无机材料学报 ›› 2025, Vol. 40 ›› Issue (12): 1365-1372.DOI: 10.15541/jim20240500 CSTR: 32189.14.jim20240500
• 专栏:高温燃料电池关键材料(客座编辑:凌意瀚) • 上一篇 下一篇
收稿日期:2024-12-02
修回日期:2025-01-27
出版日期:2025-12-20
网络出版日期:2025-02-25
通讯作者:
周青军, 教授. E-mail: qjzhou@usst.edu.cn作者简介:杨恒强(1998-), 男, 硕士研究生. E-mail: 719645404@qq.com
基金资助:
YANG Hengqiang( ), ZHANG Xinyue, MA Yichu, ZHOU Qingjun(
), ZHANG Xinyue, MA Yichu, ZHOU Qingjun( )
)
Received:2024-12-02
Revised:2025-01-27
Published:2025-12-20
Online:2025-02-25
Contact:
ZHOU Qingjun, professor. E-mail: qjzhou@usst.edu.cnAbout author:YANG Hengqiang (1998-), male, Master candidate. E-mail: 719645404@qq.com
Supported by:摘要:
固体氧化物燃料电池(SOFC)的性能受阴极氧还原反应(ORR)的制约, 其对整个电池性能至关重要。本研究通过容限因子和结构特征设计合成了La0.25M0.75FeO3-δ (M=Ba, Sr, Ca, 标记为LBF、LSF、LCF)钙钛矿阴极, 研究了Ba、Sr、Ca元素取代的特性及其对阴极电化学性能的影响。不同碱土元素对晶体结构有显著影响, LBF为Pm-3m立方相, LSF为R-3c菱方相, 而LCF为P21ma正交和Pcmn菱方的复合相。晶体结构的差异也导致了材料不同的热膨胀系数(TEC)和电导率, LCF具有最小的TEC(1.38×10-5 K-1), LSF具有最高的电导率, 其电导率在550 ℃达到404.4 S·cm-1。三种Fe基阴极在空气和CO2气氛下都呈现出优异的稳定性, 以及与电解质的化学兼容性。此外, 不同的碱土元素也影响着材料的催化活性, LSF和LBF具有低的面比电阻(ASR), 在800 ℃时ASR仅为0.022和0.027 Ω·cm2, 优于LCF的0.351 Ω·cm2。较高的氧还原活性归因于其晶体结构, 以及氧的吸附和解离能力。鉴于Ba2+、Sr2+、Ca2+在阴极性能上各有优缺点, 未来可以在A位有效引入中高熵设计, 充分发挥各自的优势以获得综合性能优异的SOFC阴极。
中图分类号:
杨恒强, 张馨月, 马义初, 周青军. 铁基钙钛矿La0.25M0.75FeO3-δ (M=Ba, Sr, Ca)的制备及其作为固体氧化物燃料电池阴极材料的性能研究[J]. 无机材料学报, 2025, 40(12): 1365-1372.
YANG Hengqiang, ZHANG Xinyue, MA Yichu, ZHOU Qingjun. Iron-based Perovskite Material La0.25M0.75FeO3-δ (M=Ca, Sr, Ba): Preparation and Performance as Cathode for Solid Oxide Fuel Cells[J]. Journal of Inorganic Materials, 2025, 40(12): 1365-1372.
