
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (1): 1-16.DOI: 10.15541/jim20230244
Special Issue: 【生物材料】骨骼与齿类组织修复(202506)
• PERSPECTIVE • Next Articles
ZHENG Jiaqian1,2( ), LU Xiao3,4, LU Yajie3,5, WANG Yingjun1,2, WANG Zhen1,3,5(
), LU Xiao3,4, LU Yajie3,5, WANG Yingjun1,2, WANG Zhen1,3,5( ), LU Jianxi3,4(
), LU Jianxi3,4( )
)
Received:2023-05-20
Revised:2023-06-27
Published:2024-01-20
Online:2023-07-28
Contact:
WANG Zhen, professor. E-mail: wangzhen@fmmu.edu.cn;About author:ZHENG Jiaqian (1994-), male, PhD candidate. E-mail: mszjq2021@mail.scut.edu.cn
Supported by:CLC Number:
ZHENG Jiaqian, LU Xiao, LU Yajie, WANG Yingjun, WANG Zhen, LU Jianxi. Functional Bioadaptability in Medical Bioceramics: Biological Mechanism and Application[J]. Journal of Inorganic Materials, 2024, 39(1): 1-16.
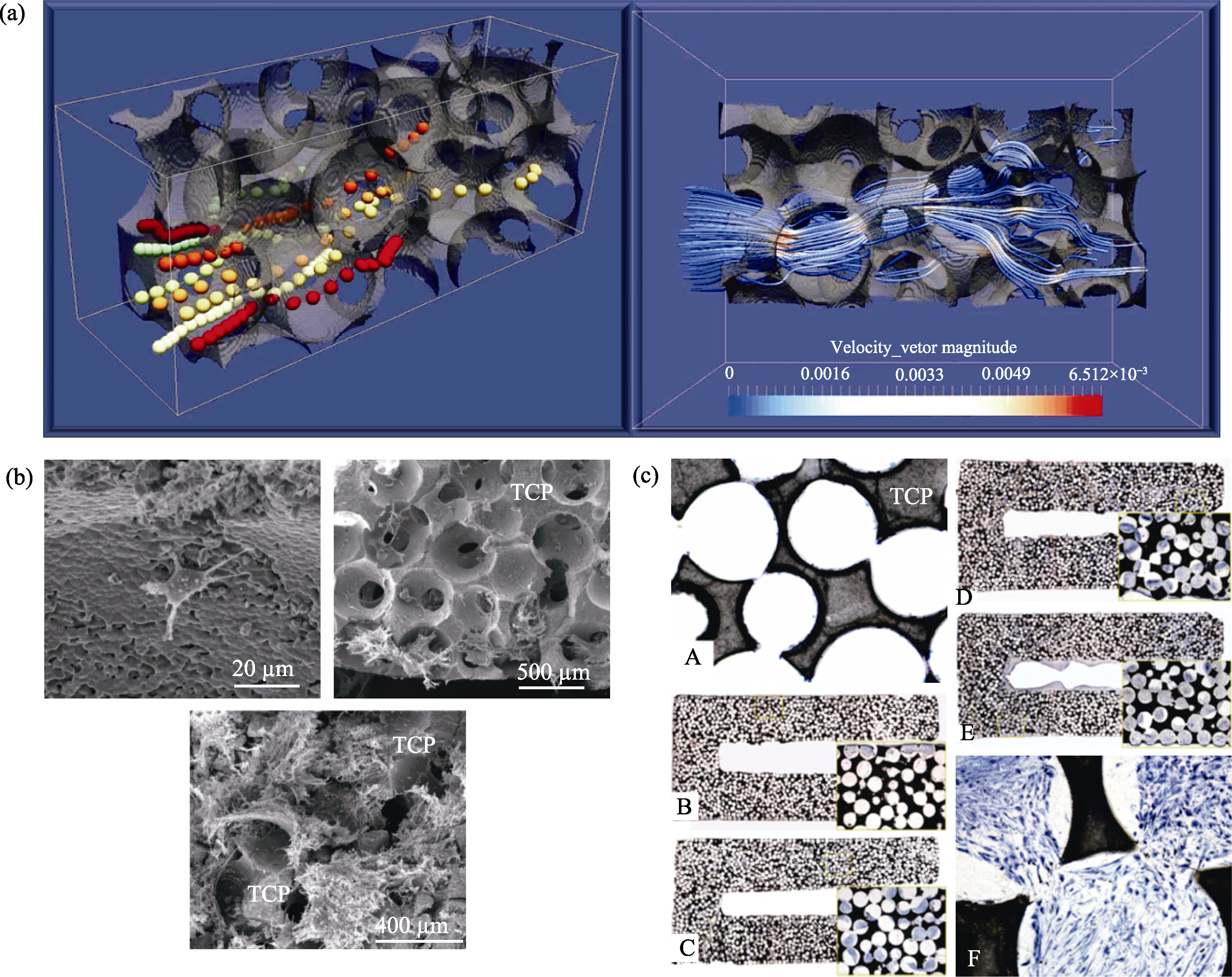
Fig. 2 Effects of microstructure on cell recombination and proliferation[21,27] (a) Cell motility in bioceramic microstructure with three-dimensional flow dynamic culture system, perfusion bioreactor; (b) SEM images of the cross section of a scaffold seeded with sheep MSCs; (c) Histological section of the cell-TCP composite stained with May-Grünwald Giemsa (A-F)

Fig. 3 Different kinds of interconnective pore size β-TCP porous ceramics with the same macroporous size (300-400 μm) evaluated in vivo[31] (a-e) Interconnective pore size is 70, 100, 120, 150, and 200 μm, respectively; (f) The lumen of new blood vessels passing through the interconnection became narrower and larger after entering the hole, resembling a string-of-beads shape. Scale bars in all images are 100 μm
