无机材料学报 ›› 2024, Vol. 39 ›› Issue (8): 911-919.DOI: 10.15541/jim20240025 CSTR: 32189.14.10.15541/jim20240025
所属专题: 【能源环境】燃料电池(202506); 【能源环境】钙钛矿(202506)
收稿日期:2024-01-11
修回日期:2024-03-08
出版日期:2024-08-20
网络出版日期:2024-03-30
通讯作者:
郇 宇, 副教授. E-mail: mse_huany@ujn.edu.cn;作者简介:潘建隆(1998-), 男, 硕士研究生. E-mail: pjl2812054@163.com
基金资助:
PAN Jianlong( ), MA Guanjun, SONG Lemei, HUAN Yu(
), MA Guanjun, SONG Lemei, HUAN Yu( ), WEI Tao(
), WEI Tao( )
)
Received:2024-01-11
Revised:2024-03-08
Published:2024-08-20
Online:2024-03-30
Contact:
HUAN Yu, associate professor. E-mail: mse_huany@ujn.edu.cn;About author:PAN Jianlong (1998-), male, Master candidate. E-mail: pjl2812054@163.com
Supported by:摘要:
受固体氧化物燃料电池(SOFCs)原位还原脱溶纳米金属阳极技术的启发, 本工作采用煅烧后的Sr2V0.1Co0.9MoO6前驱体(包含其他相的钙钛矿)在空气气氛下与电解质共烧制备出单电池, 避免了为防止阳极氧化而需在还原/惰性气氛下制备电池的苛刻条件。制备电解质片上的阳极前驱体仅需在燃料侧原位还原4 h, 便可形成纯相Sr2V0.1Co0.9MoO6 (R-SVCMO)阳极。结果表明, R-SVCMO在活化能显著降低的同时电导率由2.7 S•cm-1提高至21.6 S•cm-1。当R-SVCMO为阳极的单电池分别以H2和湿CH4为燃料气时, 在850 ℃的最大功率密度(Pmax)分别达到862和514 mW•cm-2, 显示出优秀的催化性能。还原前后阳极在100~850 ℃的平均热膨胀系数(TEC)分别为1.15×10-5和1.23×10-5 K-1, 均与传统SOFC电解质相近。因此, 还原过程不会导致阳极层体积产生变化, 可以显著提高电池结构稳定性(退化率仅为0.13%)。加之R-SVCMO是在燃料气氛下合成的, 其作为阳极表现出极高的长期稳定性和催化活性。R-SVCMO对湿CH4的催化效率达到60%, 并能够稳定运行1450 h, 相应的单电池可在0.7 V稳定运行450 h。综上所述, 本研究采用燃料原位还原法制备了具有优异电化学性能和结构稳定性的单电池。
中图分类号:
潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919.
PAN Jianlong, MA Guanjun, SONG Lemei, HUAN Yu, WEI Tao. High Stability/Catalytic Activity Co-based Perovskite as SOFC Anode: In-situ Preparation by Fuel Reducing Method[J]. Journal of Inorganic Materials, 2024, 39(8): 911-919.
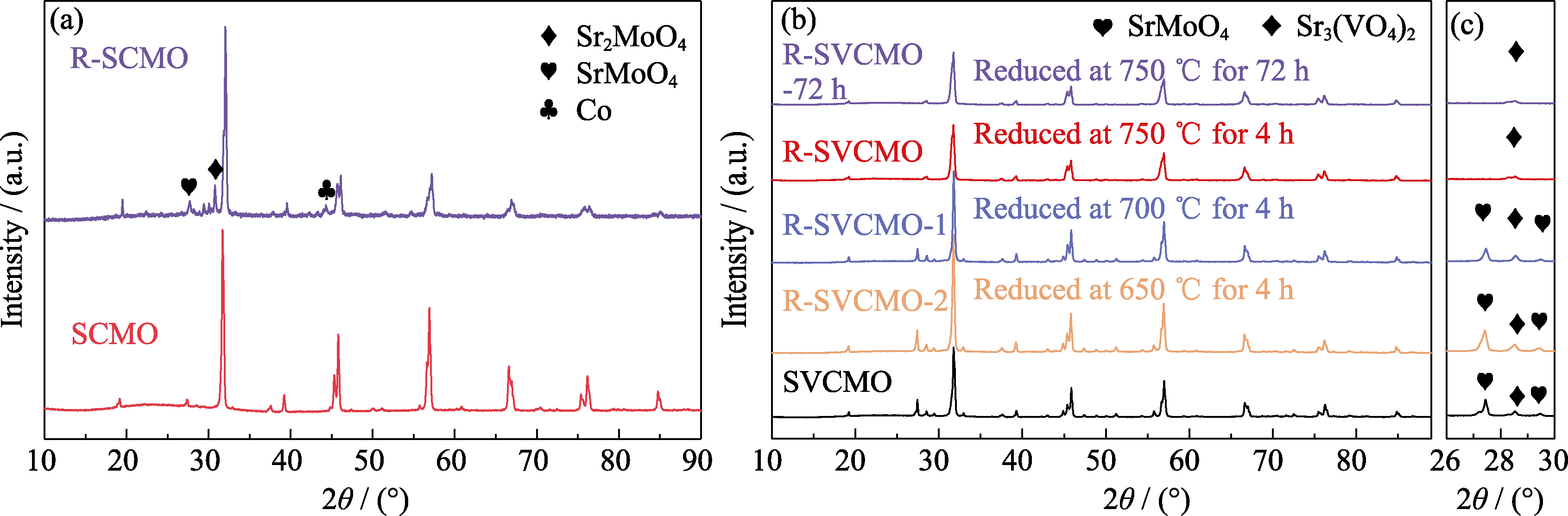
图1 SCMO和SVCMO在不同条件下煅烧前后的XRD谱图
Fig. 1 XRD patterns of SCMO and SVCMO before and after calcination under different conditions (a) SCMO powders before and after H2 reduction at 750 ℃ for 4 h; (b) SVCMO powders before and after H2 reduction at different temperatures for 4 and 72 h; (c) Enlarged patterns of 2θ=26°-30° in (b)