| Sample | Space group | a/Å | b/Å | c/Å | Volume/Å3 | Rwp/% | GOF |
|---|---|---|---|---|---|---|---|
| LBF | Pm-3m | 3.964 | 3.964 | 3.964 | 62.264 | 8.59 | 1.28 |
| LSF | R-3c | 5.480 | 5.480 | 13.428 | 349.249 | 8.12 | 1.62 |
| LCF-1 | P21ma | 5.450 | 11.265 | 5.560 | 341.347 | 14.33 | 2.12 |
| LCF-2 | Pcmn | 5.425 | 14.775 | 5.593 | 448.293 | 14.33 | 2.12 |
表1 LBF、LSF和LCF粉末的晶胞参数
Table 1 Lattice parameters of LBF, LSF and LCF powders
| Sample | Space group | a/Å | b/Å | c/Å | Volume/Å3 | Rwp/% | GOF |
|---|---|---|---|---|---|---|---|
| LBF | Pm-3m | 3.964 | 3.964 | 3.964 | 62.264 | 8.59 | 1.28 |
| LSF | R-3c | 5.480 | 5.480 | 13.428 | 349.249 | 8.12 | 1.62 |
| LCF-1 | P21ma | 5.450 | 11.265 | 5.560 | 341.347 | 14.33 | 2.12 |
| LCF-2 | Pcmn | 5.425 | 14.775 | 5.593 | 448.293 | 14.33 | 2.12 |
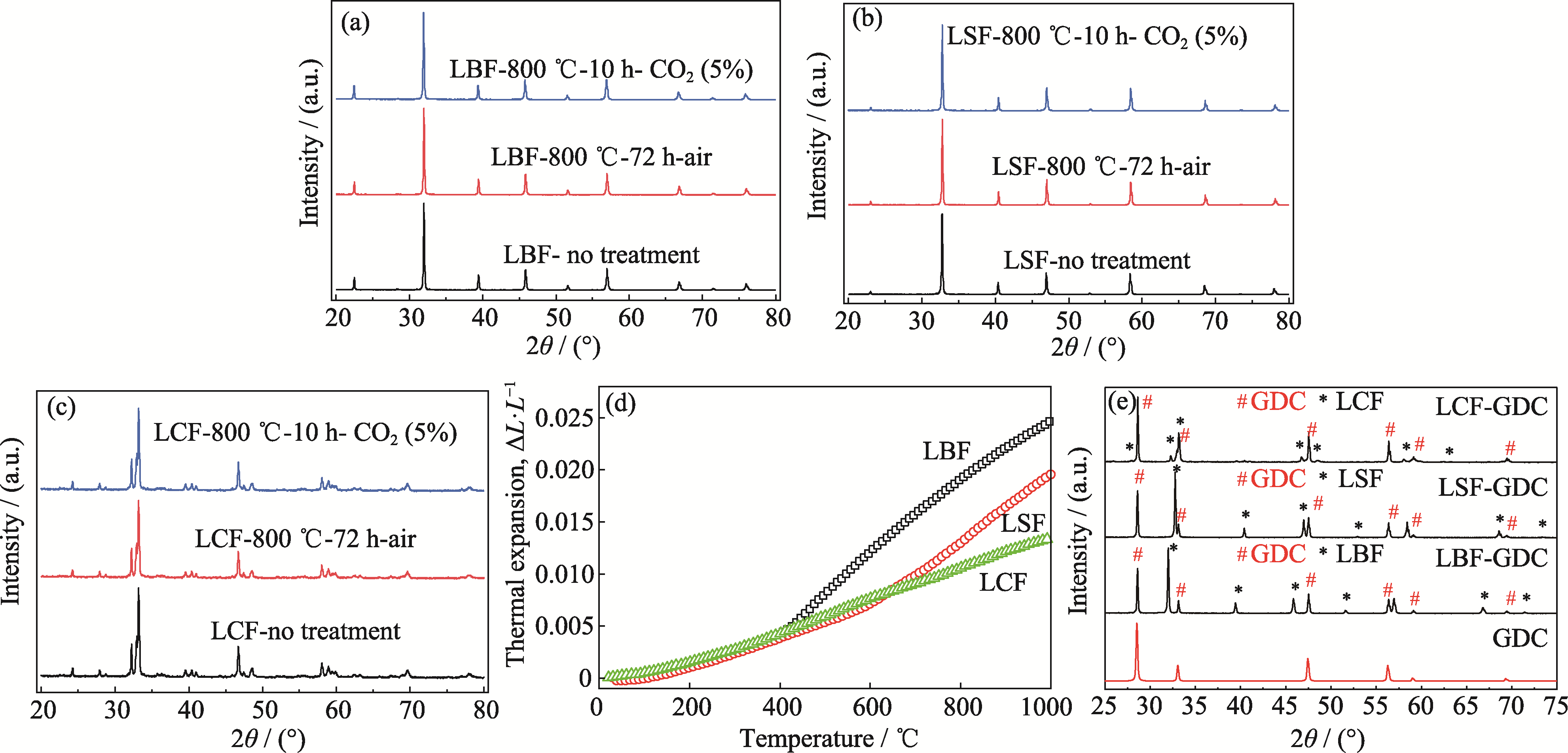
图2 LBF、LSF、LCF样品的稳定性及其与GDC的兼容性
Fig. 2 Stability of LBF, LSF and LCF samples and their compatibility with GDC (a-c) XRD patterns of (a) LBF, (b) LSF and (c) LCF calcined in 5% CO2-air at 800 ℃ for 10 h, and calcined in air at 800 ℃ for 72 h; (d) Thermal expansion curves of LBF, LSF and LCF; (e) XRD patterns of LBF-GDC, LSF-GDC and LCF-GDC calcined at 1100 ℃ for 5 h
| Sample | Oad/eV | Olat/eV | Fe3+/eV | Fe4+/eV |
|---|---|---|---|---|
| LBF | 531.77(70.40%) | 529.13(29.60%) | 710.25; 723.25(55.85%) | 712.69; 725.57(44.15%) |
