 32189.14.jim20240410
刘弘明1( ), 张金柯2, 陈正鹏1, 李明飞1, 钱秀洋1, 孙传骐1, 熊凯3, 饶睦敏1, 陈创庭1, 高源2(
), 张金柯2, 陈正鹏1, 李明飞1, 钱秀洋1, 孙传骐1, 熊凯3, 饶睦敏1, 陈创庭1, 高源2( ), 凌意瀚2(
), 凌意瀚2( )
)
收稿日期:2024-09-14
修回日期:2024-12-11
出版日期:2025-12-20
网络出版日期:2024-12-27
通讯作者:
高 源, 博士后. E-mail: tbh371@cumt.edu.cn;作者简介:刘弘明(1996-), 男, 博士. E-mail: liuhongming@geg.com.cn
LIU Hongming1( ), ZHANG Jinke2, CHEN Zhengpeng1, LI Mingfei1, QIAN Xiuyang1, SUN Chuanqi1, XIONG Kai3, RAO Mumin1, CHEN Chuangting1, GAO Yuan2(
), ZHANG Jinke2, CHEN Zhengpeng1, LI Mingfei1, QIAN Xiuyang1, SUN Chuanqi1, XIONG Kai3, RAO Mumin1, CHEN Chuangting1, GAO Yuan2( ), LING Yihan2(
), LING Yihan2( )
)
Received:2024-09-14
Revised:2024-12-11
Published:2025-12-20
Online:2024-12-27
Contact:
GAO Yuan, post doctor. E-mail: tbh371@cumt.edu.cn;About author:LIU Hongmin (1996-), male, PhD. E-mail: liuhongming@geg.com.cn
Supported by:摘要: 作为经典的固体氧化物燃料电池(SOFC)阴极材料, 铁基钙钛矿材料具有成本低、热膨胀系数低和稳定性高的优点。本研究采用柠檬酸-硝酸盐燃烧法制备了B位高熵钙钛矿氧化物La0.7Sr0.3(FeNiCo)0.8Mo0.1Ti0.1O3-δ(LSFNCMT)。由于高熵材料的氧表面交换速率更快, LSFNCMT阴极显示出优异的氧还原反应(ORR)活性, 800 ℃的极化电阻(Rp)为0.11 Ω·cm2, 远低于LSF阴极的0.31 Ω·cm2。更重要的是, 由于掺入高酸度的Ni、Co和Mo离子以及平均金属氧键能(ABE)更高的Ti离子, 高熵材料表现出更出色的稳定性。以LSFNCMT为阴极的对称电池在含铬气氛中进行22 h的稳定性测试, Rp仅从1.07 Ω·cm2增加到2.98 Ω·cm2, 而LSF对称电池的Rp从2.62 Ω·cm2增加到7.90 Ω·cm2。LSFNCMT阴极单电池在800 ℃下的最大功率密度(MPD)为1105.26 mW·cm-2, 显著高于LSF阴极单电池(830.74 mW·cm-2), 800 ℃下Rp为0.24 Ω·cm2, 也低于相同条件下LSF阴极单电池(0.36 Ω·cm2), 证实高熵阴极具有卓越的耐毒化性。结果表明, B位高熵是提高阴极材料催化活性和耐铬性的有效途径。
中图分类号:
刘弘明, 张金柯, 陈正鹏, 李明飞, 钱秀洋, 孙传骐, 熊凯, 饶睦敏, 陈创庭, 高源, 凌意瀚. B位高熵策略提高La0.7Sr0.3FeO3-δ基阴极性能[J]. 无机材料学报, 2025, 40(12): 1433-1442.
LIU Hongming, ZHANG Jinke, CHEN Zhengpeng, LI Mingfei, QIAN Xiuyang, SUN Chuanqi, XIONG Kai, RAO Mumin, CHEN Chuangting, GAO Yuan, LING Yihan. Enhanced Performance of La0.7Sr0.3FeO3-δ Cathode for SOFC via Implementation of B-site High-entropy Strategy[J]. Journal of Inorganic Materials, 2025, 40(12): 1433-1442.
