
Journal of Inorganic Materials ›› 2026, Vol. 41 ›› Issue (3): 273-288.DOI: 10.15541/jim20250135
• REVIEW • Next Articles
WEI Lianjin( ), QI Zhijie, WANG Xin, ZHU Junwu, FU Yongsheng(
), QI Zhijie, WANG Xin, ZHU Junwu, FU Yongsheng( )
)
Received:2025-03-31
Revised:2025-07-10
Published:2025-08-26
Online:2025-08-26
Contact:
FU Yongsheng, professor. E-mail: fuyongsheng@njust.edu.cnAbout author:WEI Lianjin (2003-), male, PhD candidate. E-mail: 3259728845@qq.com
Supported by:CLC Number:
WEI Lianjin, QI Zhijie, WANG Xin, ZHU Junwu, FU Yongsheng. Modification of Nanodiamond and Its Application in Electrocatalytic Oxygen Reduction Reaction[J]. Journal of Inorganic Materials, 2026, 41(3): 273-288.
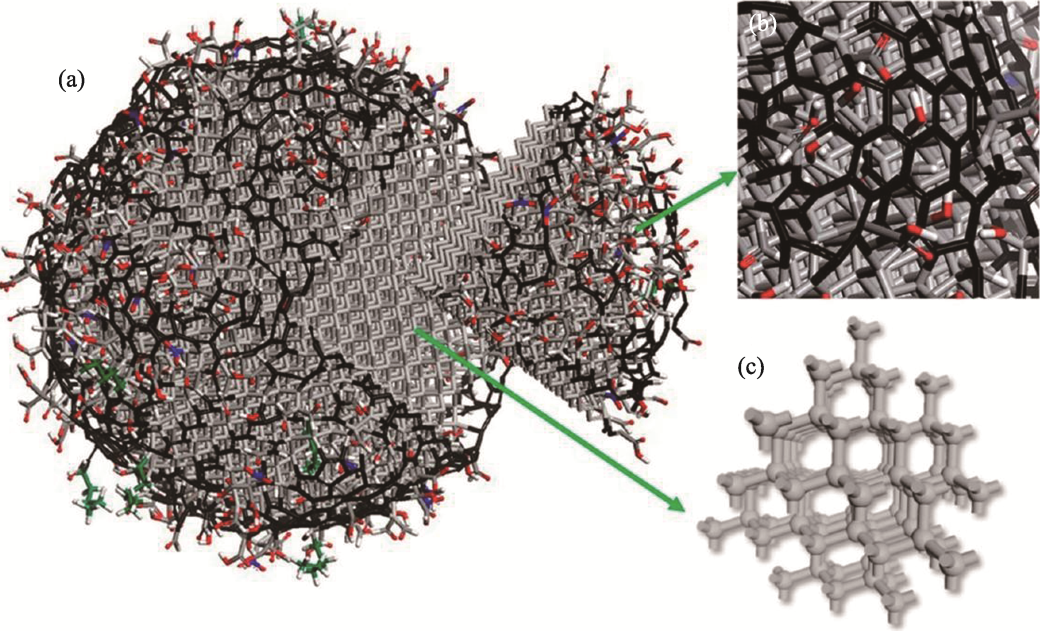
Fig. 2 Schematic model of the ND structure[14] (a) Schematic model illustrating structure of detonation ND; (b) Closer view of surface region of ND covered with surface functional groups and sp2 carbon; (c) Illustration of sp3 carbon framework in the core
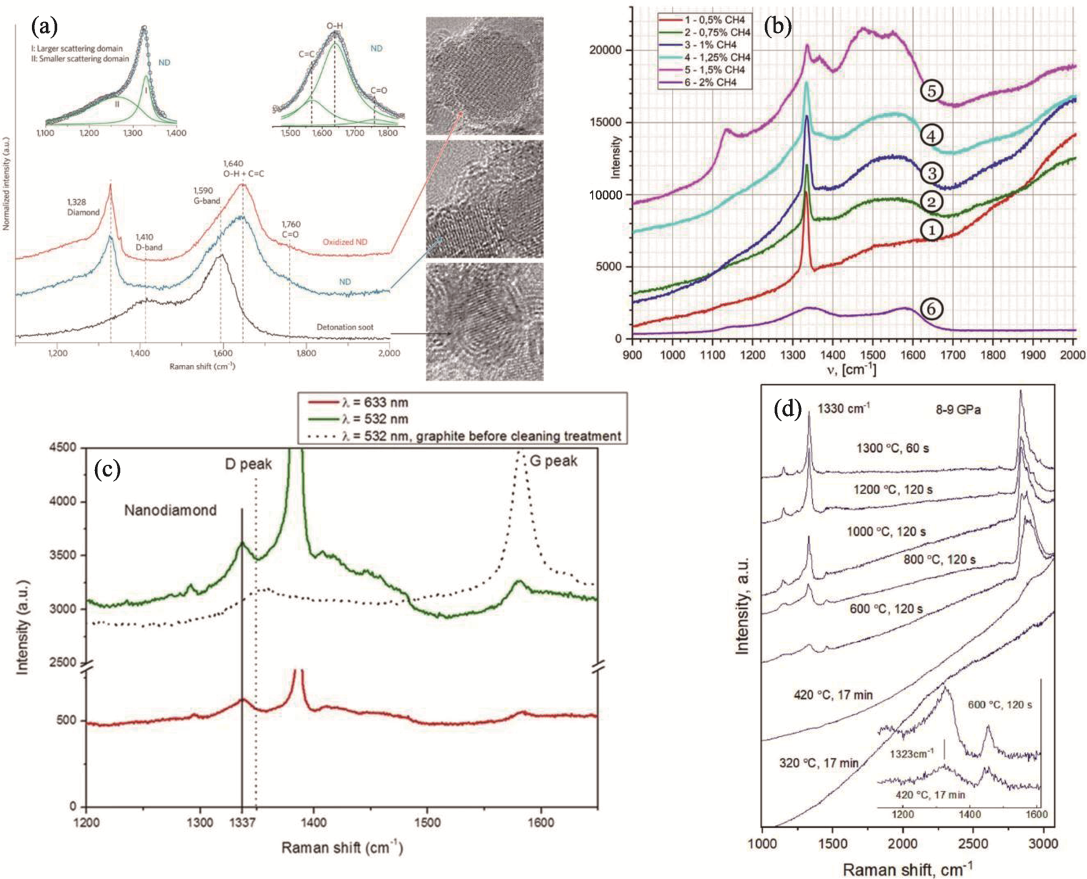
Fig. 3 Raman spectra of ND prepared by different methods[34,37,43,49] (a) DND[34]; (b) CVD under different methane concentrations[37]; (c) PLA[43]; (d) HPHT at different temperatures[49]
| ORR pathway | 2e- | 4e- |
|---|---|---|
| Acid electrolyte | O2+2H++2e-→H2O2 (E0=0.67 V (vs. RHE)) O2+*→*O2 *O2+H++e-→*OOH *OOH+H++e-→H2O2+* | O2+4H++4e-→2H2O (E0=1.23 V (vs. RHE)) O2+*→*O2 |
| *O2+H++e-→*OOH | ||
| *OOH+H+e-→*O+H2O | ||
| *O+H++e-→*OH | ||
| *OH+H++e-→H2O+* | ||
| Alkaline electrolyte | O2+2H2O+2e-→HO2-+OH- (E0=0.065 V (vs. RHE)) | O2+2H2O+4e-→4OH- (E0=0.40 V (vs. RHE)) O2+*→*O2 *O2+H2O+e-→*OOH+OH- *OOH+e-→*O+OH- *O+H2O+e-→*OH-+OH- *OH-+e-→OH-+* |
| HO2-+H2O+2e-→3OH- (E0=0.867 V (vs. RHE)) |
Table 1 Cathodic ORR path in acid and alkaline electrolytes[55] (E0: standard potential)
| ORR pathway | 2e- | 4e- |
|---|---|---|
| Acid electrolyte | O2+2H++2e-→H2O2 (E0=0.67 V (vs. RHE)) O2+*→*O2 *O2+H++e-→*OOH *OOH+H++e-→H2O2+* | O2+4H++4e-→2H2O (E0=1.23 V (vs. RHE)) O2+*→*O2 |
| *O2+H++e-→*OOH | ||
| *OOH+H+e-→*O+H2O | ||
| *O+H++e-→*OH | ||
| *OH+H++e-→H2O+* | ||
| Alkaline electrolyte | O2+2H2O+2e-→HO2-+OH- (E0=0.065 V (vs. RHE)) | O2+2H2O+4e-→4OH- (E0=0.40 V (vs. RHE)) O2+*→*O2 *O2+H2O+e-→*OOH+OH- *OOH+e-→*O+OH- *O+H2O+e-→*OH-+OH- *OH-+e-→OH-+* |
| HO2-+H2O+2e-→3OH- (E0=0.867 V (vs. RHE)) |
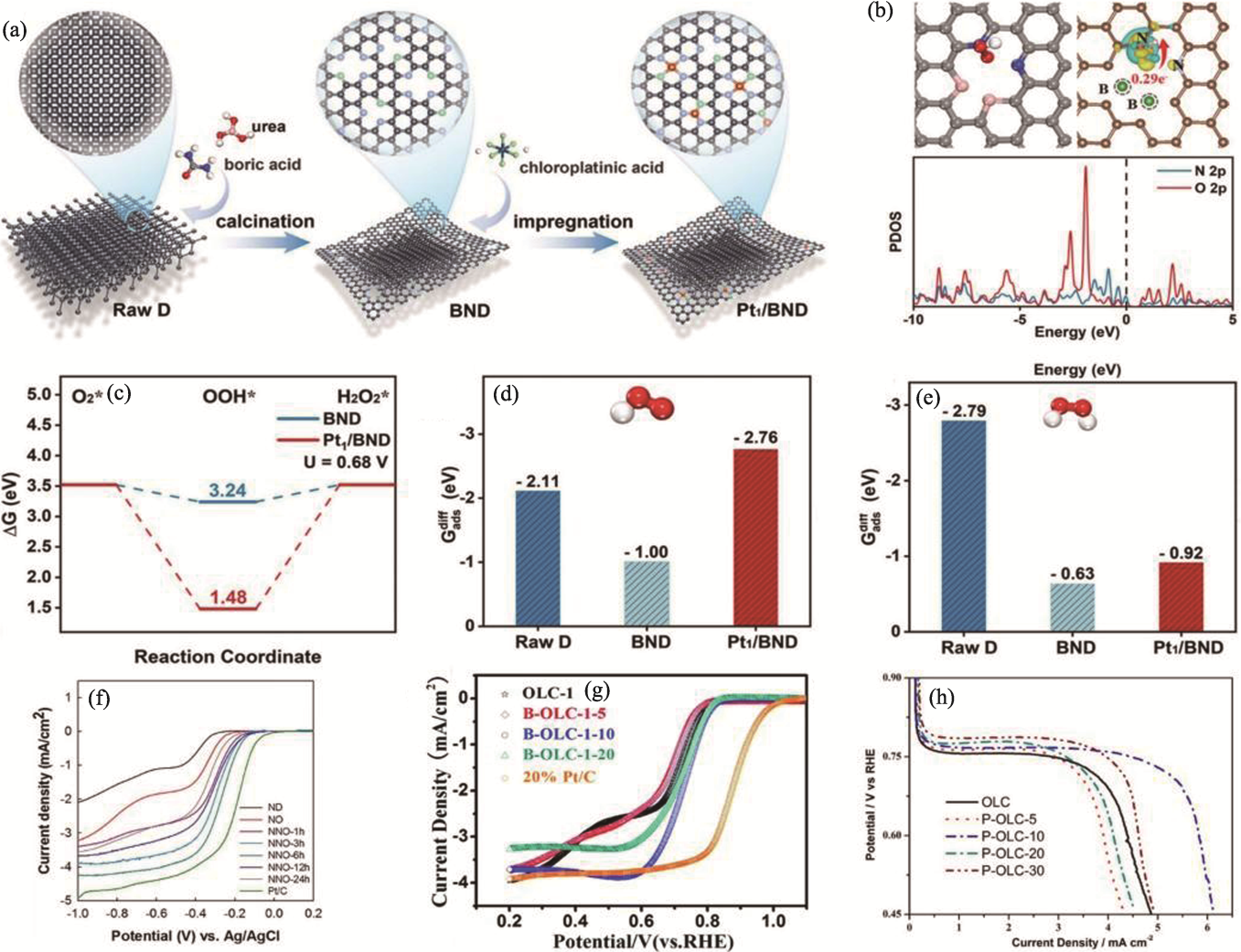
Fig. 5 Various studies based on heteroatom doping[64,68,70-71] (a) Schematic diagram of preparation process of BND and Pt1/BND[64]; (b) Structure of OOH adsorption, charge density (up) and PDOS (down) for OOH adsorption on BND surface[64]; (c) Free-energy profile of 2e− ORR at 0.68 V (vs. RHE) on BND and Pt1/BND[64]; (d, e) Adsorption energies of (d) ΔG*OOH and (e) ΔG*H2O2 on the surface of raw D, BND, and Pt1/BND[64]; (f) LSV curves of ND, NO, NNO-xh, and Pt/C samples[68]; (g) RDE voltammograms recorded with OLC-1, B-OLC-1-5, B-OLC-1-10, B-OLC-1-20, and Pt/C in O2-saturated 0.1 mol·L-1 KOH at 900 r·min-1 with a scan rate of 5 mV·s−1[70]; (h) LSV curves of OLC with different phosphate loading[71]
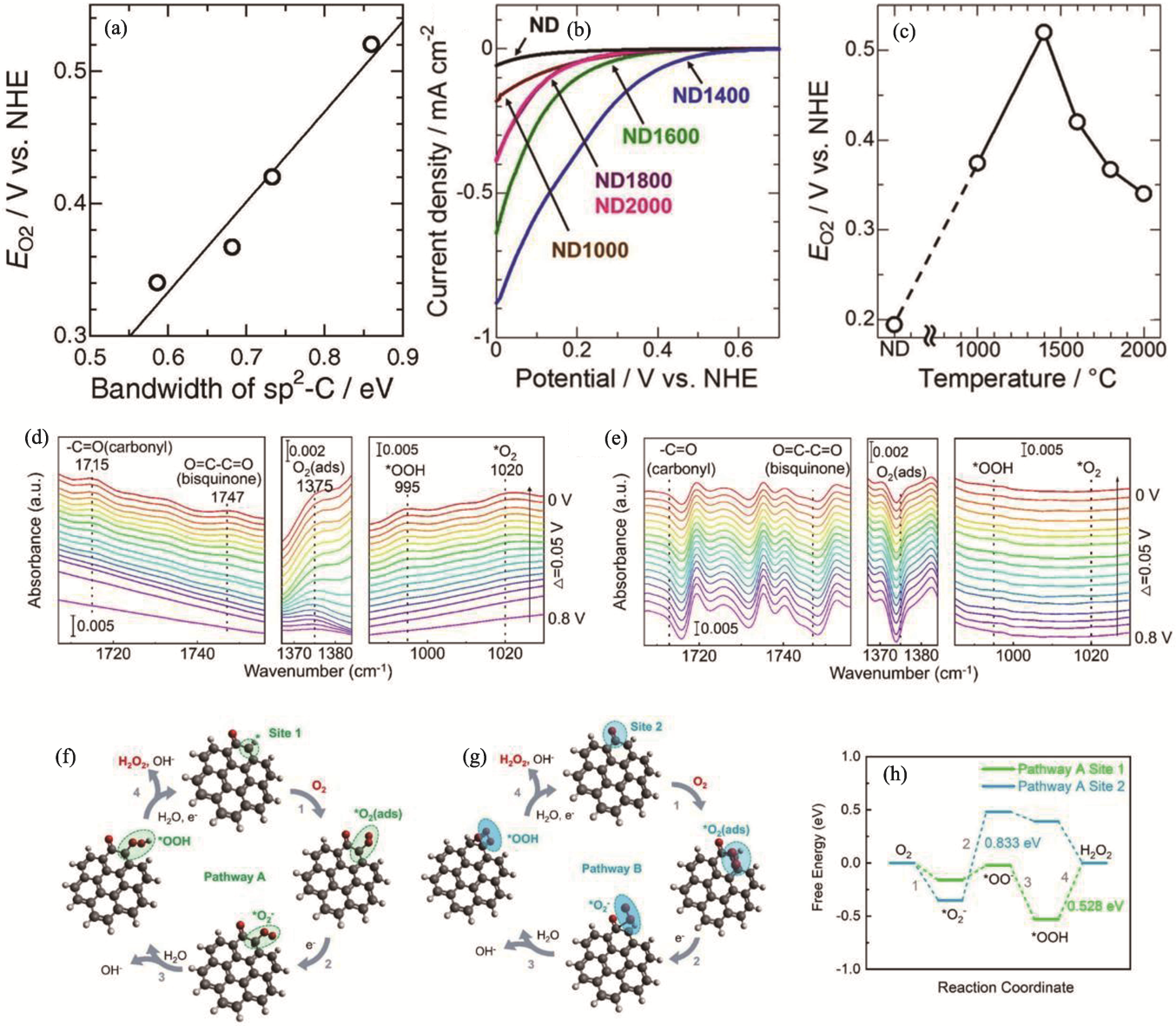