| LSF | 531.87(71.67%) | 529.03(28.33%) | 710.29; 723.59(62.71%) | 712.12; 724.95(37.29%) |
| LCF | 531.50(66.93%) | 529.15(33.07%) | 710.18; 723.17(45.36%) | 712.15; 725.40(54.64%) |
表2 LBF、LSF和LCF样品中O1s和Fe2p的结合能及离子面积含量百分比
Table 2 Binding energies of O1s and Fe2p and the ionic area content percentages in LBF, LSF and LCF samples
| Sample | Oad/eV | Olat/eV | Fe3+/eV | Fe4+/eV |
|---|---|---|---|---|
| LBF | 531.77(70.40%) | 529.13(29.60%) | 710.25; 723.25(55.85%) | 712.69; 725.57(44.15%) |
| LSF | 531.87(71.67%) | 529.03(28.33%) | 710.29; 723.59(62.71%) | 712.12; 724.95(37.29%) |
| LCF | 531.50(66.93%) | 529.15(33.07%) | 710.18; 723.17(45.36%) | 712.15; 725.40(54.64%) |
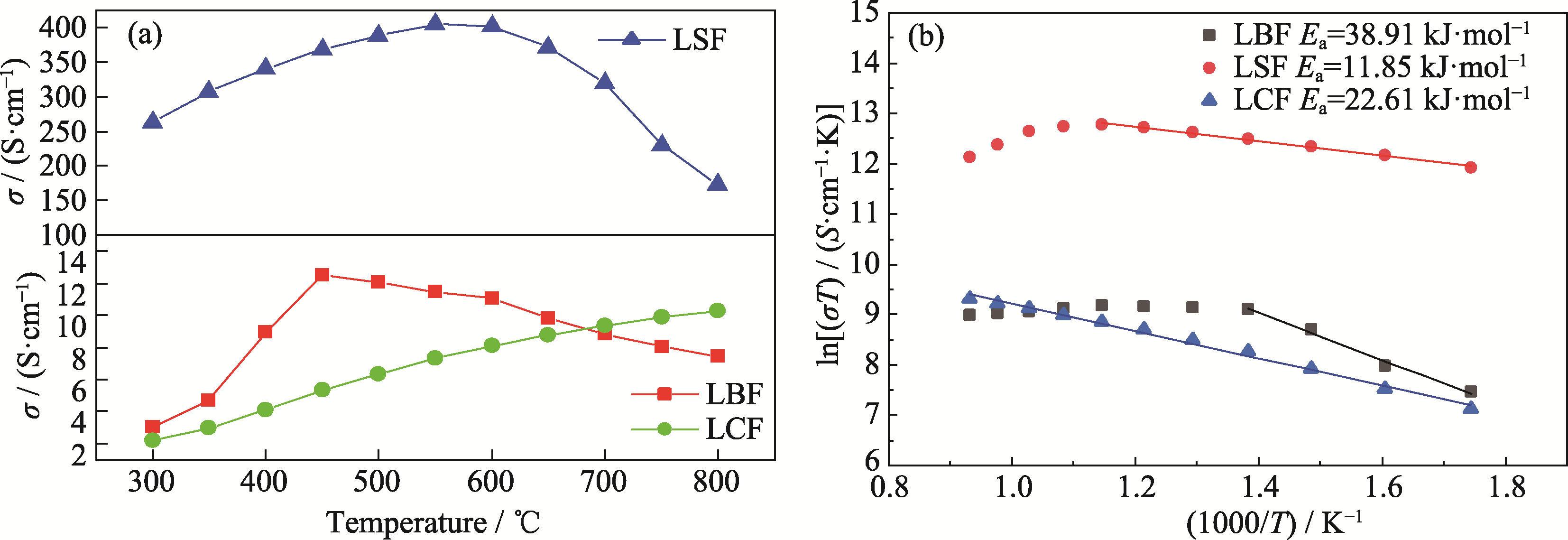
图4 LBF、LSF、LCF的电导率和Arrhenius曲线
Fig. 4 Conductivities and Arrhenius curves of LBF, LSF and LCF (a) Temperature dependence of electrical conductivity; (b) Arrhenius curves

图5 LBF、LSF、LCF的对称电池阻抗
Fig. 5 Impedance of symmetric cells for LBF, LSF and LCF (a-c) EIS results of LBF, LSF and LCF cathodes at different temperatures; (d) Equivalent circuit; (e) Corresponding Arrhenius plots of the ASR; (f, g) DRT curves of EIS at 600 and 700 ℃

图6 LSF为阴极的单电池性能
Fig. 6 Performance of the single cell using LSF cathode (a) I-V and I-P curves at 600-800 ℃; (b) Cross-sectional SEM image of the LSF and GDC after performance test