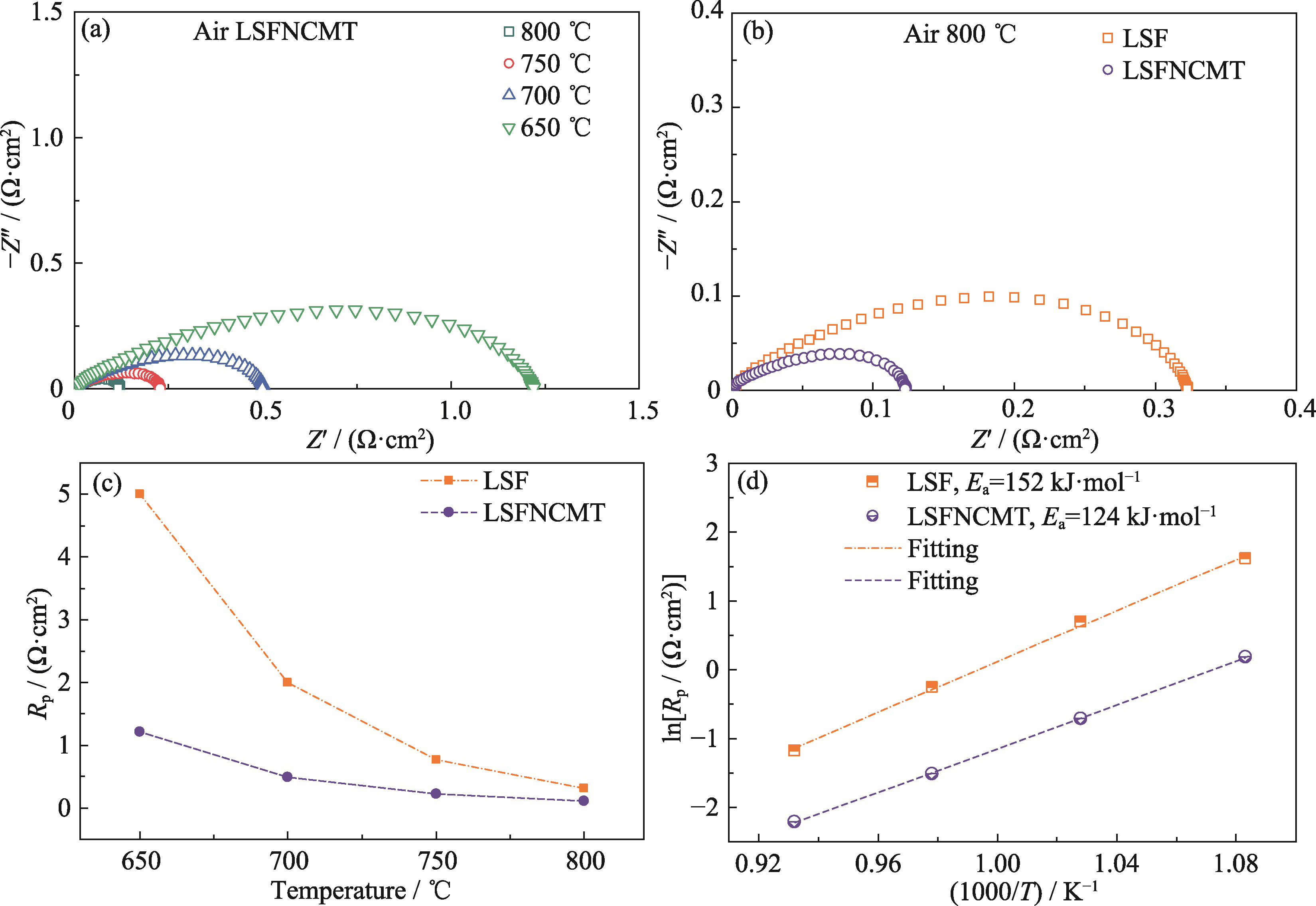
Fig. 3 Electrochemical performance of symmetric cell under different temperatures in air (a) EIS spectra of LSFNCMT electrode; (b) EIS spectra of LSF and LSFNCMT electrodes at 800 ℃; (c) Rp and (d) corresponding Arrhenius plots of two symmetric cells
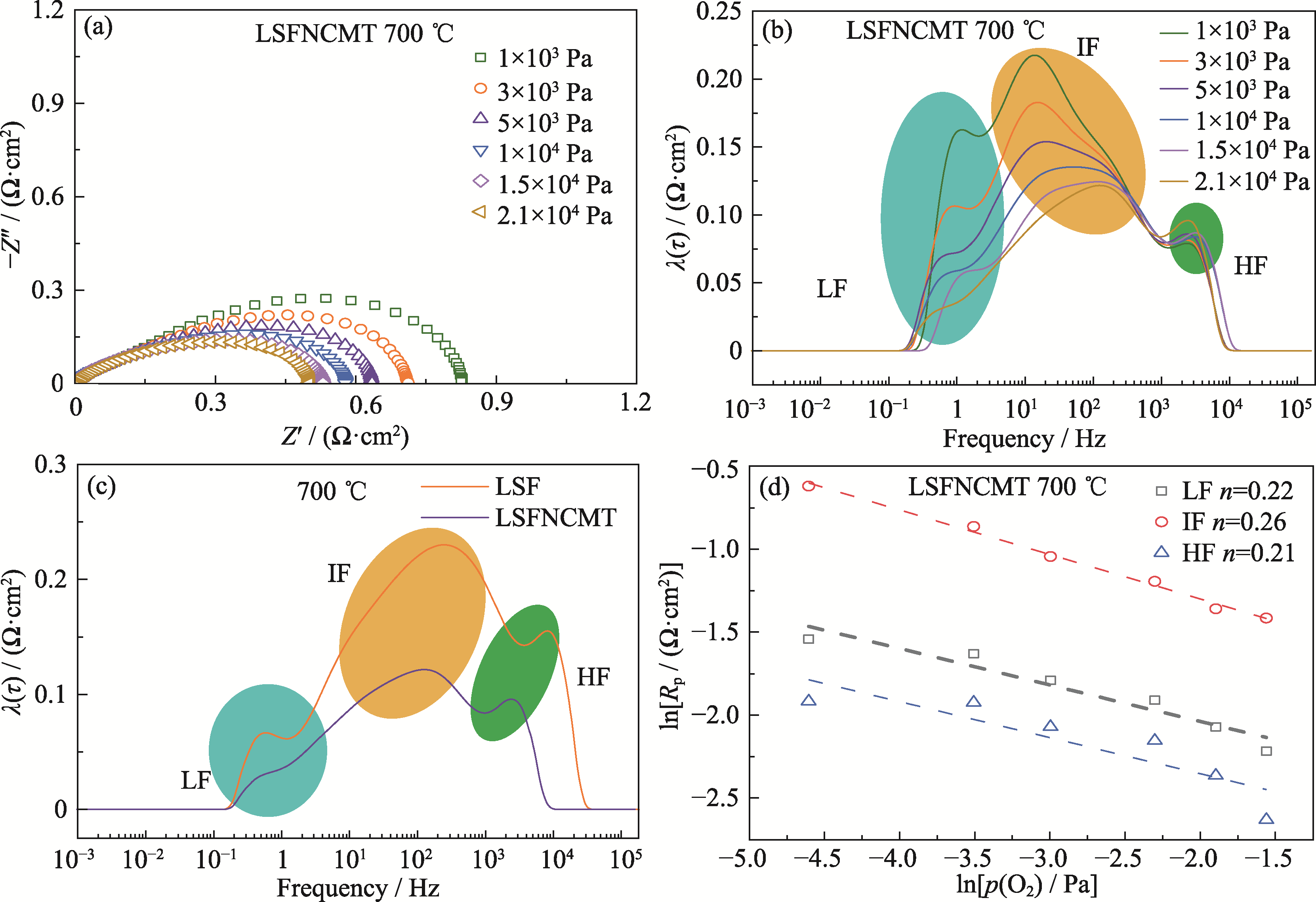
Fig. 4 Electrochemical performance of symmetrical cell under different p(O2) at 700 ℃ (a, b) EIS spectra (a) and DRT curves (b) of LSFNCMT; (c) DRT curves of LSF and LSFNCMT under p(O2) of 2.1×104 Pa at 700 ℃; (d) Dependence of each Rp as a function of p(O2) for LSFNCMT. Colorful figures are available on website
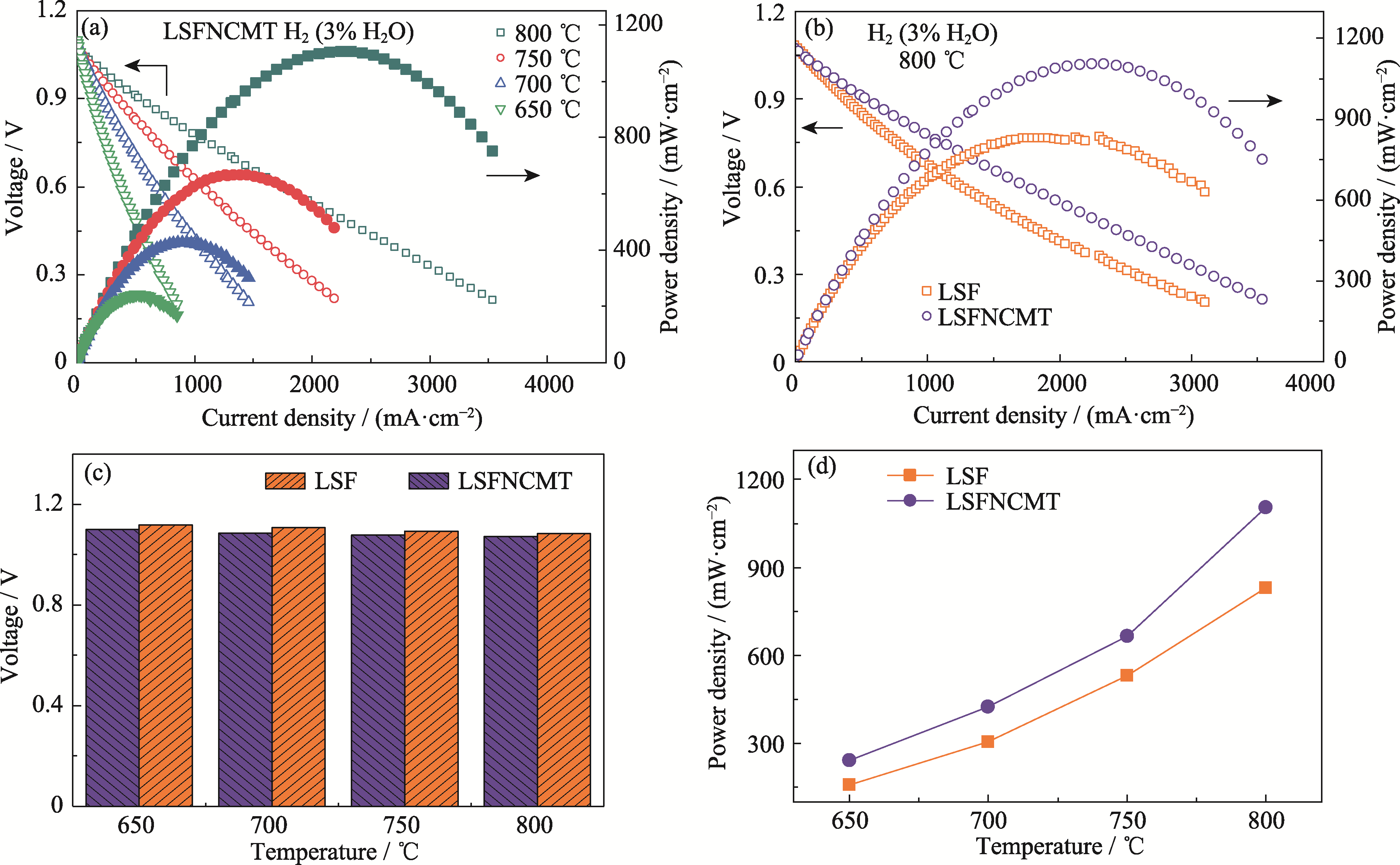
Fig. 6 Electrochemical performance of the single cell at 650-800 ℃ in wet H2 (3% (in volume) H2O) (a) I-V and power density curves of LSFNCMT