| Parameter | SCMO | R-SVCMO |
|---|---|---|
| Space group | I4/m | I4/m |
| a=b/Å | 5.6374 | 5.6218 |
| c/Å | 7.9128 | 7.8823 |
| α/(º) | 90 | 90 |
| β/(º) | 90 | 90 |
| γ/(º) | 90 | 90 |
| Sr-O/Å | 2.8130 | 2.8071 |
| Co-O/Å | 2.0765 | 2.0532 |
| V-O/Å | — | 1.7758 |
| Mo-O/Å | 1.8945 | 1.9191 |
表1 SCMO以及R-SVCMO粉体XRD精修后的晶格参数
Table 1 Lattice parameters of SCMO and R-SVCMO obtained by XRD Rietveld refinement
| Parameter | SCMO | R-SVCMO |
|---|---|---|
| Space group | I4/m | I4/m |
| a=b/Å | 5.6374 | 5.6218 |
| c/Å | 7.9128 | 7.8823 |
| α/(º) | 90 | 90 |
| β/(º) | 90 | 90 |
| γ/(º) | 90 | 90 |
| Sr-O/Å | 2.8130 | 2.8071 |
| Co-O/Å | 2.0765 | 2.0532 |
| V-O/Å | — | 1.7758 |
| Mo-O/Å | 1.8945 | 1.9191 |
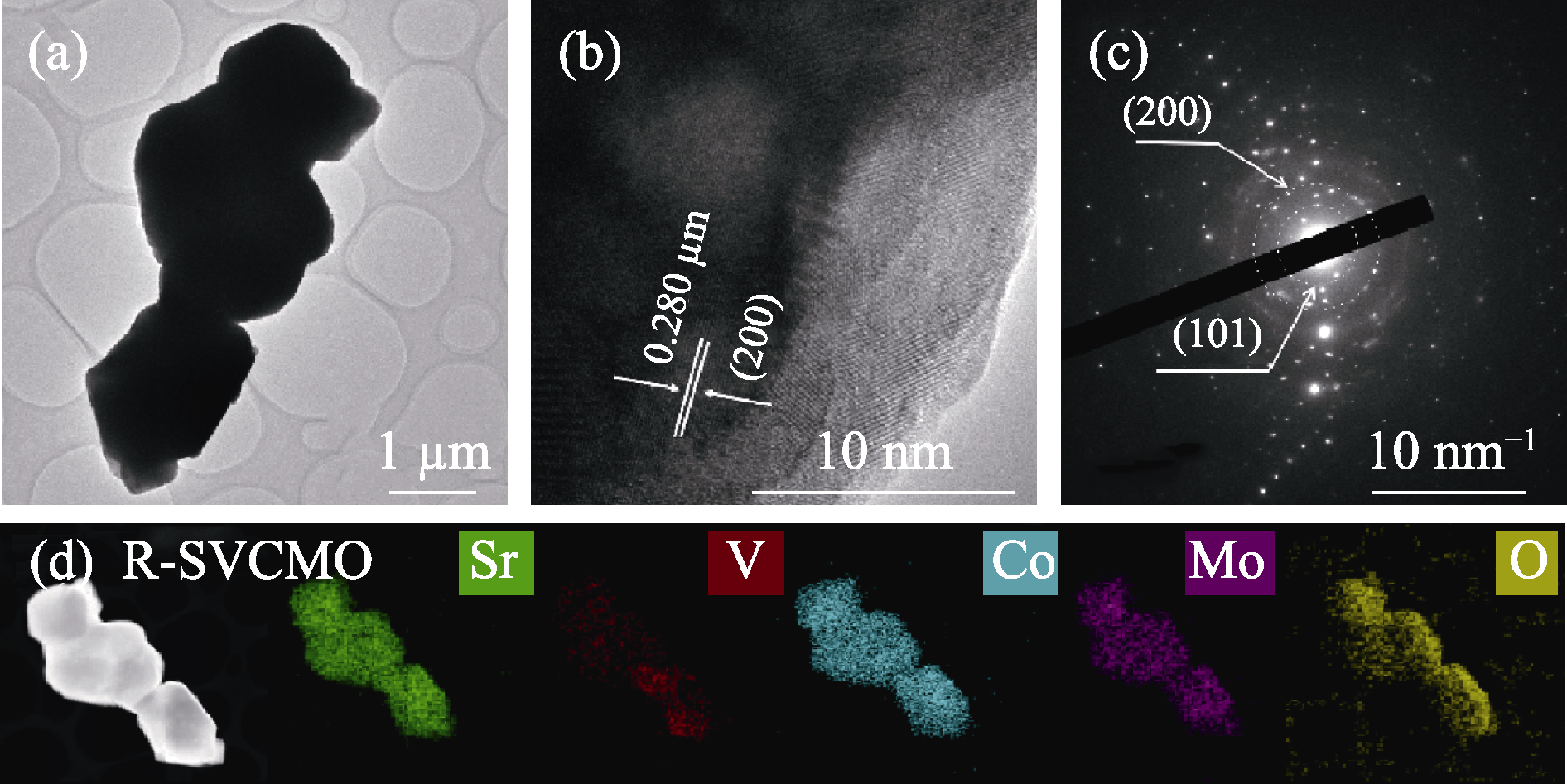
图2 R-SVCMO样品的微观结构
Fig. 2 Microstructure of R-SVCMO sample (a) TEM image; (b) HRTEM image and (c) corresponding SAED pattern; (d) STEM image and corresponding EDX elemental mappings
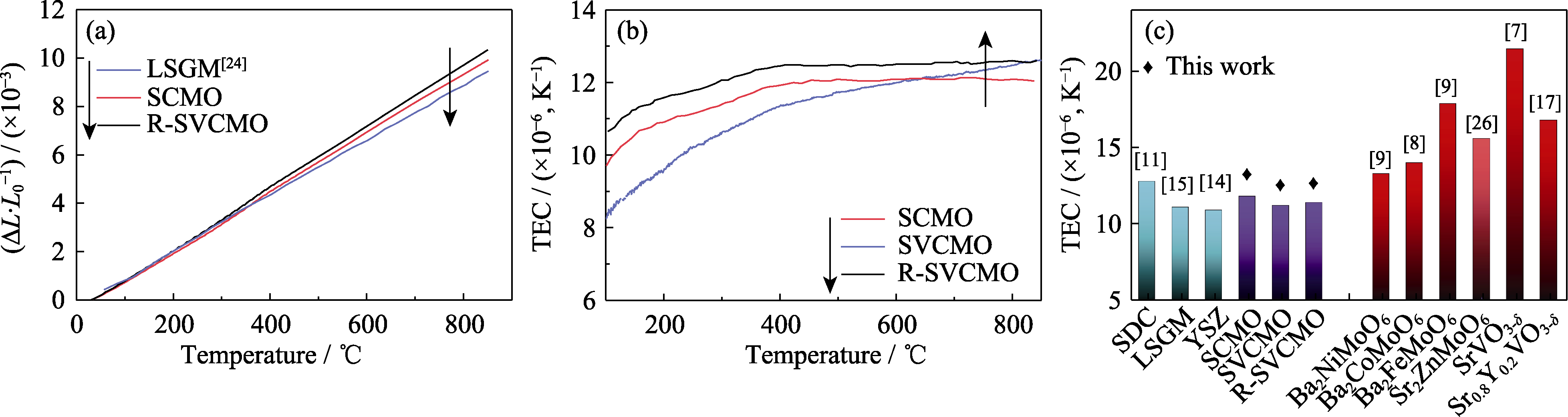
图3 不同材料的热膨胀现象
Fig. 3 Thermal expansion of different materials (a) Thermal expansion (ΔL/L0) curves of electrolyte LSGM, anodes SCMO and R-SVCMO from room temperature to 850 ℃; (b) TEC curves of SCMO, SVCMO and R-SVCMO in the range of 100−850 ℃; (c) Comparison of TEC of SCMO, SVCMO and R-SVCMO with conventional electrolytes and other perovskite anode materials[7⇓-9,11,14 -15,17,26]
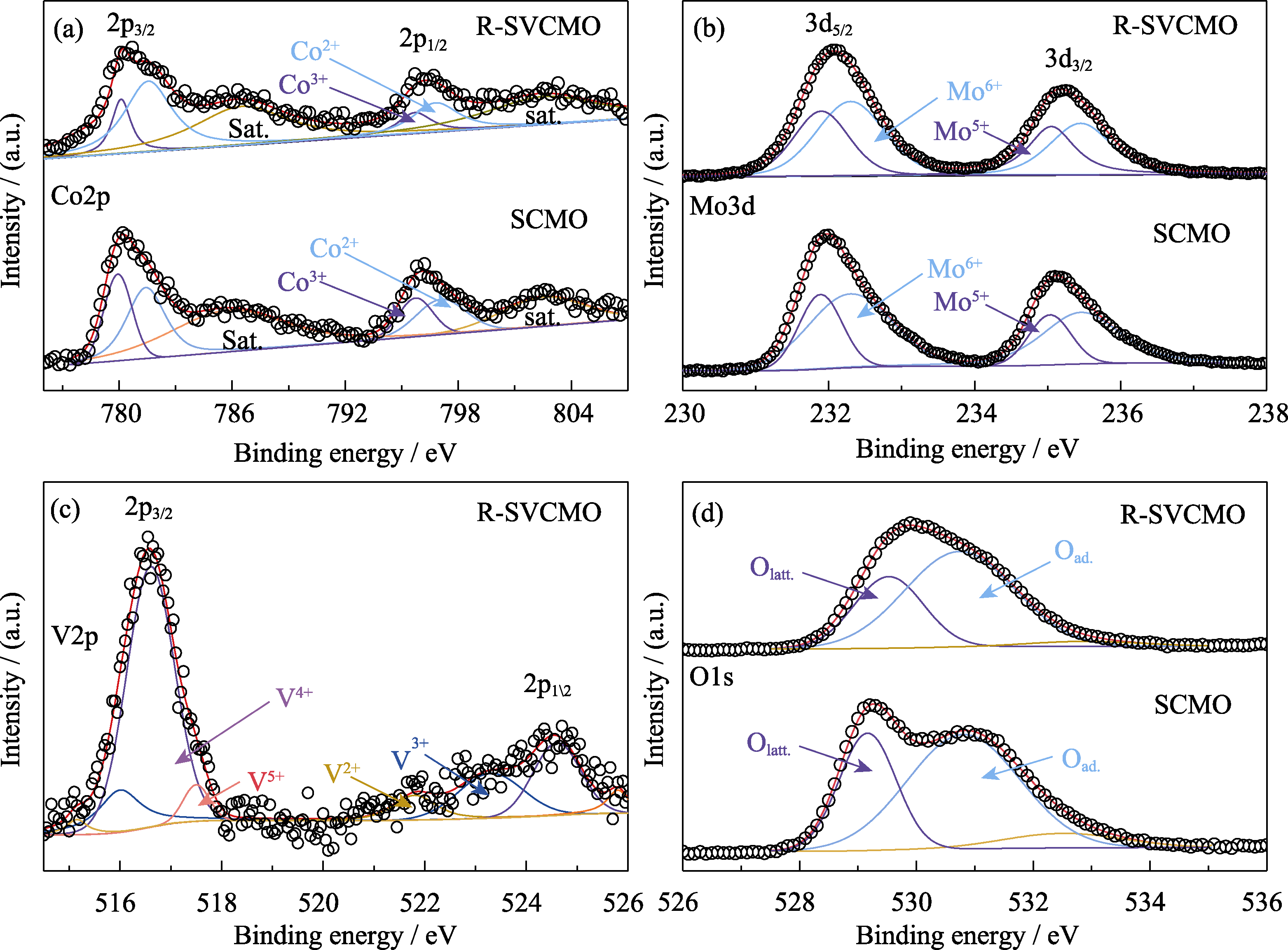
图4 SCMO和R-SVCMO材料的XPS谱图
Fig. 4 XPS spectra for SCMO and R-SVCMO (a) Co2p, (b) Mo3d, (d) O1s XPS spectra for SCMO and R-SVCMO; (c) V2p XPS spectrum for R-SVCMO
| Sample | Valence ratio/% | ||||||
|---|---|---|---|---|---|---|---|
| Mo | O | Co | |||||
| Mo5+ | Mo6+ | Olatt. | Oad. | H2O | Co2+ | Co3+ | |
| SCMO | 34.1 | 65.9 | 31.4 | 60.5 | 8.2 | 74.0 | 26.0 |