Fig. 7 Surface treatment of ND[77,79] (a) Relationship between ORR activity and degree of defect based on sp2-C bandwidth in C1s XPS spectra[77]; (b) ORR voltammograms of samples prepared at different temperatures[77]; (c) Changes in ORR activity at-10 μA·cm-2 for heat-treated NDs at different preparation temperatures[77]; (d, e) In situ attenuated total reflectance infrared (ATR-IR) spectra under different potential conditions in O2-saturated environment of (d) ND and (e) ND(PH)[79]; (f, g) Schematic diagrams of possible reaction pathways for carbonyl sites on ND (gray: C atom, red: O atom, white: H atom, *: active site)[79]; (h) Free energies of key intermediates occurring at two sites on pathway A and pathway B[79]
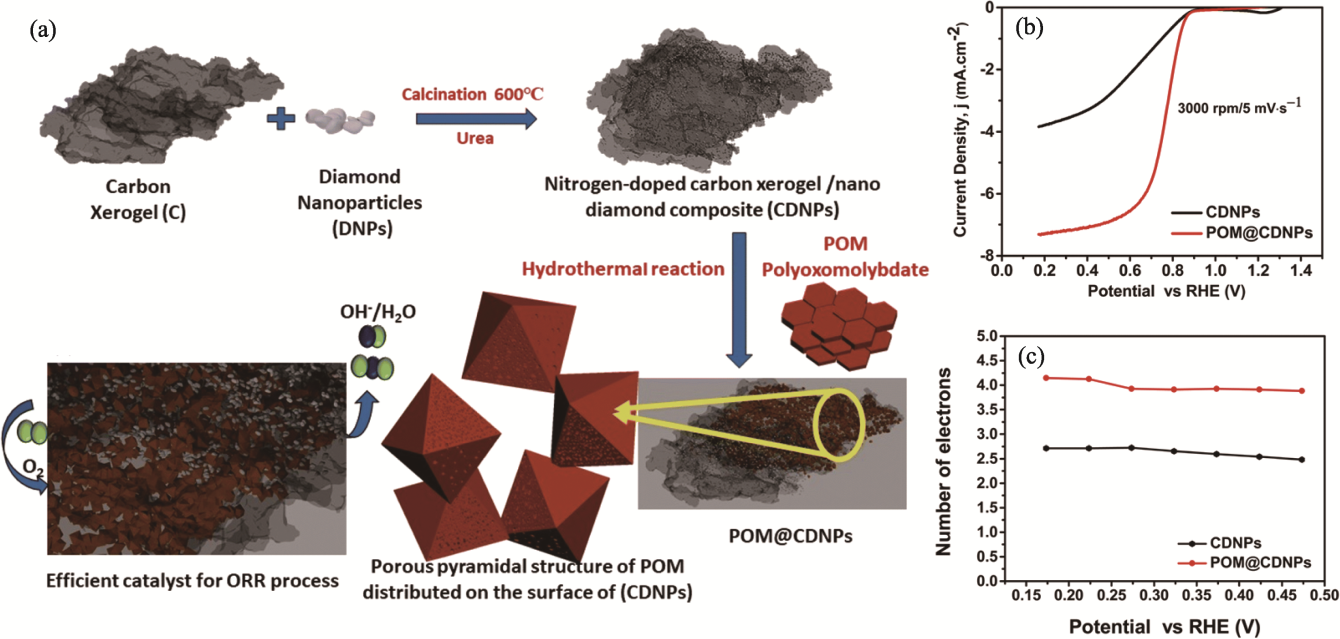
Fig. 8 Preparation and property of POM@CDNPs composites[83] (a) Preparative schematic of POM@CDNPs composite; (b) LSV comparison of electrocatalysts recorded at 3000 r·min-1 with a scan rate of 5 mV·s-1; (c) Transfer electron numbers of CDNP and POM@CDNPs
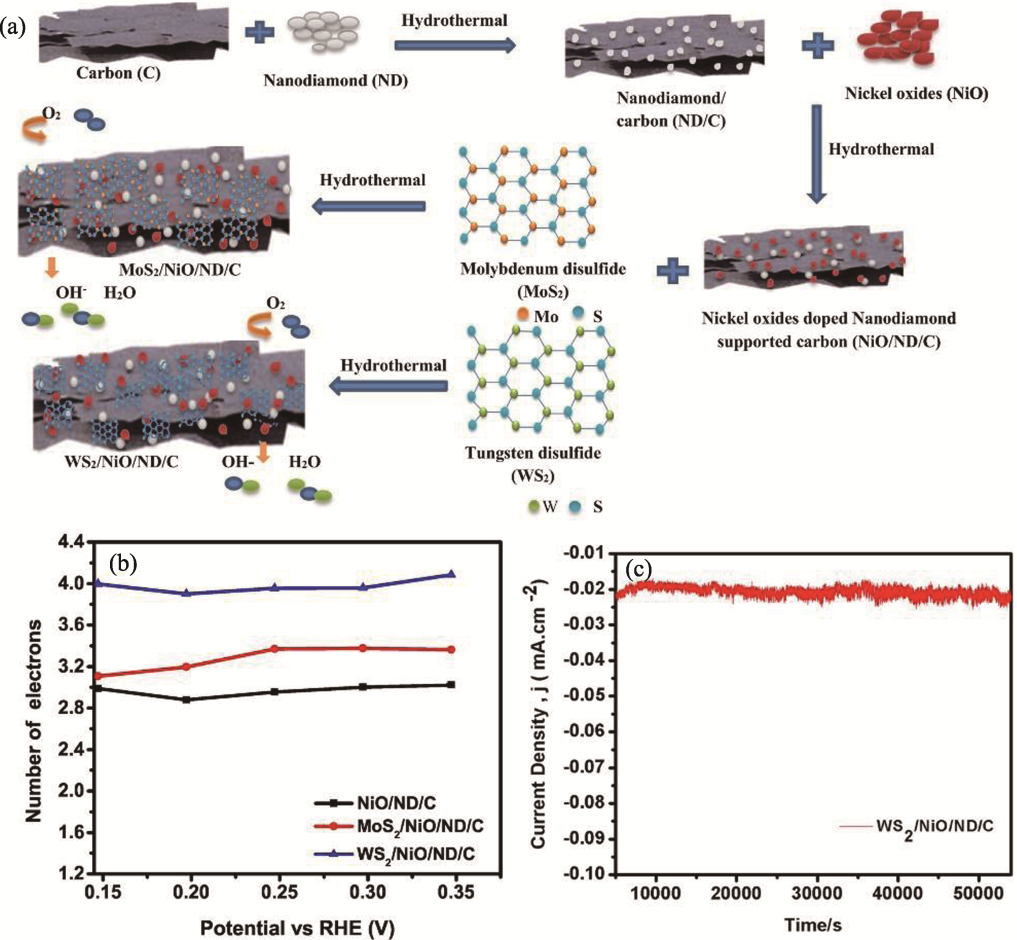
Fig. 9 Preparation and performance of WS2/NiO/ND/C and MoS2/NiO/ND/C[84] (a) Schematic illustration for preparation of MoS2/NiO/ND/C and WS2/NiO/ND/C nanocomposites; (b) Number of transferred electrons; (c) Chronoamperometric curve of WS2/NiO/ND/C in 0.1 mol·L-1 KOH for 54000 s
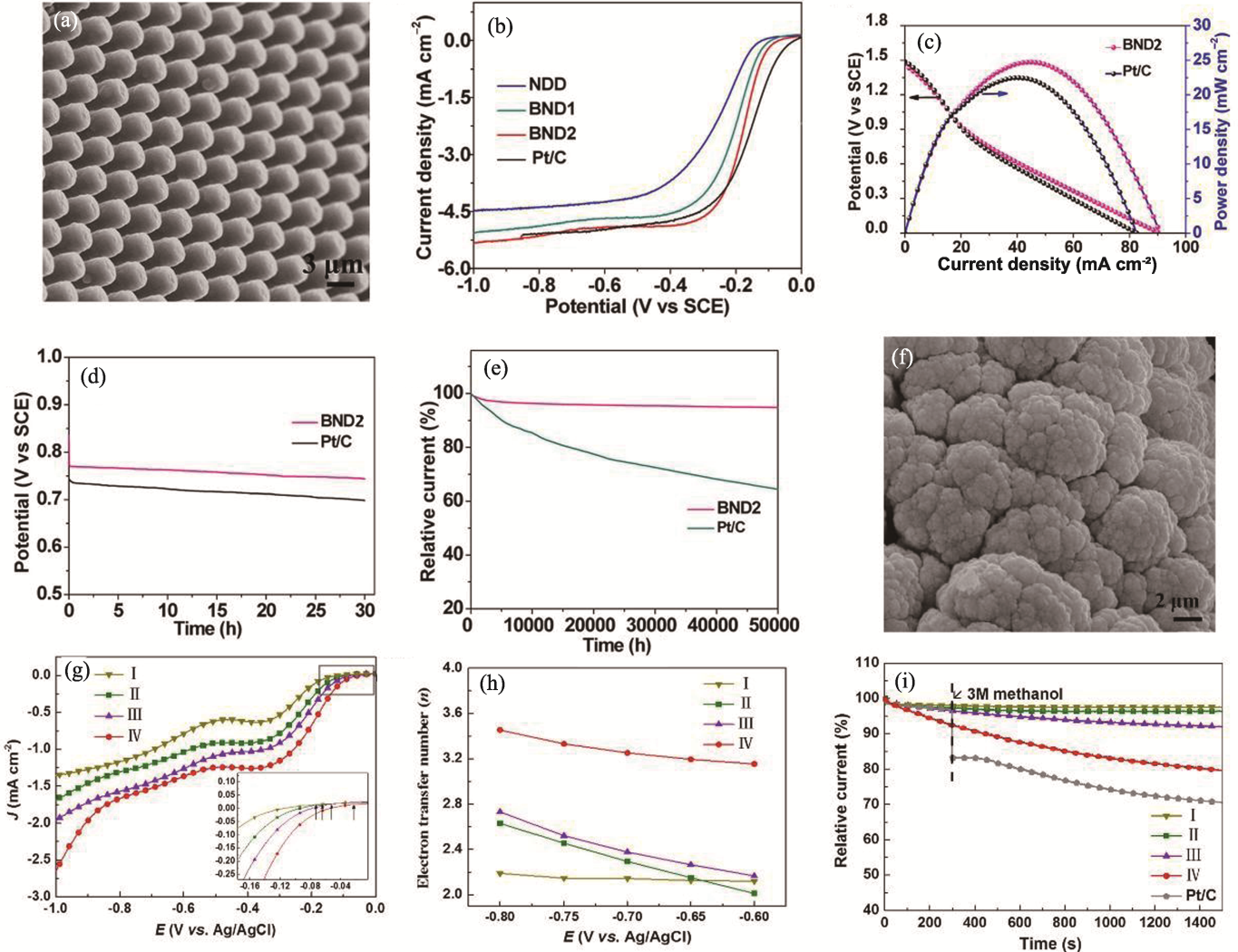
Fig. 10 Preparation and performance of ND electrocatalyst based on morphology regulation[91-92] (a) SEM image of BND2[91]; (b) ORR polarization curves of different catalysts at a rotational speed of 1400 r·min-1[91]; (c) Polarization and power density curves for zinc-air batteries with BND2 and Pt/C catalysts[91]; (d) Evaluation of durability of BND2 and Pt/C catalysts at-0.2 V (0.1 mol·L-1 KOH in saturated O2)[91]; (e) Discharge curves of zinc-air batteries with BND2 and Pt/C catalysts at a current density of 30.0 mA·cm-2[91]; (f) SEM image of BDD catalyst deposited on a foamed nickel substrate[92]; (g) LSV curves, (h) electron transfer numbers and (i) methanol tolerance tests for different SSA samples and Pt/C (I to IV represent samples with increasing SSA)[92]
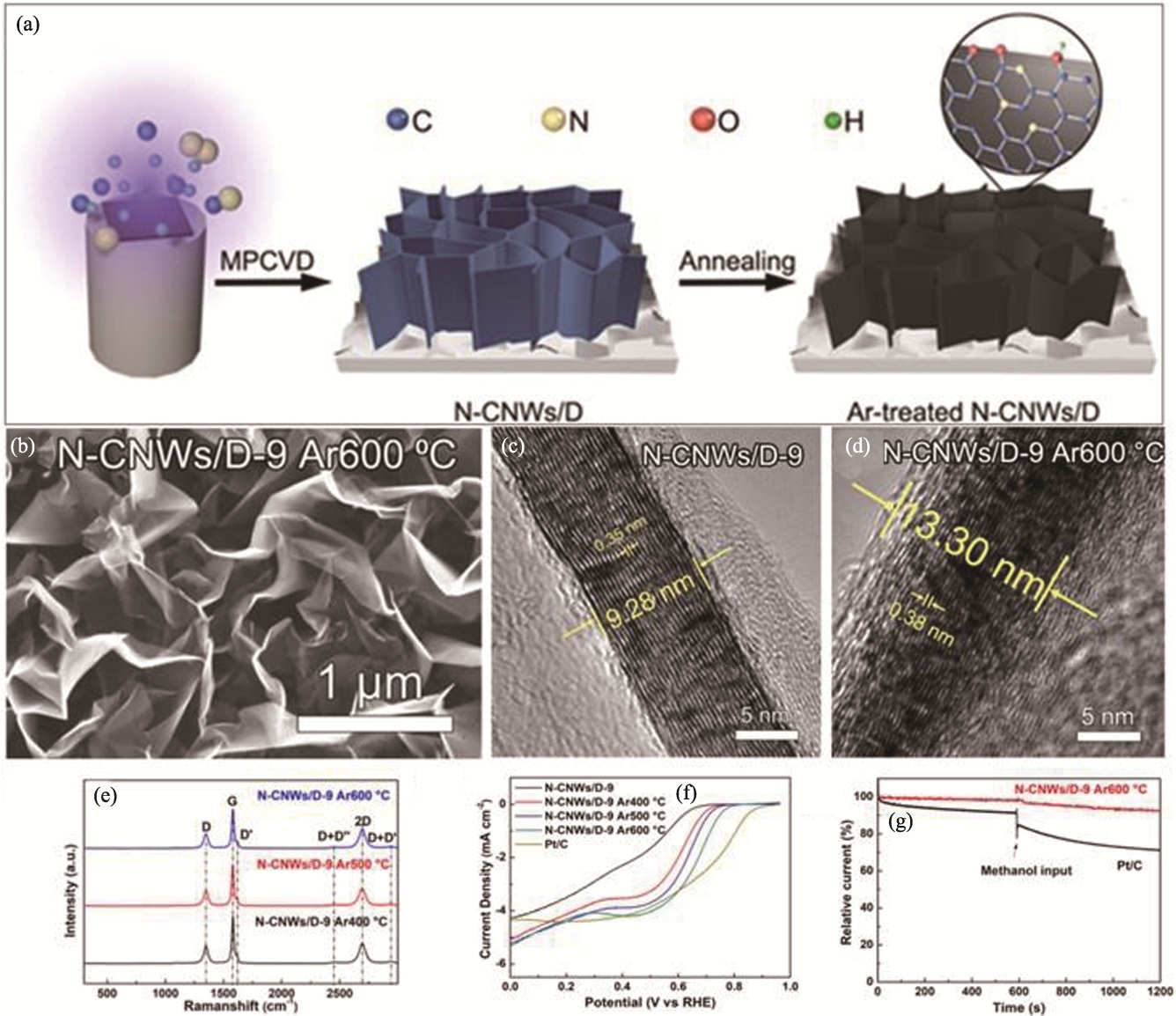
Fig. 11 Preparation and performance of N-CNWs/D material[93] (a) Illustration of preparation of Ar-treated N-CNWs/D films; (b) SEM image of N-CNWs/D-9 films after Ar annealing treatment at 600 ℃; (c, d) HRTEM images of N-CNWs/D-9 (c) before and (d) after Ar annealing at 600 ℃; (e) Raman spectra of N-CNWs/D-9 films after Ar annealing at different temperatures; (f) LSV curves of N-CNWs/D-9 films treated with Ar at different temperatures; (g) Chronoamperometric curves of Ar-treated N-CNWs/D-9 films and Pt/C in 0.1 mol·L-1 KOH solution before and after addition of methanol
| Catalyst | Eonset/V (vs. RHE) | E1/2/V (vs. RHE) | JL/(mA·cm−2) | Transferred electrons, n | Ref. |
|---|---|---|---|---|---|
| BN-C/ND | 0.75 | 0.69 | −5.98 | 3.8 | [ |
| Co-N-C/ND | 0.89 | 0.76 | −4.31 | 3.8 | [ |
| ND/Fe3C@Fe-N-C | 0.89 | 0.80 | −4.25 | 3.9 | [ |
| NNO-6h | 0.81 | 0.71 | −4.23 | 3.3 | [ |
| B-OLC-1-10 | 0.80 | 0.734 | ‒3.74 | 3.95 | [ |
| N-GND | 0.89 | 0.82 | ‒4.63 | 3.8 | [ |
| NDGNSs | 0.93 | 0.783 | ‒4.45 | 3.95 | [ |
| POM@CDNPs | 0.85 | 0.773 | −7.30 | 4.1 | [ |
| ND/N-G | 0.97 | 0.773 | ‒4.61 | 3.65 | [ |
| WS2/NiO/ND/C | 0.76 | 0.63 | −7.47 | 4.0 | [ |
| BND2 | 0.96 | 0.83 | ‒5.34 | 3.96 | [ |
| BDD | 0.94 | 0.80 | ‒2.62 | 3.45 | [ |
| N-CNWs/D-9 | 0.84 | 0.72 | −4.18 | 3.86 | [ |
| BND | 0.73 | - | - | 2.19 | [ |
Table 2 Summary of properties of some ND catalysts
| Catalyst | Eonset/V (vs. RHE) | E1/2/V (vs. RHE) | JL/(mA·cm−2) | Transferred electrons, n | Ref. |
|---|---|---|---|---|---|
| BN-C/ND | 0.75 | 0.69 | −5.98 | 3.8 | [ |
| Co-N-C/ND | 0.89 | 0.76 | −4.31 | 3.8 | [ |
| ND/Fe3C@Fe-N-C | 0.89 | 0.80 | −4.25 | 3.9 | [ |