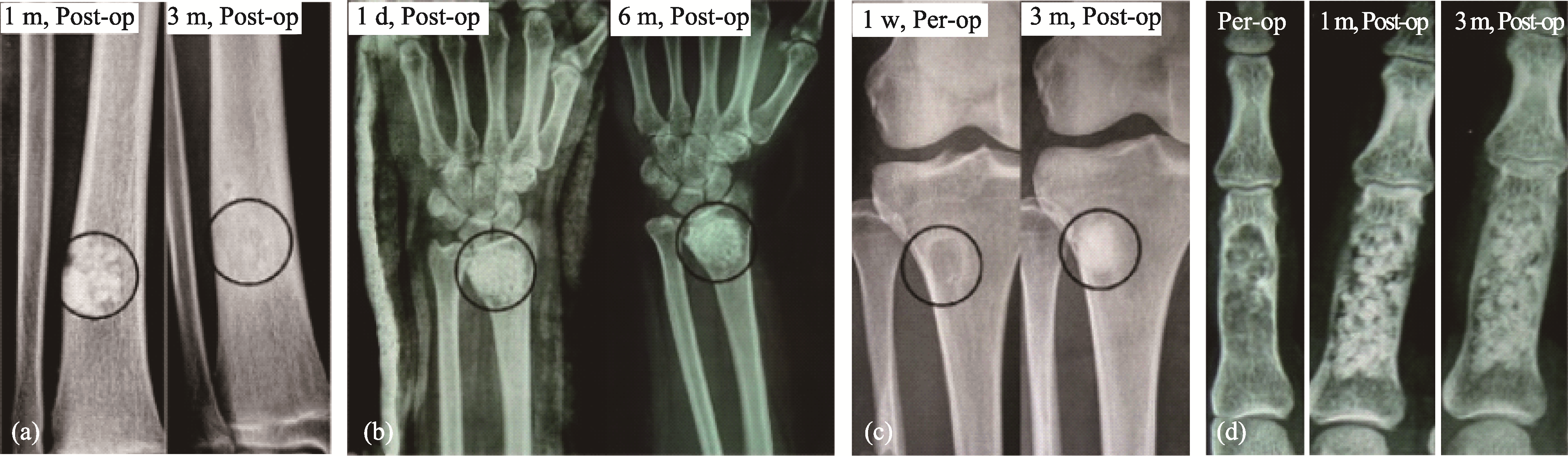
Fig. 5 Effect of packing method on degradation[63] (a, d) Radiographs of cases with loose packing; (b, c) Radiographs of cases implanted with β-TCP granules by dense packing
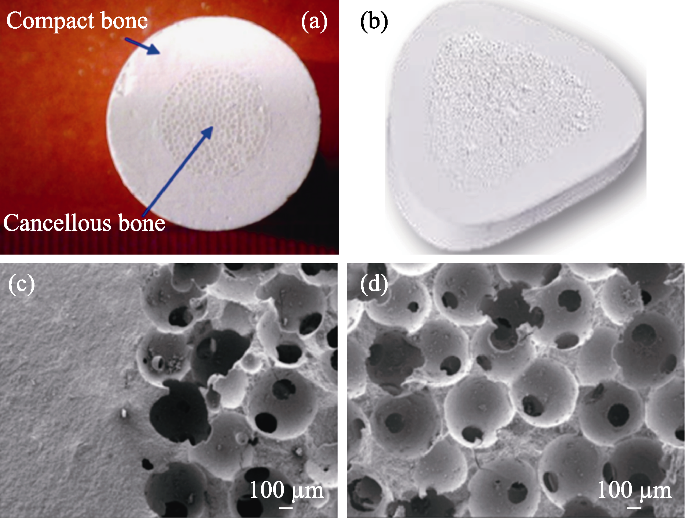
Fig. 6 Mechanically enhanced bioceramics[72] (a) Structure of mechanically enhanced bioceramics; (b) Wedge- shaped implant for high tibial osteotomy (HTO); (c, d) Microstructures of the bioinspired β-TCP bioceramics showing the dense/porous interface (c), and macroporous structure (d)
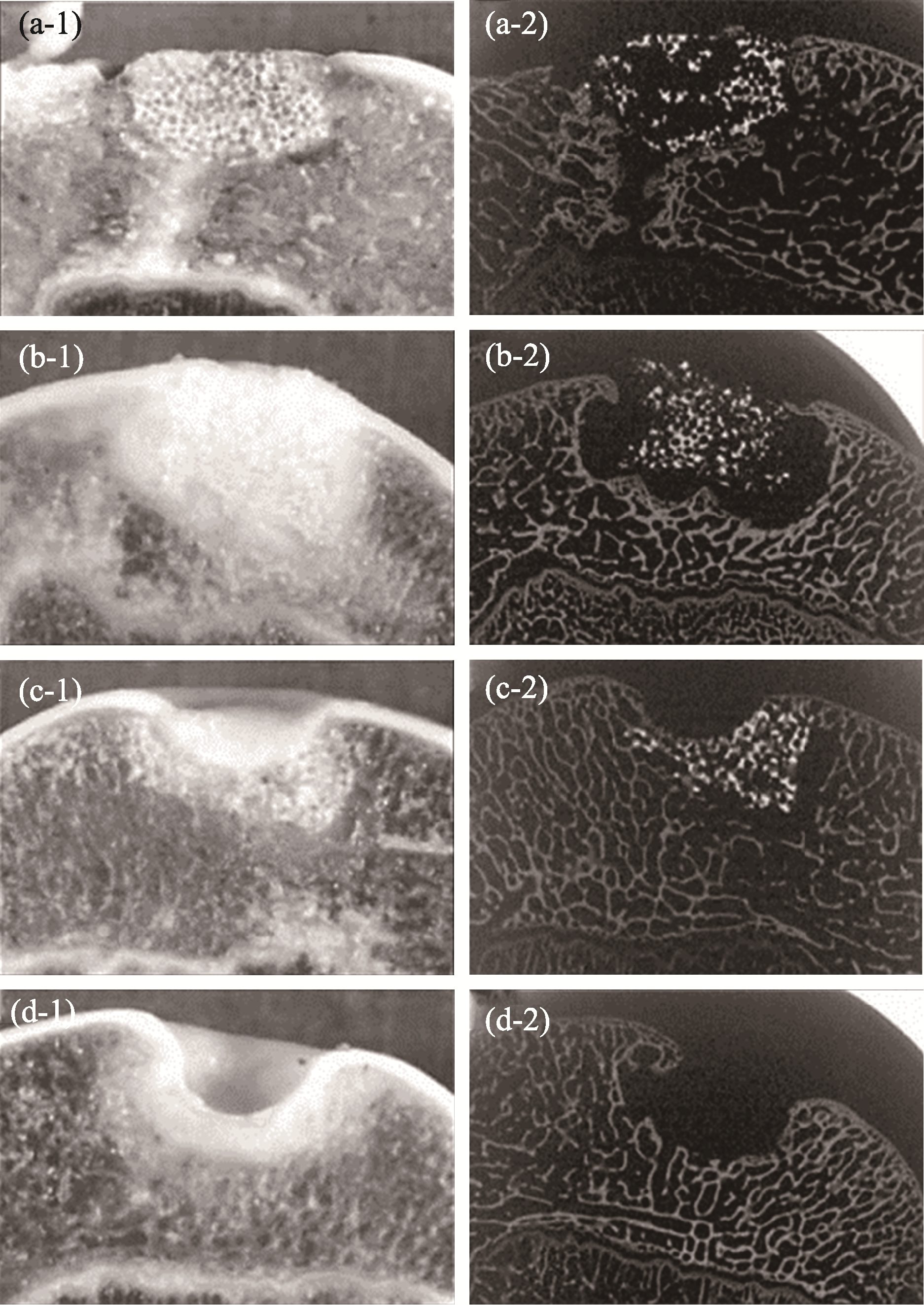