| R-SVCMO | 58.5 | 41.5 | 31.1 | 65.5 | 3.4 | 62.9 | 37.1 |
表2 XPS计算的SCMO与R-SVCMO材料中不同价态Mo和Co元素以及不同形式O的面积含量百分比
Table 2 Area content percentages of Mo and Co elements in different valences, and different O types for SCMO and R-SVCMO samples based on XPS data
| Sample | Valence ratio/% | ||||||
|---|---|---|---|---|---|---|---|
| Mo | O | Co | |||||
| Mo5+ | Mo6+ | Olatt. | Oad. | H2O | Co2+ | Co3+ | |
| SCMO | 34.1 | 65.9 | 31.4 | 60.5 | 8.2 | 74.0 | 26.0 |
| R-SVCMO | 58.5 | 41.5 | 31.1 | 65.5 | 3.4 | 62.9 | 37.1 |
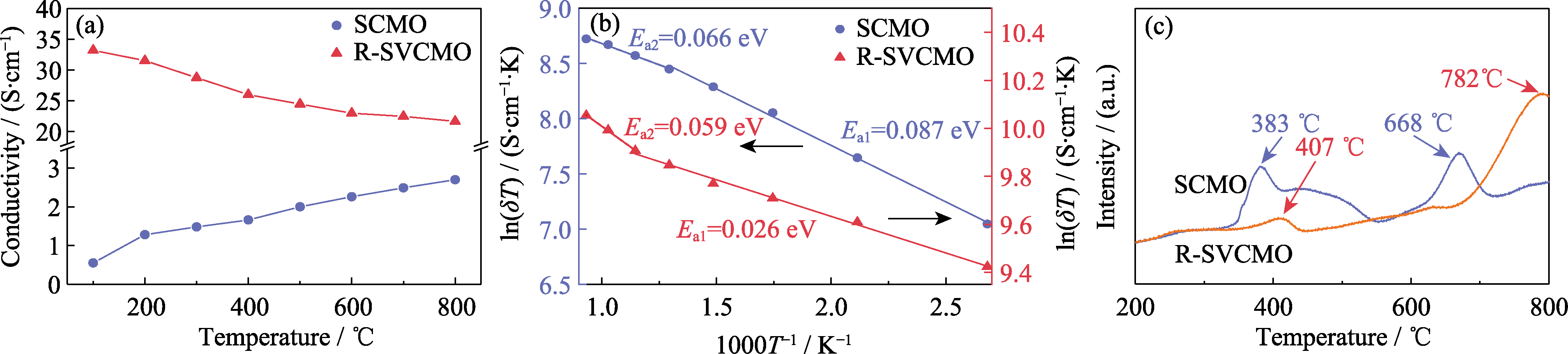
图5 SCMO和R-SVCMO在H2气氛下测试的电导率和H2-TPR曲线
Fig. 5 Conductivity and H2-TPR curves for SCMO and R-SVCMO in testing H2 (a) Temperature dependence of conductivity curves; (b) Arrhenius curves; (c) H2-TPR curves; Colorful figures are available on website
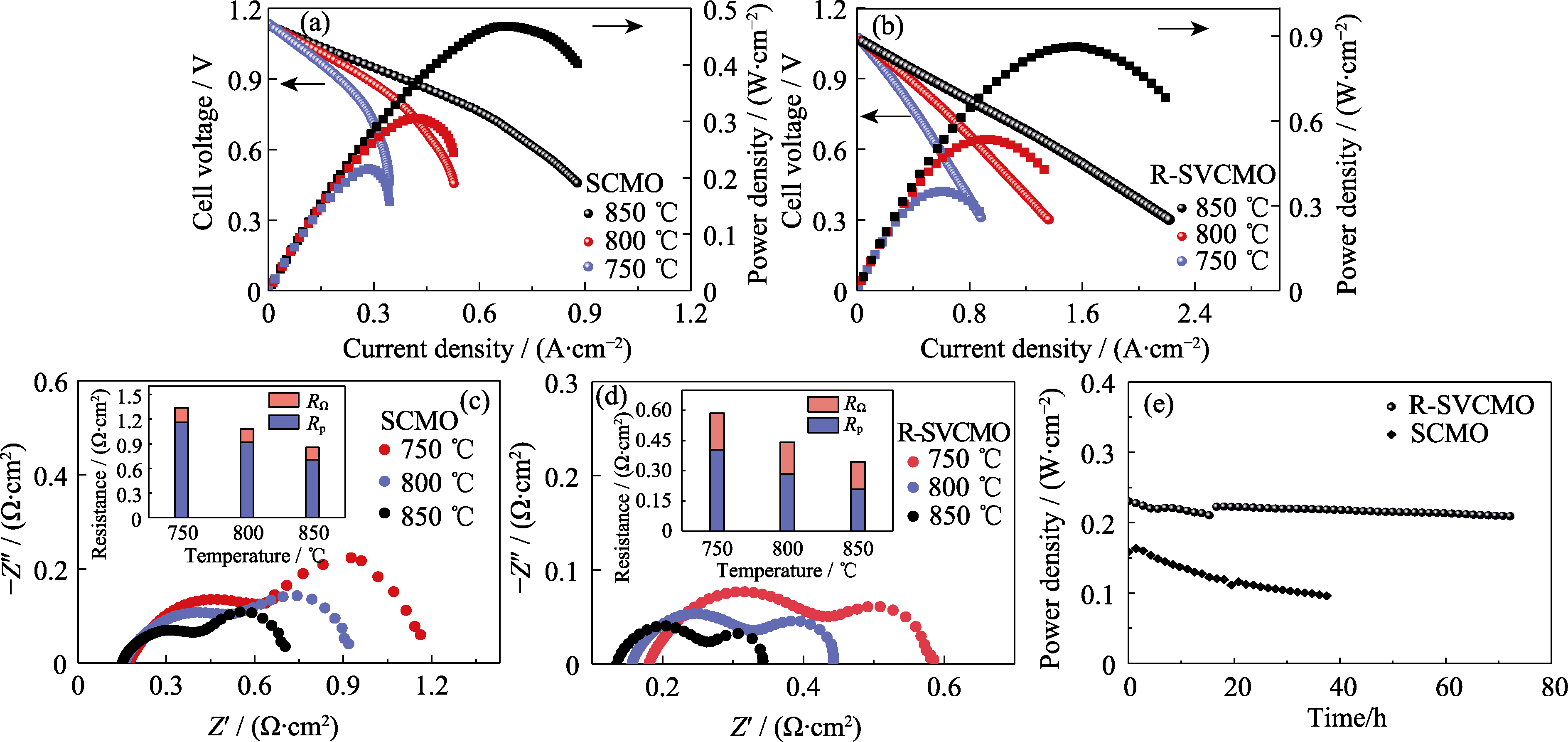
图6 以SCMO和R-SVCMO为阳极的单电池电化学性能
Fig. 6 Electrochemical performance of single cells with SCMO and R-SVCMO as anode materials (a, b) I-V-P curves for single cells with (a) SCMO and (b) R-SVCMO as anodes obtained under H2 at different temperatures; (c, d) Electrochemical Impedance spectra for (c) SCMO and (d) R-SVCMO single cells under H2 at different temperatures; (e) Durability of single cells with SCMO and R-SVCMO as anodes under 0.7 V at 750 ℃; Colorful figures are available on website
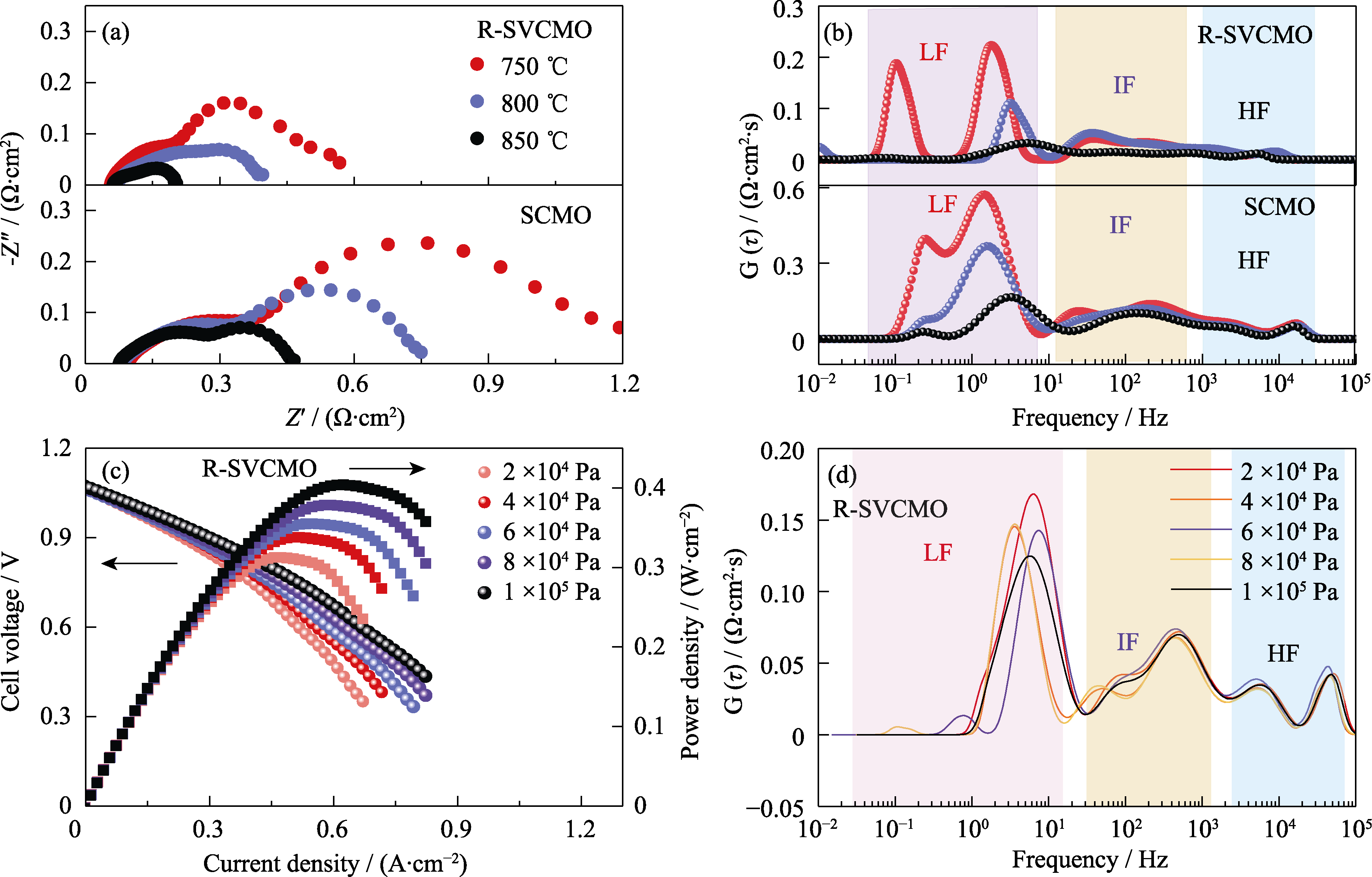
图7 以SCMO、R-SVCMO为阳极或对称电极的单电池或对称电池的电化学性能
Fig. 7 Electrochemical performance of single cells or symmetric cells with SCMO and R-SVCMO as anodes or symmetric electrodes (a) EIS and (b) DRT spectra of SCMO and R-SVCMO symmetric cells under H2 at different temperatures; (c) I-V-P curves and (d) DRT spectra of R-SVCMO single cell under different H2 partial pressures at 750 ℃; Colorful figures are available on website
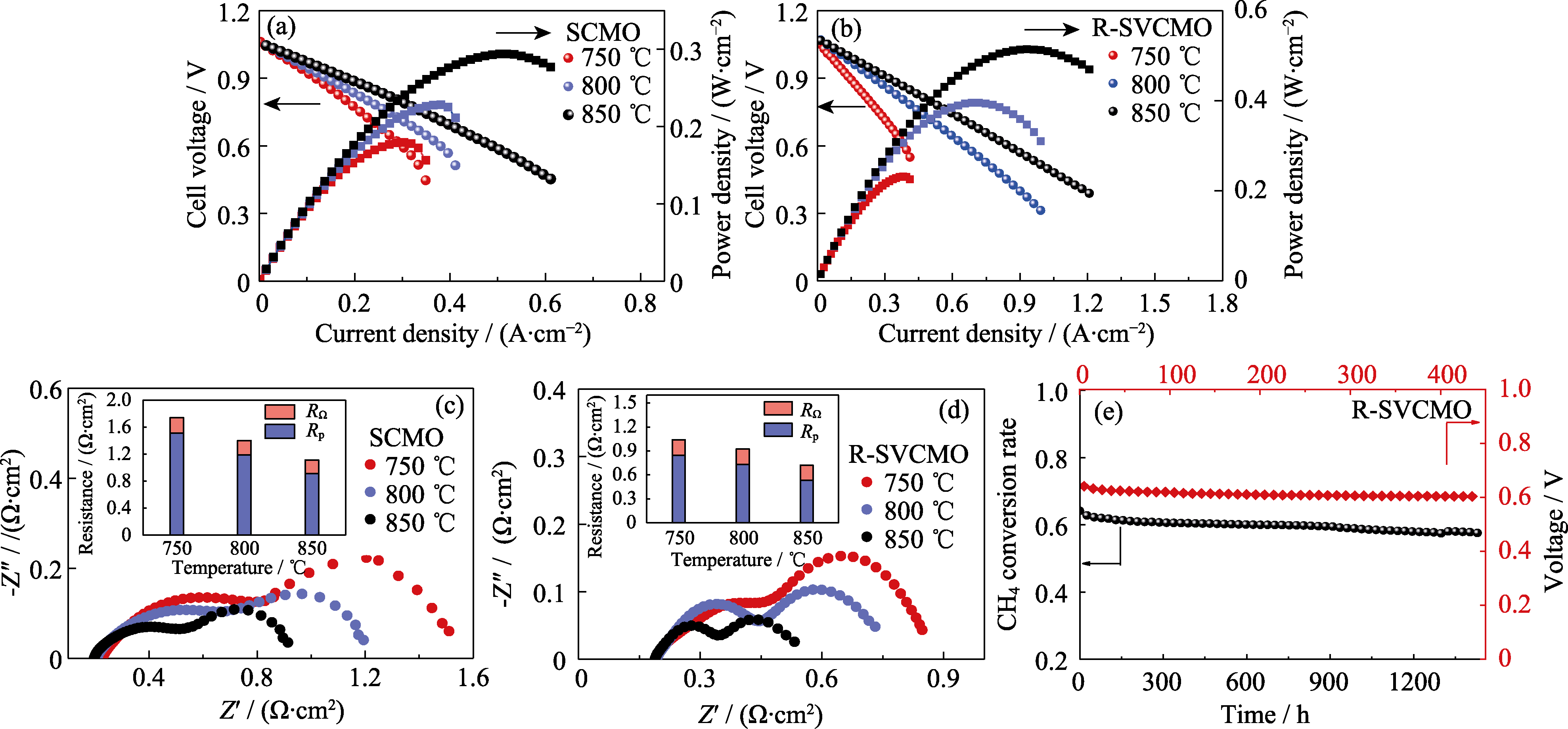