cathodes; (b) I-V and power density curves of LSF and LSFNCMT cathodes at 800 ℃; (c) OCV and (d) MPD varied with temperature of different single cells
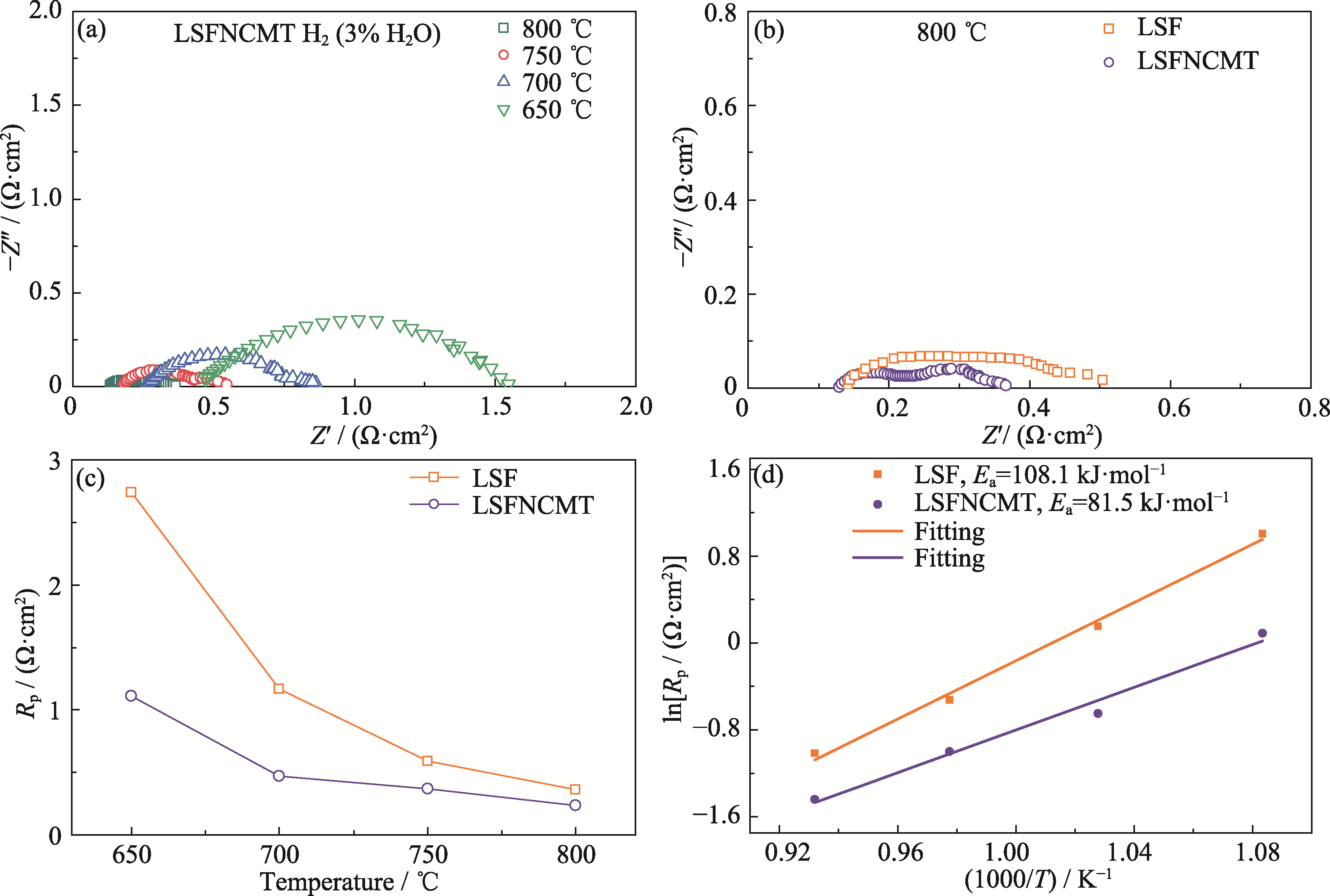
Fig. 7 Electrochemical performance of the anode-supported single cell at 650-800 ℃ in wet H2 (3% (in volume) H2O) (a) EIS spectra of LSFNCMT cathode; (b) EIS spectra of LSF and LSFNCMT cathodes at 800 ℃; (c) Rp at different temperatures and (d) corresponding Arrhenius plots of different single cells
| [1] |
PAN Z, LIU Q, NI M, et al. Activation and failure mechanism of La0.6Sr0.4Co0.2Fe0.8O3-δ air electrode in solid oxide electrolyzer cells under high-current electrolysis. International Journal of Hydrogen Energy, 2018, 43(11): 5437.
DOI URL |
| [2] |