| [1] |
NDUBUISI A, ABOUALI S, SINGH K, et al. Recent advances, practical challenges, and perspectives of intermediate temperature solid oxide fuel cell cathodes. Journal of Materials Chemistry A, 2022, 10(5): 2196.
DOI URL |
| [2] |
CHEN Z, JIN F, LI M, et al. Double perovskite Sr2CoFeO5+δ: preparation and performance as cathode material for intermediate-temperature solid oxide fuel cells. Journal of Inorganic Materials, 2024, 39(3): 337.
DOI URL |
| [3] |
SAMREEN A, ALI M S, HUZAIFA M, et al. Advancements in perovskite-based cathode materials for solid oxide fuel cells: a comprehensive review. The Chemical Record, 2023, 24(1): e202300247.
DOI PMID |
| [4] | SONG Y, CHEN Y, XU M, et al. A cobalt-free multi-phase nanocomposite as near-ideal cathode of intermediate-temperature solid oxide fuel cells developed by smart self-assembly. Advanced Materials, 2020, 32(8): 1906979. |
| [5] |
WANG J, ZHANG D, LIU T, et al. Self-assembled FeRu bimetallic nanocatalysts for efficient and durable mutual CO-CO2 conversion in a reversible solid oxide electrochemical cell. Science China Materials, 2024, 67(5): 1471.
DOI |
| [6] |
PAN J, MA G, SONG L, et al. High stability/catalytic activity Co-based perovskite as SOFC anode: in-situ preparation by fuel reducing method. Journal of Inorganic Materials, 2024, 39(8): 911.