图8 以SCMO和R-SVCMO为阳极的单电池在CH4气氛下的电化学性能
Fig. 8 Electrochemical performance of single cells with SCMO and R-SVCMO as anodes in CH4 atmosphere (a, b) I-V-P curves of (a) SCMO and (b) R-SVCMO based SOFC with humidified CH4 as fuel gas at different temperatures; (c, d) EIS spectra of (c) SCMO and (d) R-SVCMO based SOFC in humidified CH4 at different temperatures; (e) CH4 conversion rate of R-SVCMO catalyst for CH4 reforming and R-SVCMO based single cell working at 0.7 V under humidified CH4 at 750 ℃ as a function of testing time; Colorful figures are available on website
| [1] | DWIVEDI S. Solid oxide fuel cell: materials for anode, cathode and electrolyte. International Journal of Hydrogen Energy, 2020, 45(44): 23988. |
| [2] | PAN K, HUSSAIN A M, HUANG Y L, et al. High performance SrFe0.2Co0.4Mo0.4O3-δ ceramic anode supported low-temperature SOFCs. Journal of the Electrochemical Society, 2021, 168(11): 114503. |
| [3] | BILAL H M, MOTOLA M, QAYYUM S, et al. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chemical Engineering Journal, 2022, 428: 132603. |
| [4] | ZHOU S M, MIAO X B, ZHAO X, et al. Engineering electrocatalytic activity in nanosized perovskite cobaltite through surface spin-state transition. Nature Communications, 2016, 7: 11510. |
| [5] | NIU B, JIN F, FU R, et al. Pd-impregnated Sr1.9VMoO6-δ double perovskite as an efficient and stable anode for solid-oxide fuel cells operating on sulfur-containing syngas. Electrochimica Acta, 2018, 274: 91. |
| [6] | ZHENG K, ŚWIERCZEK K. Physicochemical properties of rock salt-type ordered Sr2MMoO6 (M=Mg, Mn, Fe, Co, Ni) double perovskites. Journal of the European Ceramic Society, 2014, 34(16): 4273. |
| [7] | ZHANG Q, WEI T, HUANG Y H. Electrochemical performance of double-perovskite Ba2MMoO6 (M=Fe, Co, Mn, Ni) anode materials for solid oxide fuel cells. Journal of Power Sources, 2012, 198: 59. |