CAOO D, ZHOU M, LIU Z, et al. Fabrication and characterization of anode-supported solid oxide fuel cell based on proton conductor electrolyte. Journal of Inorganic Materials, 2020, 35(9): 1047.
DOI |
| [3] |
LAGUNA-BERCERO M A, CAMPANA R, LARREA A, et al. Electrolyte degradation in anode supported microtubular yttria stabilized zirconia-based solid oxide steam electrolysis cells at high voltages of operation. Journal of Power Sources, 2011, 196(21): 8942.
DOI URL |
| [4] |
SINGH V, MUROYAMA H, MATSUI T, et al. Feasibility of alternative electrode materials for high temperature CO2 reduction on solid oxide electrolysis cell. Journal of Power Sources, 2015, 293: 642.
DOI URL |
| [5] |
LV H, ZHOU Y, ZHANG X, et al. Infiltration of Ce0.8Gd0.2O1.9 nanoparticles on Sr2Fe1.5Mo0.5O6-δ cathode for CO2 electroreduction in solid oxide electrolysis cell. Journal of Energy Chemistry, 2019, 35: 71.
DOI URL |
| [6] |
YE Z, ZOU G, WU Q, et al. Preparation and performances of tubular cone-shaped anode-supported segmented-in-series direct carbon solid oxide fuel cell. Journal of Inorganic Materials, 2024, 39(7): 819.
DOI URL |
| [7] |
XUE L, LI S, AN S, et al. Ca-doping cobalt-free double perovskite oxide as a cathode material for intermediate-temperature solid oxide fuel cell. Molecules, 2024, 29(13): 2991.
DOI URL |
| [8] |
PRAŻUCH J, PYZALSKI M, FERNÁNDEZ GONZÁLEZ D, et al. Physicochemical properties of (La,Sr)CoO3 thick films on Fe-25Cr steel under exposure to SOFC cathode operating conditions. Materials, 2024, 17(15): 3791.
DOI URL |
| [9] |
PINSKY R, SABHARWALL P, HARTVIGSEN J, et al. Comparative review of hydrogen production technologies for nuclear hybrid energy systems. Progress in Nuclear Energy, 2020, 123: 103317.