Fig. 10 Bioceramics used in combination with chondrocytes to achieve better cartilage tissue repair[85] (a) Repaired with bioceramic-chondrocyte constructs implant, 2 w postsurgery; (b) Repaired with bioceramic-chondrocyte constructs implant, 24 w postsurgery; (c) Repaired with bioceramic without cells implant, 24 w postsurgery; (d) Defect without any implant (control), 24 w postsurgery
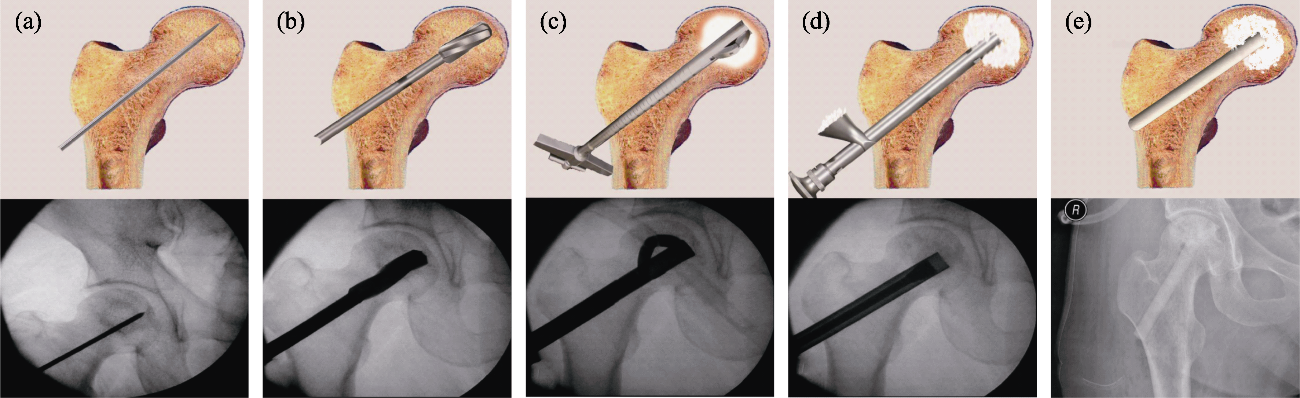
Fig. 11 Standard surgical procedure for ONFH treatment, performed with surgical ancillary instruments[44] (a) Insertion of the Kirschner wire under fluoroscopy; (b) Core decompression by drilling; (c) Necrosis debridement by minimal reamers; (d) Bioceramic granules packing; (e) Insertion of the porous bioceramic rods
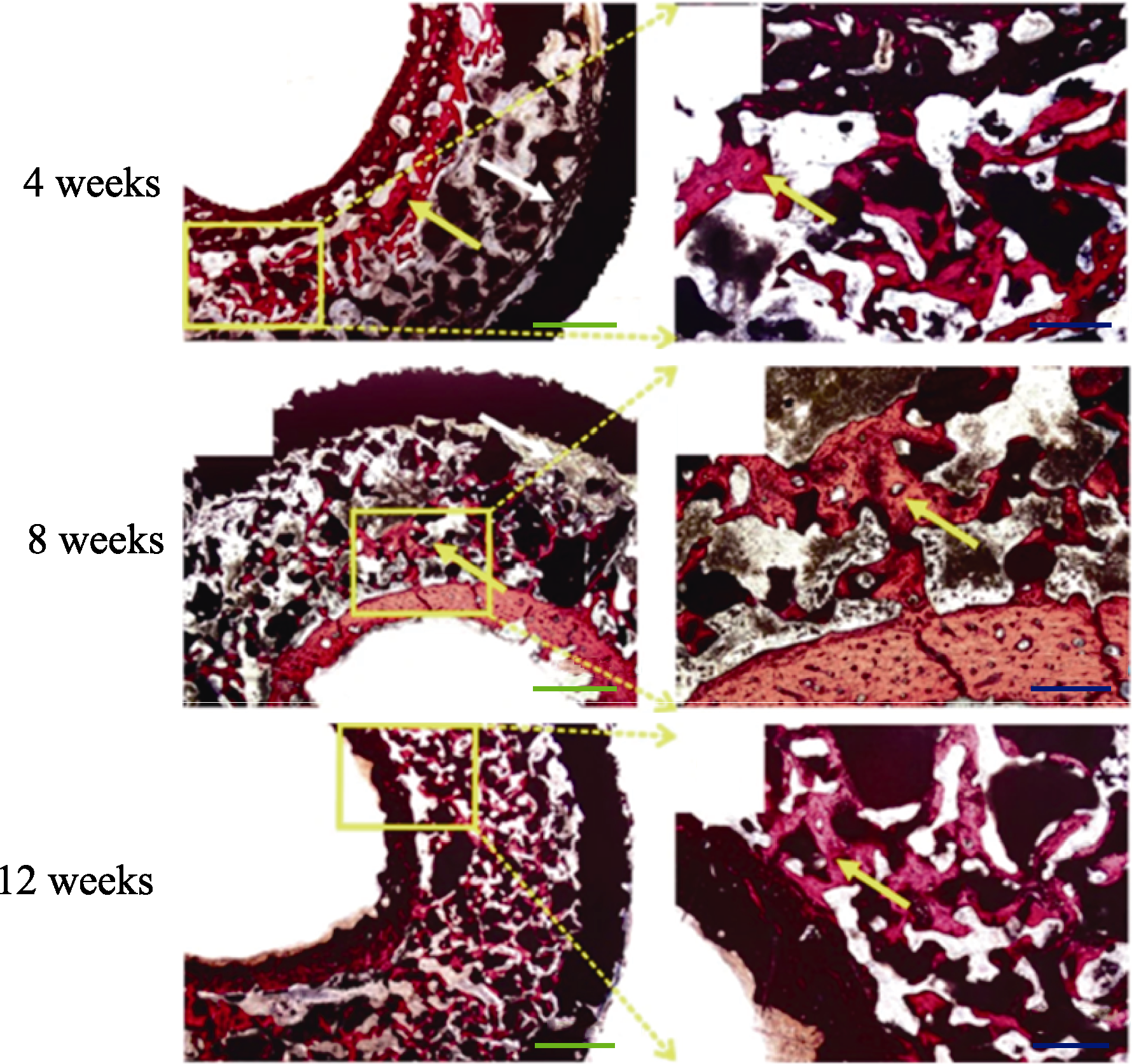