| [8] | SUBOTIĆ V, BALDINELLI A, BARELLI L, et al. Applicability of the SOFC technology for coupling with biomass-gasifier systems: short- and long-term experimental study on SOFC performance and degradation behaviour. Applied Energy, 2019, 256: 113904 |
| [9] | SHIRATORI Y. YSZ-MgO composite electrolyte with adjusted thermal expansion coefficient to other SOFC components. Solid State Ionics, 2003, 164(1/2): 27. |
| [10] | SUN K, ZHANG J, JIANG T, et al. Flash-sintering and characterization of La0.8Sr0.2Ga0.8Mg0.2O3-δ electrolytes for solid oxide fuel cells. Electrochimica Acta, 2016, 196: 487. |
| [11] | ZHANG J, PAYDAR S, AKBAR N, et al. Electrical properties of Ni-doped Sm2O3 electrolyte. International Journal of Hydrogen Energy, 2021, 46(15): 9758. |
| [12] | HOU N, YAO T, LI P, et al. A-site ordered double perovskite with in situ exsolved core-shell nanoparticles as anode for solid oxide fuel cells. ACS Applied Materials & Interfaces, 2019, 11(7): 6995. |
| [13] | XU K, ZHANG H, DENG W, et al. Self-hydrating of a ceria-based catalyst enables efficient operation of solid oxide fuel cells on liquid fuels. Science Bulletin, 2023, 68(21): 2574. |
| [14] | SONG L, CHEN D, PAN J, et al. B-site super-excess design Sr2V0.4Fe0.9Mo0.7O6-δ-Ni0.4 as a highly active and redox-stable solid oxide fuel cell anode. ACS Applied Materials & Interfaces, 2023, 15(41): 48296. |
| [15] | YAREMCHENKO A A, BRINKMANN B, JANSSEN R, et al. Electrical conductivity, thermal expansion and stability of Y- and Al-substituted SrVO3 as prospective SOFC anode material. Solid State Ionics, 2013, 247: 86. |
| [16] | WANG F Y, ZHONG G B, LUO S, et al. Porous Sr2MgMo1-xVxO6-δ ceramics as anode materials for SOFCs using biogas fuel. Catalysis Communications, 2015, 67: 108. |