| NNO-6h | 0.81 | 0.71 | −4.23 | 3.3 | [ |
| B-OLC-1-10 | 0.80 | 0.734 | ‒3.74 | 3.95 | [ |
| N-GND | 0.89 | 0.82 | ‒4.63 | 3.8 | [ |
| NDGNSs | 0.93 | 0.783 | ‒4.45 | 3.95 | [ |
| POM@CDNPs | 0.85 | 0.773 | −7.30 | 4.1 | [ |
| ND/N-G | 0.97 | 0.773 | ‒4.61 | 3.65 | [ |
| WS2/NiO/ND/C | 0.76 | 0.63 | −7.47 | 4.0 | [ |
| BND2 | 0.96 | 0.83 | ‒5.34 | 3.96 | [ |
| BDD | 0.94 | 0.80 | ‒2.62 | 3.45 | [ |
| N-CNWs/D-9 | 0.84 | 0.72 | −4.18 | 3.86 | [ |
| BND | 0.73 | - | - | 2.19 | [ |
| [1] | WAN X, LIU X, LI Y, et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nature Catalysis, 2019, 2(3): 259. |
| [2] | ANSON C W, STAHL S S. Processes for electrochemical production of electrolyte-free hydrogen peroxide. Joule, 2019, 3(12): 2889. |
| [3] | SUN W, WANG F, ZHANG B, et al. A rechargeable zinc-air battery based on zinc peroxide chemistry. Science, 2021, 371(6524): 46. |
| [4] | SHAO M, CHANG Q, DODELET J P, et al. Recent advances in electrocatalysts for oxygen reduction reaction. Chemical Reviews, 2016, 116(6): 3594. |
| [5] | HUANG X, WANG Y, LI W, et al. Noble metal-free catalysts for oxygen reduction reaction. Science China Chemistry, 2017, 60(12): 1494. |
| [6] | PAN T, LIU H, REN G, et al. Metal-free porous nitrogen-doped carbon nanotubes for enhanced oxygen reduction and evolution reactions. Science Bulletin, 2016, 61(11): 889. |
| [7] | HU C, PAUL R, DAI Q, et al. Carbon-based metal-free electrocatalysts: from oxygen reduction to multifunctional electrocatalysis. Chemical Society Reviews, 2021, 50(21): 11785. |
| [8] | LIU X, DAI L. Carbon-based metal-free catalysts. Nature Reviews Materials, 2016, 1(11): 16064. |
| [9] | LAM E, LUONG J H T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catalysis, 2014, 4(10): 3393. |
| [10] | DENG D, YU L, CHEN X, et al. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angewandte Chemie International Edition, 2013, 52(1): 371. |
| [11] | ABIDA B, CHIRCHI L, BARANTON S, et al. Preparation and characterization of Pt/TiO2 nanotubes catalyst for methanol electro-oxidation. Applied Catalysis B: Environmental, 2011, 106(3): 609. |
| [12] | LIN Y, SUN X, SU D S, et al. Catalysis by hybrid sp2/sp3 nanodiamonds and their role in the design of advanced nanocarbon materials. Chemical Society Reviews, 2018, 47(22): 8438. |
| [13] | GAO X W, ZHAO Z W, HE Y, et al. Nanodiamond: a promising metal-free nanoscale material in photocatalysis and electrocatalysis. Rare Metals, 2024, 43(8): 3501. |
| [14] | KOREPANOV V I, HAMAGUCHI H O, OSAWA E, et al. Carbon structure in nanodiamonds elucidated from Raman spectroscopy. Carbon, 2017, 121: 322. |
| [15] | FANG Y X, WANG X C. Metal-free boron-containing heterogeneous catalysts. Angewandte Chemie International Edition, 2017, 56(49): 15506. |
| [16] | DUAN X, AO Z, ZHANG H, et al. Nanodiamonds in sp2/sp3 configuration for radical to nonradical oxidation: core-shell layer dependence. Applied Catalysis B: Environmental, 2018, 222: 176. |
| [17] | GRAYDON O. Sensitive nanodiamonds. Nature Photonics, 2019, 13(7): 438. |
| [18] | PIÑA-SALAZAR E Z, KUKOBAT R, FUTAMURA R, et al. Water-selective adsorption sites on detonation nanodiamonds. Carbon, 2018, 139: 853. |
| [19] | KARAMI P, KHASRAGHI S S, HASHEMI M, et al. Polymer/nanodiamond composites-a comprehensive review from synthesis and fabrication to properties and applications. Advances in Colloid and Interface Science, 2019, 269: 122. |