DOI URL |
| [7] | ZHANG B, ZHANG S, HAN H, et al. Cobalt-free double perovskite oxide as a promising cathode for solid oxide fuel cells. ACS Applied Materials & Interfaces, 2023, 15(6): 8253. |
| [8] | CRUMLIN E J, AHN S J, LEE D, et al. Oxygen electrocatalysis on epitaxial La0.6Sr0.4CoO3-δperovskite thin films for solid oxide fuel cells. Journal of the Electrochemical Society, 2012, 159(7): F219. |
| [9] |
ZHOU W, RAN R, SHAO Z. Progress in understanding and development of Ba0.5Sr0.5Co0.8Fe0.2O3-δ-based cathodes for intermediate-temperature solid-oxide fuel cells: a review. Journal of Power Sources, 2009, 192(2): 231.
DOI URL |
| [10] |
QI S, CHEN Y, LIN Z, et al. PrSr3Fe3O10-δ as cobalt-free cathode for intermediate-temperature solid oxide fuel cell. Materials Letters, 2020, 279: 128489.
DOI URL |
| [11] |
ZHOU Q, CHENG Y, LI W, et al. Investigation of cobalt-free perovskite Sr2FeTi0.75Mo0.25O6-δ as new cathode for solid oxide fuel cells. Materials Research Bulletin, 2016, 74: 129.
DOI URL |
| [12] |
ZHOU Q, CHEN L, CHENG Y, et al. Cobalt-free quintuple perovskite Sm1.875Ba3.125Fe5O15-δ as a novel cathode for intermediate temperature solid oxide fuel cells. Ceramics International, 2016, 42(8): 10469.
DOI URL |
| [13] |
WANG C, MIAO H, ZHANG X, et al. On Fe-based perovskite electrodes for symmetrical reversible solid oxide cells - a review. Journal of Power Sources, 2024, 596: 234112.
DOI URL |
| [14] |
ZHOU Q, XU L, GUO Y, et al. La0.6Sr0.4Fe0.8Cu0.2O3-δ perovskite oxide as cathode for IT-SOFC. International Journal of Hydrogen Energy, 2012, 37(16): 11963.
DOI URL |
| [15] |
HOU S E, AGUADERO A, ALONSO J A, et al. Fe-based perovskites as electrodes for intermediate-temperature solid oxide fuel cells. Journal of Power Sources, 2011, 196(13): 5478.
DOI URL |
| [16] |
PETRIC A, HUANG P, TIETZ F. Evaluation of La-Sr-Co-Fe-O perovskites for solid oxide fuel cells and gas separation membranes. Solid State Ionics, 2000, 135: 719.
DOI URL |
| [17] |
GOLDSCHMIDT V M. Die gesetze der krystallochemie. Die Naturwissenschaften, 1926, 14(21): 477.
DOI URL |
| [18] |
WANG Y C, WANG Y T, QI H Y, et al. Tuning the ORR catalytic activity of LaFeO3-δ-based perovskite cathode for solid oxide fuel cells by doping with alkaline-earth metal elements. Ceramics International, 2024, 50(3): 5818.
DOI URL |
| [19] |
ECIJA A, VIDAL K, LARRAÑAGA A, et al. Characterization of Ln0.5M0.5FeO3-δ (Ln=La, Nd, Sm; M=Ba, Sr) perovskites as SOFC cathodes. Solid State Ionics, 2011, 201(1): 35.
DOI URL |
| [20] |
HUNG M H, RAO M V M, TSAI D S. Microstructures and electrical properties of calcium substituted LaFeO3 as SOFC cathode. Materials Chemistry and Physics, 2007, 101(2/3): 297.
DOI URL |
| [21] |
ZHOU Q, ZHANG X, WANG Y, et al. A thermal-expansion offset to cobalt-based cathode materials for solid oxide fuel cells. Next Energy, 2024, 5: 100168.