DOI URL |
| [10] |
DING P, LI W, ZHAO H, et al. Review on Ruddlesden-Popper perovskites as cathode for solid oxide fuel cells. Journal of Physics: Materials, 2021, 4(2): 022002.
DOI |
| [11] |
GREELEY J, MARKOVIC N M. The road from animal electricity to green energy: combining experiment and theory in electrocatalysis. Energy and Environmental Science, 2012, 5(11): 9246.
DOI URL |
| [12] |
SHEN R, NIE J, WANG K, et al. Applying multifunctional perovskite LaNiO3 as electrolyte and anode for low‐temperature solid oxide fuel cell. Journal of Materials Science: Materials in Electronics, 2021, 32(4): 4196.
DOI |
| [13] |
ZHANG K, WANG Y, ZHU T, et al. LaNi0.6Fe0.4O3 cathode contact material: electrical conducting property manipulation and its effect on SOFC electrochemical performance. Journal of Inorganic Materials, 2024, 39(4): 367.
DOI URL |
| [14] |
ZHANG Y, KNIBBE R, SUNARSO J, et al. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 ℃. Advanced Materials, 2017, 29(48): 1700132.
DOI URL |
| [15] |
ISTOMIN S Y, ANTIPOV E V. Cathode materials based on perovskite-like transition metal oxides for intermediate temperature solid oxide fuel cells. Russian Chemical Reviews, 2013, 82(7): 686.
DOI URL |
| [16] |
ZHANG J, SONG K, GAO Y, et al. Tuning the thermo-mechanical synergies effect of solid oxide fuel cells with negative thermal expansion NdMnO3 in La0.6Sr0.4Co0.2Fe0.8O3-δ-based symmetric electrode. Chemical Engineering Journal, 2024, 491: 152063.
DOI URL |
| [17] |
CHEN Y, ZHU J, XIA T, et al. Improved electrocatalytic activity and CO2 tolerance of iron-based perovskite as an intermediate temperature SOFC cathode. Fuel, 2024, 375: 132546.
DOI URL |
| [18] | XIA Z, ZHANG Y, XIONG X, et al. Realizing B-site high-entropy air electrode for superior reversible solid oxide cells. Applied Catalysis B: Environmental, 2024, 357: 124410. |
| [19] | JIN F, LIU X, TIAN Y, et al. Enhancing oxygen reduction activity and CO2 tolerance by a bismuth doping strategy for solid oxide fuel cell cathodes. Advanced Functional Materials, 2024, 34(29): 2400519. |
| [20] |
CHU Z, GAO J, LI Q, et al. Highly oxygen reduction activity and CO2 resistance of Fe-based cathode electrocatalysts for solid oxide fuel cells. Journal of Materials Science and Technology, 2025, 212: 303.
DOI URL |
| [21] |
ZHANG H X, YAO C G, ZHANG Z, et al. Lithium doping enhanced ORR kinetics and CO2 tolerance of iron-based double perovskite cathode for solid oxide fuel cells. Journal of Alloys and Compounds, 2024, 980: 173632.
DOI URL |
| [22] |
BAI J, NIU L, ZHU Q, et al. Ni-doped Fe-based perovskite to obtain multifunctional and highly efficient electrocatalytic active IT-SOFC electrode. Fuel, 2024, 365: 131334.
DOI URL |
| [23] |
SUN C, SHEN Y, WANG F, et al. Optimization of a cobalt-free La0.7Sr0.3FeO3-δ-BaZr0.1Ce0.7Y0.2O3-δ composite cathode for proton-conducting solid oxide fuel cells. Journal of Alloys and Compounds, 2022, 923: 166447.
DOI URL |
| [24] |
YANG C, WANG Z, TAN Y, et al. Interface engineering of La0.6Sr0.4Co0.2Fe0.8O3-δ/Gd0.1Ce0.9O1.95 heterostructure oxygen electrode for solid oxide electrolysis cells with enhanced CO2 electrolysis performance. Chemical Engineering Journal, 2024, 498: 155461.
DOI URL |
| [25] |
WANG J, FU L, YANG J, et al. Cerium and ruthenium co-doped La0.7Sr0.3FeO3-δ as a high-efficiency electrode for symmetrical solid oxide fuel cell. Journal of Rare Earths, 2021, 39(9): 1095.
DOI URL |
| [26] |
YUAN M, WANG Z, GAO J, et al. Configuration entropy tailored beneficial surface segregation on double perovskite cathode with enhanced Cr-tolerance for SOFC. Ceramics International, 2024, 50(9): 15076.