| [20] | ZHANG Y, CUI Z, KONG H, et al. One-shot immunomodulatory nanodiamond agents for cancer immunotherapy. Advanced Materials, 2016, 28(14): 2699. |
| [21] | LIN Z, XIAO J, LI L, et al. Nanodiamonds: nanodiamond-embedded p-type copper(I) oxide nanocrystals for broad-spectrum photocatalytic hydrogen evolution. Advanced Energy Materials, 2016, 6(4): 1501865. |
| [22] | VLASOV I I, SHIRYAEV A A, RENDLER T, et al. Molecular-sized fluorescent nanodiamonds. Nature Nanotechnology, 2014, 9(1): 54. |
| [23] | BARBIERO M, CASTELLETTO S, GAN X, et al. Spin-manipulated nanoscopy for single nitrogen-vacancy center localizations in nanodiamonds. Light: Science & Applications, 2017, 6(11): e17085. |
| [24] | LAZOVIC J, GOERING E, WILD A M, et al. Nanodiamond-enhanced magnetic resonance imaging. Advanced Materials, 2024, 36(11): 2470085. |
| [25] | HUANG G, GHALEI B, ISFAHANI A P, et al. Overcoming humidity-induced swelling of graphene oxide-based hydrogen membranes using charge-compensating nanodiamonds. Nature Energy, 2021, 6(12): 1176. |
| [26] | ZHANG X Q, LAM R, XU X, et al. Multimodal nanodiamond drug delivery carriers for selective targeting, imaging, and enhanced chemotherapeutic efficacy. Advanced Materials, 2011, 23(41): 4770. |
| [27] | DUAN X, TIAN W, ZHANG H, et al. sp2/sp3 framework from diamond nanocrystals: a key bridge of carbonaceous structure to carbocatalysis. ACS Catalysis, 2019, 9(8): 7494. |
| [28] | KUMAR S, NEHRA M, KEDIA D, et al. Nanodiamonds: emerging face of future nanotechnology. Carbon, 2019, 143: 678. |
| [29] | BAGGE-HANSEN M, BASTEA S, HAMMONS J A, et al. Detonation synthesis of carbon nano-onions via liquid carbon condensation. Nature Communications, 2019, 10: 3819. |
| [30] | WHITLOW J, PACELLI S, PAUL A. Multifunctional nanodiamonds in regenerative medicine: recent advances and future directions. Journal of Controlled Release, 2017, 261: 62. |
| [31] | BERNAT-QUESADA F, VALLÉS-GARCÍA C, MONTERO-LANZUELA E, et al. Hybrid sp2/sp3 nanodiamonds as heterogeneous metal-free ozonation catalysts in water. Applied Catalysis B: Environmental, 2021, 299: 120673. |
| [32] | DANILENKO V V. On the history of the discovery of nanodiamond synthesis. Physics of the Solid State, 2004, 46(4): 595. |
| [33] | DOLMATOV V Y. The influence of detonation synthesis conditions on the yield of condensed carbon and detonation nanodiamond through the example of using TNT-RDX explosive mixture. Journal of Superhard Materials, 2018, 40(4): 290. |
| [34] | MOCHALIN V N, SHENDEROVA O, HO D, et al. The properties and applications of nanodiamonds. Nature Nanotechnology, 2012, 7(1): 11. |
| [35] | GUNAWAN M A, MONCEA O, POINSOT D, et al. Nanodiamond-palladium core-shell organohybrid synthesis: a mild vapor-phase procedure enabling nanolayering metal onto functionalized sp3-carbon. Advanced Functional Materials, 2018, 28(13): 1705786. |
| [36] | BASSO L, CAZZANELLI M, ORLANDI M, et al. Nanodiamonds: synthesis and application in sensing, catalysis, and the possible connection with some processes occurring in space. Applied Sciences, 2020, 10(12): 4094. |
| [37] | EMELYANOV A A, PINAEV V A, PLOTNIKOV M Y, et al. Effect of methane flow rate on gas-jet MPCVD diamond synthesis. Journal of Physics D: Applied Physics, 2022, 55(20): 205202. |
| [38] | TRUSHEIM M E, LI L, LARAOUI A, et al. Scalable fabrication of high purity diamond nanocrystals with long-spin-coherence nitrogen vacancy centers. Nano Letters, 2014, 14(1): 32. |
| [39] | TZENG T Y, ZHANG J L, LU H, et al. Vertical-substrate MPCVD epitaxial nanodiamond growth. Nano Letters, 2017, 17(3): 1489. |