Fig. 12 Van Gieson staining of “In Vivo Bioreactor” in rabbits showed the “Rebar Coagulated Bone” structure[102] Yellow arrow: Newly formed bone; White arrow: Connective. Scale bar: 50 μm (blue), 20 μm (green); Colorful figures are available on website
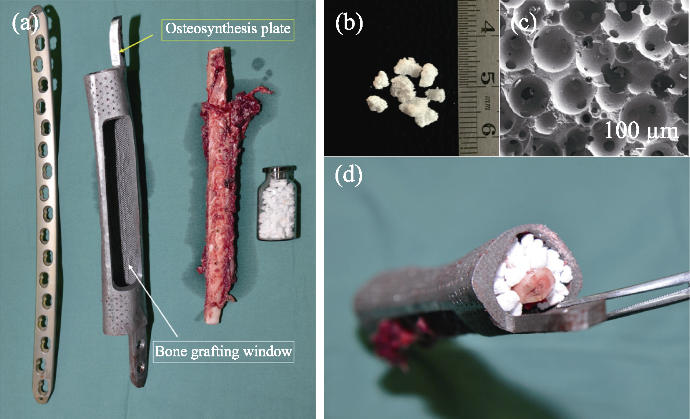
Fig. 13 Application of“In vivo Bioreactor” in operation[77] (a) Multi-material, structural, and technical bone defect repair solution; (b) Bioceramics granules being used; (c) Bioceramics microstructure; (d) Composite in operation
| [1] | LEMONS J E. Ceramics: past, present, and future. Bone, 1996,19(1 Suppl): 121S. |
| [2] | WILLIAMS D F. The plasticity of biocompatibility. Biomaterials, 2023, 296: 122077. |
| [3] |
WILLIAMS D. Revisiting the definition of biocompatibility. Med. Device Technol., 2003, 14(8):10.