DOI URL |
| [22] |
HROVAT M, HOLC J, KOLAR D. Thick film ruthenium oxide/yttria-stabilized zirconia-based cathode material for solid oxide fuel cells. Solid State Ionics, 1994, 68: 99.
DOI URL |
| [23] |
WANG J Q, ZHOU D F, GAO J Q, et al. Effect of A/B-site non- stoichiometry on the structure and properties of La0.9Sr0.1Ga0.9Mg0.1O3-δ solid electrolyte in intermediate‐ temperature solid oxide fuel cells. ChemElectroChem, 2018, 5(4): 665.
DOI URL |
| [24] |
BAI J, ZHOU D, ZHU X, et al. In-situ segregation of A-site defect (La0.6Sr0.4)0.90Co0.2Fe0.8O3-δ to form a high-performance solid oxide fuel cell cathode material with heterostructure. Ceramics International, 2023, 49(4): 5687.
DOI URL |
| [25] |
PEI Y, WANG H, GONG J, et al. Co and Hf co-doped BaFeO3 cathode with obviously enhanced catalytic activity and CO2tolerance for solid oxide fuel cell. International Journal of Hydrogen Energy, 2022, 47(89): 37945.
DOI URL |
| [26] |
DONG F, CHEN D, CHEN Y, et al. La-doped BaFeO3-δ perovskite as a cobalt-free oxygen reduction electrode for solid oxide fuel cells with oxygen-ion conducting electrolyte. Journal of Materials Chemistry, 2012, 22: 15071.
DOI URL |
| [27] |
WEI B, LU Z, HUANG X, et al. Synthesis, electrical and electrochemical properties of Ba0.5Sr0.5Zn0.2Fe0.8O3-δ perovskite oxide for IT-SOFC cathode. Journal of Power Sources, 2008, 176: 1.
DOI URL |
| [28] |
UNGER L S, NIEDRIG C, WAGNER S F, et al. Yttrium doping of Ba0.5Sr0.5Co0.8Fe0.2O3-δ part I: influence on oxygen permeation, electrical properties, reductive stability, and lattice parameters. Journal of the European Ceramic Society, 2018, 38(5): 2378.
DOI URL |
| [29] |
ZHANG W, ZHANG L, GUAN K, et al. Effective promotion of oxygen reduction activity by rare earth doping in simple perovskite cathodes for intermediate-temperature solid oxide fuel cells. Journal of Power Sources, 2020, 446: 227360.
DOI URL |
| [30] |
MOHSIN M, YOUSAF A, RAZA R, et al. Highly conducting perovskite structured (M-SrCoFe-O3-δ, M=Ce, Ba) cathode for solid oxide fuel cell. Journal of Alloys and Compounds, 2019, 791: 248.
DOI URL |
| [31] |
ZHANG T, CHEN H, XIAO C, et al. Comparative investigation of composition, microstructure and property influences on electrocatalysis of two representative cathodes: BaCo0.4Fe0.4Zr0.1Y0.1O3-δversus Ba0.5Sr0.5Co0.8Fe0.2O3-δ. Journal of Electroanalytical Chemistry, 2024, 975: 118735.
DOI URL |
| [32] |
LUO Y, ZHANG D, LIU T, et al. In situ exsolution of quaternary alloy nanoparticles for CO2-CO mutual conversion using reversible solid oxide cells. Advanced Functional Materials, 2024, 34(40): 2403922.
DOI URL |
| [33] |
ZHOU Q, SHI Y, HU J, et al. Preparation, characterization, and electrochemical properties of YBaCo3.4Al0.3Ga0.3O7+δ and YBaCo3.2Al0.4Ga0.4O7+δ cathodes for IT-SOFCs. Ceramics International, 2014, 40(8): 13481.