| [40] | YOGESH G K, SHUKLA S, SASTIKUMAR D, et al. Progress in pulsed laser ablation in liquid (PLAL) technique for the synthesis of carbon nanomaterials: a review. Applied Physics A, 2021, 127(11): 810. |
| [41] | 郑腊梅, 吕豫文, 唐少雄, 等. 激光法制备超细纳米金刚石的相变机理. 激光技术, 2016, 40(1): 25. |
| [42] | YANG G W, WANG J B, LIU Q X. Preparation of nano-crystalline diamonds using pulsed laser induced reactive quenching. Journal of Physics: Condensed Matter, 1998, 10(35): 7923. |
| [43] | GORRINI F, CAZZANELLI M, BAZZANELLA N, et al. On the thermodynamic path enabling a room-temperature, laser-assisted graphite to nanodiamond transformation. Scientific Reports, 2016, 6: 35244. |
| [44] | BORUAH A, SAIKIA B K. Synthesis, characterization, properties, and novel applications of fluorescent nanodiamonds. Journal of Fluorescence, 2022, 32(3): 863. |
| [45] | GUO Z, GUO B, ZHANG J, et al. CVD diamond processing tools: a review. Journal of Advanced Research, 2025, 74: 333. |
| [46] | CRANE M J, PETRONE A, BECK R A, et al. High-pressure, high-temperature molecular doping of nanodiamond. Science Advances, 2019, 5(5): eaau6073. |
| [47] | ELDEMRDASH S, THALASSINOS G, ALZAHRANI A, et al. Fluorescent HPHT nanodiamonds have disk-and rod-like shapes. Carbon, 2023, 206: 268. |
| [48] | ZHANG Y, RHEE K Y, HUI D, et al. A critical review of nanodiamond based nanocomposites: synthesis, properties and applications. Composites Part B: Engineering, 2018, 143: 19. |
| [49] | EKIMOV E A, SHIRYAEV A A, SIDOROV V A, et al. Synthesis and properties of nanodiamonds produced by HPHT carbonization of 1-fluoroadamantane. Diamond and Related Materials, 2023, 136: 109907. |
| [50] | SHEN Y, SU S, ZHAO W, et al. Sub-4 nm nanodiamonds from graphene-oxide and nitrated polycyclic aromatic hydrocarbons at 423 K. ACS Nano, 2021, 15(11): 17392. |
| [51] | FENG Y, DAVIDSON D J, SUN W, et al. Formation of nanodiamonds during pyrolysis of butanosolv lignin. ACS Nano, 2024, 18(36): 24803. |
| [52] | YANG X, ZENG Y, ALNOUSH W, et al. Tuning two-electron oxygen-reduction pathways for H2O2 electrosynthesis via engineering atomically dispersed single metal site catalysts. Advanced Materials, 2022, 34(23): 2107954. |
| [53] | DING Y, ZHOU W, XIE L, et al. Pulsed electrocatalysis enables an efficient 2-electron oxygen reduction reaction for H2O2 production. Journal of Materials Chemistry A, 2021, 9(29): 15948. |
| [54] | YAN M, YANG H, GONG Z, et al. Sulfur-tuned main-group Sb-N-C catalysts for selective 2-electron and 4-electron oxygen reduction. Advanced Materials, 2024, 36(27): 2402963. |
| [55] | ALLENDORF M D. Oxygen reduction reaction: a framework for success. Nature Energy, 2016, 1(5): 16058. |
| [56] | LUO E, YANG T, LIANG J, et al. Selective oxygen electroreduction to hydrogen peroxide in acidic media: the superiority of single-atom catalysts. Nano Research, 2024, 17(6): 4668. |
| [57] | DEY S, MONDAL B, CHATTERJEE S, et al. Molecular electrocatalysts for the oxygen reduction reaction. Nature Reviews Chemistry, 2017, 1(12): 0098. |
| [58] | LIU Y, TZENG Y K, LIN D, et al. An ultrastrong double-layer nanodiamond interface for stable lithium metal anodes. Joule, 2018, 2(8): 1595. |
| [59] | WANG H, TZENG Y K, JI Y, et al. Synergistic enhancement of electrocatalytic CO2 reduction to C2 oxygenates at nitrogen-doped nanodiamonds/Cu interface. Nature Nanotechnology, 2020, 15(2): 131. |