| [17] | DOS SANTOS-GÓMEZ L, LEÓN-REINA L, PORRAS-VÁZQUEZ J M, et al. Chemical stability and compatibility of double perovskite anode materials for SOFCs. Solid State Ionics, 2013, 239: 1. |
| [18] | MA G J, CHEN D Z, JI S J, et al. Medium-entropy SrV1/3Fe1/3Mo1/3O3 with high conductivity and strong stability as SOFCs- high-performance anode. Materials, 2022, 15(6): 2298. |
| [19] | DEWA M, YU W, DALE N, et al. Recent progress in integration of reforming catalyst on metal-supported SOFC for hydrocarbon and logistic fuels. International Journal of Hydrogen Energy, 2021, 46(67): 33523. |
| [20] | FARES A, BARAMA A, BARAMA S, et al. Synthesis and characterization of Ba0.5Sr0.5NixCo0.8-xFe0.2O3-δ (x=0 and 0.2) perovskites as electro-catalysts for methanol oxidation in alkaline media. Electroanalysis, 2017, 29(10): 2323. |
| [21] | XIA W W, LI Q, SUN L P, et al. Electrochemical performance of Sn-doped Bi0.5Sr0.5FeO3-δ perovskite as cathode electrocatalyst for solid oxide fuel cells. Journal of Alloys and Compounds, 2020, 835: 155406. |
| [22] | LI K, LI X, LI J, et al. Structural stability of Ni-Fe supported solid oxide fuel cells based on stress analysis. Journal of Inorganic Materials, 2019, 34(6): 611. |
| [23] | MORI M, SAMMES N M J S S I. Sintering and thermal expansion characterization of Al-doped and Co-doped lanthanum strontium chromites synthesized by the Pechini method. Solid State Ionics, 2002, 146(3/4): 301. |
| [24] | LI Y, YIN B, FAN Y, et al. Achieving high mechanical-strength CH4-based SOFCs by low-temperature sintering (1100 ℃). International Journal of Hydrogen Energy, 2020, 45(4): 3086. |
| [25] | FLORES-LASLUISA J X, HUERTA F, CAZORLA-AMORÓS D, et al. Structural and morphological alterations induced by cobalt substitution in LaMnO perovskites. Journal of Colloid and Interface Science, 2019, 556: 658. |