DOI URL |
| [34] |
SUN Y, YAO C, ZHANG Z, et al. Non-metal doping enhsances oxygen reduction kinetics and CO2 tolerance of SrFeO3-δ perovskite as high-performance cathodes for solid oxide fuel cells. Fuel, 2024, 378: 132917.
DOI URL |
| [35] | GOU Y, LI G, REN R, et al. Pr-doping motivating the phase transformation of the BaFeO3-δ perovskite as a high-performance solid oxide fuel cell cathode. ACS Applied Materials & Interfaces, 2021, 13: 20174. |
| [1] | 高源, 魏波, 金芳军, 吕喆, 凌意瀚. Ag掺杂调控中温固体氧化物燃料电池阴极酸性位点增强耐铬能力[J]. 无机材料学报, 2026, 41(1): 70-78. |
| [2] | 闫共芹, 王晨, 蓝春波, 洪雨昕, 叶维超, 付向辉. Al掺杂P2型Na0.8Ni0.33Mn0.67-xAlxO2钠离子电池正极材料的制备与电化学性能[J]. 无机材料学报, 2025, 40(9): 1005-1012. |
| [3] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [4] | 柴润宇, 张镇, 王孟龙, 夏长荣. 直接组装法制备氧化铈基金属支撑固体氧化物燃料电池[J]. 无机材料学报, 2025, 40(7): 765-771. |
| [5] | 何国强, 张恺恒, 王震涛, 包健, 席兆琛, 方振, 王昌昊, 王威, 王鑫, 姜佳沛, 李祥坤, 周迪. Ba(Nd1/2Nb1/2)O3: 一种被低估的K40微波介质陶瓷[J]. 无机材料学报, 2025, 40(6): 639-646. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 渠吉发, 王旭, 张维轩, 张康喆, 熊永恒, 谭文轶. 掺杂改性NaYTiO4增强固体氧化物燃料电池阳极抗硫中毒性能[J]. 无机材料学报, 2025, 40(5): 489-496. |
| [8] | 万俊池, 杜路路, 张永上, 李琳, 刘建德, 张林森. Na4FexP4O12+x/C钠离子电池正极材料的结构演变及其电化学性能[J]. 无机材料学报, 2025, 40(5): 497-503. |
| [9] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [10] | 刘弘明, 张金柯, 陈正鹏, 李明飞, 钱秀洋, 孙传骐, 熊凯, 饶睦敏, 陈创庭, 高源, 凌意瀚. B位高熵策略提高La0.7Sr0.3FeO3-δ基阴极性能[J]. 无机材料学报, 2025, 40(12): 1433-1442. |
| [11] | 王哲, 郝鸿儒, 吴宗辉, 徐玲玲, 吕喆, 魏波. 构型熵工程增强双钙钛矿型氧电极抗Cr中毒能力[J]. 无机材料学报, 2025, 40(12): 1341-1348. |
| [12] | 姜玥宏, 宋云峰, 张磊磊, 马季, 宋昭远, 龙文. 质子传导型固体氧化物燃料电池BaZr0.1Ce0.7Y0.1Yb0.1O3电解质的氟化研究[J]. 无机材料学报, 2025, 40(12): 1356-1364. |
| [13] | 薛子轩, 殷超凡, 姚跃超, 王彦敏, 孙跃跃, 刘峥嵘, 周玉存, 周峻, 吴锴. 泛氢燃料质子导体固体氧化物燃料电池研究进展[J]. 无机材料学报, 2025, 40(12): 1324-1340. |
| [14] | 刘通, 黄溯, 朱诗悦, 查方林, 胡学雷, 王瑶. 一锅法合成高温氢燃料电池用高效无钴复合阴极[J]. 无机材料学报, 2025, 40(12): 1349-1355. |
| [15] | 凌意瀚, 郭胜, 曹志强, 田云峰, 刘方升, 金芳军, 高源. 固体氧化物电池直孔电极结构的制备技术与性能研究进展[J]. 无机材料学报, 2025, 40(12): 1311-1323. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||