| [60] | XING X, LIU R, ANJASS M, et al. Bimetallic manganese-vanadium functionalized N,S-doped carbon nanotubes as efficient oxygen evolution and oxygen reduction electrocatalysts. Applied Catalysis B: Environmental, 2020, 277: 119195. |
| [61] | BHARDWAJ K, PARVIS F, WANG Y, et al. Effect of surface oxygen on the wettability and electrochemical properties of boron-doped nanocrystalline diamond electrodes in room-temperature ionic liquids. Langmuir, 2020, 36(21): 5717. |
| [62] | LIU Y, ZHANG Y, CHENG K, et al. Selective electrochemical reduction of carbon dioxide to ethanol on a boron-and nitrogen-co-doped nanodiamond. Angewandte Chemie International Edition, 2017, 56(49): 15607. |
| [63] | WU K H, LIU Y, LUO J, et al. The Coulombic nature of active nitrogen sites in N-doped nanodiamond revealed in situ by ionic surfactants. ACS Catalysis, 2017, 7(5): 3295. |
| [64] | LU M, LIU X, JING C, et al. Efficient electrochemical ozone and hydrogen peroxide production by synergistic effect of atomically dispersed Pt, boron and nitrogen doped 2D diamonds. Advanced Functional Materials, 2025, 35(2): 2412170. |
| [65] | LIU X, WANG Y, DONG L, et al. One-step synthesis of shell/core structural boron and nitrogen co-doped graphitic carbon/ nanodiamond as efficient electrocatalyst for the oxygen reduction reaction in alkaline media. Electrochimica Acta, 2016, 194: 161. |
| [66] | PRAUS P. On electronegativity of graphitic carbon nitride. Carbon, 2021, 172: 729. |
| [67] | HUANG Y K, JENA A, CHEN Y T, et al. Nanosized-Fe3PtN supported on nitrogen-doped carbon as electro-catalyst for oxygen reduction reaction. International Journal of Hydrogen Energy, 2017, 42(24): 15761. |
| [68] | CHOI E Y, KIM C K. Fabrication of nitrogen-doped nano-onions and their electrocatalytic activity toward the oxygen reduction reaction. Scientific Reports, 2017, 7: 4178. |
| [69] | DONG L, ZANG J, SU J, et al. Nanodiamond/nitrogen-doped graphene (core/shell) as an effective and stable metal-free electrocatalyst for oxygen reduction reaction. Electrochimica Acta, 2015, 174: 1017. |
| [70] | LIN Y, ZHU Y, ZHANG B, et al. Boron-doped onion-like carbon with enriched substitutional boron: the relationship between electronic properties and catalytic performance. Journal of Materials Chemistry A, 2015, 3(43): 21805. |
| [71] | SUN X, XU J, DING Y, et al. The effect of different phosphorus chemical states on an onion-like carbon surface for the oxygen reduction reaction. ChemSusChem, 2015, 8(17): 2872. |
| [72] | WU Y, ZANG J, DONG L, et al. High performance and bifunctional cobalt-embedded nitrogen doped carbon/nanodiamond electrocatalysts for oxygen reduction and oxygen evolution reactions in alkaline media. Journal of Power Sources, 2016, 305: 64. |
| [73] | WANG Y, SU J, ZHU G, et al. Fe and N co-doped graphene coated nanodiamond/Fe3C as highly active and stable catalyst for oxygen reduction reaction. Diamond and Related Materials, 2023, 136: 109905. |
| [74] | HUANG F, PENG M, LIU H, et al. Atomically dispersed metals on nanodiamond-derived hybrid materials for heterogeneous catalysis. Accounts of Materials Research, 2023, 4(3): 223. |
| [75] | MAZA W A, BRESLIN V M, FEYGELSON T I, et al. Degradation of perfluorooctanesulfonate (PFOS) by sub-bandgap irradiation of hydrogen-terminated nanodiamond. Applied Catalysis B: Environmental, 2023, 325: 122306. |
| [76] | DONG L, ZANG J, WANG Y, et al. Graphitized nanodiamond as highly efficient support of electrocatalysts for oxygen reduction reaction. Journal of the Electrochemical Society, 2014, 161(3): F185. |