| [26] | QIN M X, XIAO Y, YANG H Y, et al. Ru/Nb co-doped perovskite anode: achieving good coking resistance in hydrocarbon fuels via core-shell nanocatalysts exsolution. Applied Catalysis B-Environmental, 2021, 299: 120613. |
| [27] | LING Y H, LI X W, CHUANG T C, et al. Double perovskite Sr2CoFeO5+δ: preparation and its performance as cathode material for intermediate-temperature solid oxide fuel cells. Journal of Inorganic Materials, 2023, 39(3): 337. |
| [28] | XU C M, SUN W, REN R Z, et al. A highly active and carbon-tolerant anode decorated with grown cobalt nano-catalyst for intermediate-temperature solid oxide fuel cells. Applied Catalysis B-Environmental, 2021, 282: 119553. |
| [29] | ZHAO H L, XU N S, CHENG Y F, et al. Investigation of mixed conductor BaCo0.7Fe0.3-xYxO3-δ with high oxygen permeability. Journal of Physical Chemistry C, 2010, 114: 17975. |
| [30] | HUAN Y, LI Y, YIN B, et al. High conductive and long-term phase stable anode materials for SOFCs: A2FeMoO6 (A = Ca, Sr, Ba). Journal of Power Sources, 2017, 359: 384. |
| [31] | SEREDA V V, TSVETKOV D S, SEDNEV A L, et al. Thermodynamics of Sr2NiMoO6 and Sr2CoMoO6 and their stability under reducing conditions. Physical Chemistry Chemical Physics, 2018, 20(30): 20108. |
| [32] | ALVAREZ M, LÓPEZ T, ODRIOZOLA J A, et al. 2, 4-Dichlorophenoxyacetic acid (2,4-D) photodegradation using an Mn+/ZrO2 photocatalyst: XPS, UV-Vis, XRD characterization. Applied Catalysis B: Environmental, 2007, 73(1/2): 34. |
| [33] | LUO L H, HU J X, CHENG L, XU X, et al. Performance of the composite cathode Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2-δ for medium- low temperature solid oxide fuel cell. Journal of Inorganic Materials, 2018, 33(4): 441. |
| [34] | XIA J, WANG C, WANG X F, et al. A perspective on DRT applications for the analysis of solid oxide cell electrodes. Electrochimica Acta, 2020, 349: 136328. |