DOI URL |
| [27] |
ZHANG X, JIN Y, JIANG Y, et al. Enhancing chromium poisoning tolerance of La0.8Sr0.2Co0.2Fe0.8O3-δ cathode by Ce0.8Gd0.2O1.9-δ coating. Journal of Power Sources, 2022, 547: 231996.
DOI URL |
| [28] |
HUANG K. A thermodynamic perspective on electrode poisoning in solid oxide fuel cells. International Journal of Minerals, Metallurgy and Materials, 2024, 31(6): 1449.
DOI |
| [29] | JIANG S P, ZHANG S, ZHEN Y D. Deposition of Cr species at (La, Sr)(Co, Fe)O3 cathodes of solid oxide fuel cells. Journal of the Electrochemical Society, 2006, 153(1): A127. |
| [30] |
GAO Y, HUANG X, YUAN M, et al. A SrCo0.9Ta0.1O3-δ derived medium-entropy cathode with superior CO2 poisoning tolerance for solid oxide fuel cells. Journal of Power Sources, 2022, 540: 231661.
DOI URL |
| [31] |
GAO Y, LING Y, WANG X, et al. Sr-deficient medium-entropy Sr1-xCo0.5Fe0.2Ti0.1Ta0.1Nb0.1O3-δ cathodes with high Cr tolerance for solid oxide fuel cells. Chemical Engineering Journal, 2024, 479: 147665.
DOI URL |
| [32] |
LI Z, GE Y, XIAO Y, et al. Fabrication and performance investigation of high entropy perovskite (Sr0.2Ba0.2Bi0.2La0.2Pr0.2)FeO3 IT-SOFC cathode material. Journal of Alloys and Compounds, 2024, 989: 174357.
DOI URL |
| [33] |
GUO T M, DONG J B, CHEN Z P, et al. Enhanced compatibility and activity of high-entropy double perovskite cathode material for IT-SOFC. Journal of Inorganic Materials, 2022, 38(6): 693.
DOI URL |
| [34] | HAN X, LING Y, YANG Y, et al. Utilizing high entropy effects for developing chromium-tolerance cobalt-free cathode for solid oxide fuel cells. Advanced Functional Materials, 2023, 33(43): 230478. |
| [35] |
WANG J, SACCOCCIO M, CHEN D, et al. The effect of A-site and B-site substitution on BaFeO3-δ: an investigation as a cathode material for intermediate-temperature solid oxide fuel cells. Journal of Power Sources, 2015, 297: 511.
DOI URL |
| [36] |
KUAI X, YANG G, CHEN Y, et al. Boosting the activity of BaCo0.4Fe0.4Zr0.1Y0.1O3-δ perovskite for oxygen reduction reactions at low-to-intermediate temperatures through tuning B-Site cation deficiency. Advanced Energy Materials, 2019, 9(38): 1902384.
DOI URL |
| [37] |
ZHU C, LIU X, YI C, et al. Novel BaCo0.7Fe0.3-yNbyO3-δ (y=0-0.12) as a cathode for intermediate temperature solid oxide fuel cell. Electrochemistry Communications, 2009, 11(5): 958.
DOI URL |
| [38] |
ZHOU Q, WEI T, SHI Y, et al. Evaluation and optimization of SrCo0.9Ta0.1O3-δ perovskite as cathode for solid oxide fuel cells. Current Applied Physics, 2012, 12(4): 1092.
DOI URL |
| [39] |
SHEN Y, WANG F, MA X, et al. SrCo1-yTiyO3-δ as potential cathode materials for intermediate-temperature solid oxide fuel cells. Journal of Power Sources, 2011, 196(18): 7420.
DOI URL |
| [40] |
ZHAO H, TENG D, ZHANG X, et al. Structural and electrochemical studies of Ba0.6Sr0.4Co1-yTiyO3-δ as a new cathode material for IT-SOFCs. Journal of Power Sources, 2009, 186(2): 305.
DOI URL |
| [41] |
YU X, LONG W, JIN F, et al. Cobalt-free perovskite cathode materials SrFe1-xTixO3-δ and performance optimization for intermediate-temperature solid oxide fuel cells. Electrochimica Acta, 2014, 123: 426.
DOI URL |
| [42] |
LI J, SUN N, LIU X, et al. Investigation on Nd1-xCaxBaCo2O5+δ double perovskite as new oxygen electrode materials for reversible solid oxide cells. Journal of Alloys and Compounds, 2022, 913: 165245.
DOI URL |
| [43] |
CHEN Z P, JIN F J, LI M F, et al. Double perovskite Sr2CoFeO5+δ: preparation and performance as cathode material for intermediate- temperature solid oxide fuel cells. Journal of Inorganic Materials, 2023, 39(3): 337.