| [77] | KANNARI N, ITAKURA T, OZAKI J I. Electrochemical oxygen reduction activity of intermediate onion-like carbon produced by the thermal transformation of nanodiamond. Carbon, 2015, 87: 415. |
| [78] | JANG D M, IM H S, BACK S H, et al. Laser-induced graphitization of colloidal nanodiamonds for excellent oxygen reduction reaction. Physical Chemistry Chemical Physics, 2014, 16(6): 2411. |
| [79] | LIU J, ZHANG M, TANG L, et al. Surface hybrid engineering of nanodiamonds for boosting electrocatalytic hydrogen peroxide production with high efficiency and stability. Journal of Energy Chemistry, 2025, 109: 15. |
| [80] | MERZ V, LENHART J, VONHAUSEN Y, et al. Zwitterion-functionalized detonation nanodiamond with superior protein repulsion and colloidal stability in physiological media. Small, 2019, 15(48): 1901551. |
| [81] | LU J. Atomic lego catalysts synthesized by atomic layer deposition. Accounts of Materials Research, 2022, 3(3): 358. |
| [82] | WANG S, JI X, AO Y, et al. Vertically aligned N-doped diamond/graphite hybrid nanosheets epitaxially grown on B-doped diamond films as electrocatalysts for oxygen reduction reaction in an alkaline medium. ACS Applied Materials & Interfaces, 2018, 10(35): 29866. |
| [83] | ALLAH A E, EL-DEEB M M, FARGHALI A A, et al. Growth of polyoxomolybdate with a porous pyramidal structure on carbon xerogel nanodiamond as an efficient electro-catalyst for oxygen reduction reaction. RSC Advances, 2023, 13(12): 8090. |
| [84] | MOSTAFA E, KHEDR M H, ABDELWAHAB A. 2D WS2 and MoS2 functionalized nickel oxide/nanodiamond supported carbon electrocatalysts for oxygen reduction reaction. Diamond and Related Materials, 2023, 139: 110386. |
| [85] | CHOI E Y, LEE D, KIM J, et al. Enhanced electrocatalytic activity of N-doped nano-onion/gold nanorod nanocomposites for the oxygen reduction reaction. Electrochimica Acta, 2022, 405: 139816. |
| [86] | LU M, LIU D, ZHANG C, et al. DFT calculations and experiments of oxidation resistance research on B, N, and Si multi-doped diamond films. Applied Surface Science, 2023, 612: 155865. |
| [87] | SOLANO J R, BAÑOS A T, DURÁN Á M, et al. DFT study of anisotropy effects on the electronic properties of diamond nanowires with nitrogen-vacancy center. Journal of Molecular Modeling, 2017, 23(10): 292. |
| [88] | BOGDANOWICZ R, FICEK M, MALINOWSKA N, et al. Electrochemical performance of thin free-standing boron-doped diamond nanosheet electrodes. Journal of Electroanalytical Chemistry, 2020, 862: 114016. |
| [89] | STURSA J, HAVLIK J, PETRAKOVA V, et al. Mass production of fluorescent nanodiamonds with a narrow emission intensity distribution. Carbon, 2016, 96: 812. |
| [90] | BASSO L, BAZZANELLA N, CAZZANELLI M, et al. On the route towards a facile fluorescent nanodiamonds laser-synthesis. Carbon, 2019, 153: 148. |
| [91] | LIU Y, CHEN S, QUAN X, et al. Boron and nitrogen codoped nanodiamond as an efficient metal-free catalyst for oxygen reduction reaction. The Journal of Physical Chemistry C, 2013, 117(29): 14992. |
| [92] | SUO N, HUANG H, WU A, et al. Porous boron doped diamonds as metal-free catalysts for the oxygen reduction reaction in alkaline solution. Applied Surface Science, 2018, 439: 329. |
| [93] | ZHANG C, HUANG N, ZHAI Z, et al. Nitrogen-doped carbon nanowalls/diamond films as efficient electrocatalysts toward oxygen reduction reaction. Nanotechnology, 2022, 33(1): 015401. |