| [35] | SHI N, SU F, HUAN D, et al. Performance and DRT analysis of P-SOFCs fabricated using new phase inversion combined tape casting technology. Journal of Materials Chemistry A, 2017, 5(37): 19664. |
| [1] | 柴润宇, 张镇, 王孟龙, 夏长荣. 直接组装法制备氧化铈基金属支撑固体氧化物燃料电池[J]. 无机材料学报, 2025, 40(7): 765-771. |
| [2] | 渠吉发, 王旭, 张维轩, 张康喆, 熊永恒, 谭文轶. 掺杂改性NaYTiO4增强固体氧化物燃料电池阳极抗硫中毒性能[J]. 无机材料学报, 2025, 40(5): 489-496. |
| [3] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [4] | 张婧慧, 陆晓彤, 毛海雁, 田亚州, 张山林. 烧结助剂对BaZr0.1Ce0.7Y0.2O3-δ电解质烧结行为及电导率的影响[J]. 无机材料学报, 2025, 40(1): 84-90. |
| [5] | 叶梓滨, 邹高昌, 吴琪雯, 颜晓敏, 周明扬, 刘江. 阳极支撑型锥管串接式直接碳固体氧化物燃料电池组的制备及性能[J]. 无机材料学报, 2024, 39(7): 819-827. |
| [6] | 张琨, 王宇, 朱腾龙, 孙凯华, 韩敏芳, 钟秦. LaNi0.6Fe0.4O3阴极接触材料导电特性调控及其对SOFC电化学性能的影响[J]. 无机材料学报, 2024, 39(4): 367-373. |
| [7] | 陈正鹏, 金芳军, 李明飞, 董江波, 许仁辞, 徐韩昭, 熊凯, 饶睦敏, 陈创庭, 李晓伟, 凌意瀚. 双钙钛矿Sr2CoFeO5+δ阴极材料的制备及其中温固体氧化物燃料电池性能研究[J]. 无机材料学报, 2024, 39(3): 337-344. |
| [8] | 薛顶喜, 伊炳尧, 李国君, 马帅, 刘克勤. 功能梯度阳极固体氧化物燃料电池热应力数值模拟研究[J]. 无机材料学报, 2024, 39(11): 1189-1196. |
| [9] | 郭天民, 董江波, 陈正鹏, 饶睦敏, 李明飞, 李田, 凌意瀚. 中温固体氧化物燃料电池的高熵双钙钛矿阴极材料: 兼容性与活性研究[J]. 无机材料学报, 2023, 38(6): 693-700. |
| [10] | 樊帅, 金天, 张山林, 雒晓涛, 李成新, 李长久. Li2O烧结助剂对固体氧化物燃料电池LSGM电解质烧结特性及离子电导率的影响[J]. 无机材料学报, 2022, 37(10): 1087-1092. |
| [11] | 曹丹,周明扬,刘志军,颜晓敏,刘江. 阳极支撑质子导体电解质固体氧化物燃料电池的制备及其性能研究[J]. 无机材料学报, 2020, 35(9): 1047-1052. |
| [12] | 夏天, 孟燮, 骆婷, 占忠亮. 固体氧化物燃料电池LaxSr2-3x/2Fe1.5Ni0.1Mo0.4O6-δ阳极性能研究[J]. 无机材料学报, 2020, 35(5): 617-622. |
| [13] | 李凯, 李霄, 李箭, 谢佳苗. 基于应力分析Ni-Fe合金支撑固体氧化物燃料电池结构稳定性研究[J]. 无机材料学报, 2019, 34(6): 611-617. |
| [14] | 汪维, 苑莉莉, 丘倩媛, 周明扬, 刘美林, 刘江. 流延法制备单片式直接碳固体氧化物燃料电池组及其性能研究[J]. 无机材料学报, 2019, 34(5): 509-514. |
| [15] | 夏天, 孟燮, 骆婷, 占忠亮. Ca掺杂Sr2Fe1.5Mo0.5O6-δ材料的合成与作为对称固体氧化物燃料电池电极催化剂的性能研究[J]. 无机材料学报, 2019, 34(10): 1109-1114. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||