DOI URL |
| [44] |
ZHANG Z, WANG H, LI X, et al. CO2/Cr-tolerance and oxygen reduction reaction of novel high-entropy perovskite cathode for intermediate temperature solid oxide fuel cell. Ceramics International, 2024, 50(7): 11360.
DOI URL |
| [45] |
SCHULER J A, YOKOKAWA H, CALDERONE C F, et al. Combined Cr and S poisoning in solid oxide fuel cell cathodes. Journal of Power Sources, 2012, 201: 112.
DOI URL |
| [46] |
LI Y, ZHANG W, ZHENG Y, et al. Controlling cation segregation in perovskite-based electrodes for high electro-catalytic activity and durability. Chemical Society Reviews, 2017, 46(20): 6345.
DOI PMID |
| [47] |
JEONG N C, LEE J S, TAE E L, et al. Acidity scale for metal oxides and Sanderson's electronegativities of lanthanide elements. Angewandte Chemie International Edition, 2008, 47(52): 10128.
DOI URL |
| [1] | 高源, 魏波, 金芳军, 吕喆, 凌意瀚. Ag掺杂调控中温固体氧化物燃料电池阴极酸性位点增强耐铬能力[J]. 无机材料学报, 2026, 41(1): 70-78. |
| [2] | 柴润宇, 张镇, 王孟龙, 夏长荣. 直接组装法制备氧化铈基金属支撑固体氧化物燃料电池[J]. 无机材料学报, 2025, 40(7): 765-771. |
| [3] | 渠吉发, 王旭, 张维轩, 张康喆, 熊永恒, 谭文轶. 掺杂改性NaYTiO4增强固体氧化物燃料电池阳极抗硫中毒性能[J]. 无机材料学报, 2025, 40(5): 489-496. |
| [4] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [5] | 杨恒强, 张馨月, 马义初, 周青军. 铁基钙钛矿La0.25M0.75FeO3-δ (M=Ba, Sr, Ca)的制备及其作为固体氧化物燃料电池阴极材料的性能研究[J]. 无机材料学报, 2025, 40(12): 1365-1372. |
| [6] | 王哲, 郝鸿儒, 吴宗辉, 徐玲玲, 吕喆, 魏波. 构型熵工程增强双钙钛矿型氧电极抗Cr中毒能力[J]. 无机材料学报, 2025, 40(12): 1341-1348. |
| [7] | 姜玥宏, 宋云峰, 张磊磊, 马季, 宋昭远, 龙文. 质子传导型固体氧化物燃料电池BaZr0.1Ce0.7Y0.1Yb0.1O3电解质的氟化研究[J]. 无机材料学报, 2025, 40(12): 1356-1364. |
| [8] | 薛子轩, 殷超凡, 姚跃超, 王彦敏, 孙跃跃, 刘峥嵘, 周玉存, 周峻, 吴锴. 泛氢燃料质子导体固体氧化物燃料电池研究进展[J]. 无机材料学报, 2025, 40(12): 1324-1340. |
| [9] | 刘通, 黄溯, 朱诗悦, 查方林, 胡学雷, 王瑶. 一锅法合成高温氢燃料电池用高效无钴复合阴极[J]. 无机材料学报, 2025, 40(12): 1349-1355. |
| [10] | 张婧慧, 陆晓彤, 毛海雁, 田亚州, 张山林. 烧结助剂对BaZr0.1Ce0.7Y0.2O3-δ电解质烧结行为及电导率的影响[J]. 无机材料学报, 2025, 40(1): 84-90. |
| [11] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [12] | 叶梓滨, 邹高昌, 吴琪雯, 颜晓敏, 周明扬, 刘江. 阳极支撑型锥管串接式直接碳固体氧化物燃料电池组的制备及性能[J]. 无机材料学报, 2024, 39(7): 819-827. |
| [13] | 张琨, 王宇, 朱腾龙, 孙凯华, 韩敏芳, 钟秦. LaNi0.6Fe0.4O3阴极接触材料导电特性调控及其对SOFC电化学性能的影响[J]. 无机材料学报, 2024, 39(4): 367-373. |
| [14] | 陈正鹏, 金芳军, 李明飞, 董江波, 许仁辞, 徐韩昭, 熊凯, 饶睦敏, 陈创庭, 李晓伟, 凌意瀚. 双钙钛矿Sr2CoFeO5+δ阴极材料的制备及其中温固体氧化物燃料电池性能研究[J]. 无机材料学报, 2024, 39(3): 337-344. |
| [15] | 薛顶喜, 伊炳尧, 李国君, 马帅, 刘克勤. 功能梯度阳极固体氧化物燃料电池热应力数值模拟研究[J]. 无机材料学报, 2024, 39(11): 1189-1196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||