| [94] | COSTA F J R, DE ALMEIDA J S. Theoretical investigation of superconductivity in diamond: effects of doping and pressure. Journal of Applied Physics, 2021, 129(4): 043903. |
| [95] | RAMOS E, SANSORES L E, MAR N, et al. Diamondoids in octahedral iron complexes: a DFT study. Computational and Theoretical Chemistry, 2016, 1078: 30. |
| [1] | LIU Zhanyi, LI Mian, OUYANG Xiaoping, CHAI Zhifang, HUANG Qing. Recent Progress on Removal of Sr/Cs from Molten Salt in Dry Reprocessing [J]. Journal of Inorganic Materials, 2026, 41(2): 150-158. |
| [2] | SUN Lian, ZHANG Leilei, XUE Zexu, WU Kun, CHEN Ye, LI Zhiyuan, WANG Lukai, WANG Zungang. Research Progress on Zero-dimensional Metal Halide Scintillators towards Radiation Detection Applications [J]. Journal of Inorganic Materials, 2026, 41(2): 159-176. |
| [3] | REN Xianpei, LI Chao, HU Qiwei, XIANG Hui, PENG Yuehong. Research Progress on Mott-Schottky Hydrogen Evolution Catalysts Based on Metal/Transition Metal Compounds [J]. Journal of Inorganic Materials, 2026, 41(2): 137-149. |
| [4] | FAN Yuzhu, WANG Yuan, WANG Linyan, XIANG Meiling, YAN Yuting, LI Benhui, LI Min, WEN Zhidong, WANG Haichao, CHEN Yongfu, QIU Huidong, ZHAO Bo, ZHOU Chengyu. Graphene Oxide-based Adsorbents for Pb(II) Removing in Water: Progresses on Synthesis, Performance and Mechanism [J]. Journal of Inorganic Materials, 2026, 41(1): 12-26. |
| [5] | XU Jintao, GAO Pan, HE Weiyi, JIANG Shengnan, PAN Xiuhong, TANG Meibo, CHEN Kun, LIU Xuechao. Recent Progress on Preparation of 3C-SiC Single Crystal [J]. Journal of Inorganic Materials, 2026, 41(1): 1-11. |
| [6] | YU Shengyang, SU Haijun, JIANG Hao, YU Minghui, YAO Jiatong, YANG Peixin. A Review of Pore Defects in Ultra-high Temperature Oxide Ceramics by Laser Additive Manufacturing: Formation and Suppression [J]. Journal of Inorganic Materials, 2025, 40(9): 944-956. |
| [7] | LIU Jiangping, GUAN Xin, TANG Zhenjie, ZHU Wenjie, LUO Yongming. Research Progress on Catalytic Oxidation of Nitrogen-containing Volatile Organic Compounds [J]. Journal of Inorganic Materials, 2025, 40(9): 933-943. |
| [8] | XIAO Xiaolin, WANG Yuxiang, GU Peiyang, ZHU Zhenrong, SUN Yong. Advances in Regulation of Damaged Skin Regeneration by Two-dimensional Inorganic Materials [J]. Journal of Inorganic Materials, 2025, 40(8): 860-870. |
| [9] | MA Jingge, WU Chengtie. Application of Inorganic Bioceramics in Promoting Hair Follicle Regeneration and Hair Growth [J]. Journal of Inorganic Materials, 2025, 40(8): 901-910. |
| [10] | ZHANG Hongjian, ZHAO Ziyi, WU Chengtie. Inorganic Biomaterials on Regulating Neural Cell Function and Innervated Tissue Regeneration: A Review [J]. Journal of Inorganic Materials, 2025, 40(8): 849-859. |
| [11] | AI Minhui, LEI Bo. Micro-nanoscale Bioactive Glass: Functionalized Design and Angiogenic Skin Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 921-932. |
| [12] | WANG Yutong, CHANG Jiang, XU He, WU Chengtie. Advances in Silicate Bioceramic/Bioglass for Wound Healing: Effects, Mechanisms and Application Ways [J]. Journal of Inorganic Materials, 2025, 40(8): 911-920. |
| [13] | MA Wenping, HAN Yahui, WU Chengtie, LÜ Hongxu. Application of Inorganic Bioactive Materials in Organoid Research [J]. Journal of Inorganic Materials, 2025, 40(8): 888-900. |
| [14] | LUO Xiaomin, QIAO Zhilong, LIU Ying, YANG Chen, CHANG Jiang. Inorganic Bioactive Materials Regulating Myocardial Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 871-887. |
| [15] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||