PMID |
| [4] |
WANG Y. Bioadaptability: an innovative concept for biomaterials. J. Mater. Sci. Technol., 2016, 32(9):801.
DOI URL |
| [5] |
XU X, JIA Z, ZHENG Y, et al. Bioadaptability of biomaterials: aiming at precision medicine. Matter, 2021, 4(8):2648.
DOI URL |
| [6] |
MASTROGIACOMO M, SCAGLIONE S, MARTINETTI R, et al. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials, 2006, 27(17):3230.
DOI URL |
| [7] |
TUAN W H, CHEN Y C, HSU P Y, et al. Microstructure design for bone void filler. Int. J. Appl. Ceram. Technol., 2022, 20(2):869.
DOI URL |
| [8] | DIAO J, OUYANG J, DENG T, et al. 3D-plotted beta-tricalcium phosphate scaffolds with smaller pore sizes improve in vivo bone regeneration and biomechanical properties in a critical-sized calvarial defect rat model. Adv. Healthc. Mater., 2018, 7(17):e1800441. |
| [9] | LI M, FU X, GAO H, et al. Regulation of an osteon-like concentric microgrooved surface on osteogenesis and osteoclastogenesis. Biomaterials, 2019, 216: 119269. |
| [10] |
XU D, WAN Y, LI Z, et al. Tailorable hierarchical structures of biomimetic hydroxyapatite micro/nano particles promoting endocytosis and osteogenic differentiation of stem cells. Biomater. Sci., 2020, 8(12):3286.
DOI URL |
| [11] |
WEI Y, GAO H, HAO L, et al. Constructing a Sr2+-substituted surface hydroxyapatite hexagon-like microarray on 3D-plotted hydroxyapatite scaffold to regulate osteogenic differentiation. Nanomaterials (Basel), 2020, 10(9):1672.
DOI URL |
| [12] | LIU X, ZHAO N, LIANG H, et al. Bone tissue engineering scaffolds with HUVECs/hBMSCs cocultured on 3D-printed composite bioactive ceramic scaffolds promoted osteogenesis/ angiogenesis. J. Orthop. Translat., 2022, 37: 152. |
| [13] | LU Q, DIAO J, WANG Y, et al. 3D printed pore morphology mediates bone marrow stem cell behaviors via RhoA/ROCK2 signaling pathway for accelerating bone regeneration. Bioact. Mater., 2023, 26: 413. |
| [14] | DIAO J J, DING H W, HUANG M Q, et al. Bone defect model dependent optimal pore sizes of 3D-plotted beta-tricalcium phosphate scaffolds for bone regeneration. Small Methods, 2019, 3(11):11. |
| [15] | SONG C, LIU L, DENG Z, et al. Research progress on the design and performance of porous titanium alloy bone implants. J. Mater. Res. Technol., 2023, 23: 2626. |
| [16] |
LIN K, CHEN L, QU H, et al. Improvement of mechanical properties of macroporous β-tricalcium phosphate bioceramic scaffolds with uniform and interconnected pore structures. Ceram. Int., 2011, 37(7):2397.
DOI URL |
| [17] | LU J, WANG Z. Microstructure of bioceramics:biological effects and clinical application. Shanghai: Shanghai Science and Technology Press, 2020: 26. |
| [18] |
MOHAMMADI H, SEPANTAFAR M, MUHAMAD N, et al. How does scaffold porosity conduct bone tissue regeneration. Adv. Eng. Mater., 2021, 23(10):2100463.
DOI URL |
| [19] |
LU J X, FLAUTRE B, ANSELME K, et al. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J. Mater. Sci. Mater. Med., 1999, 10(2):111.
DOI URL |
| [20] |
CHA J M, HWANG Y S, KANG D K, et al. Development of a novel perfusion rotating wall vessel bioreactor with ultrasound stimulation for mass-production of mineralized tissue constructs. Tissue Eng. Regen. Med., 2022, 19(4):739.
DOI PMID |
| [21] |
XIE Y, HARDOUIN P, ZHU Z, et al. Three-dimensional flow perfusion culture system for stem cell proliferation inside the critical-size beta-tricalcium phosphate scaffold. Tissue Eng., 2006, 12(12):3535.
PMID |
| [22] | XIE Y, ZHU Z, TANG T, et al. Using perfusion bioreactor for mesenchymal stem cell proliferation in large tricalcium phosphate scaffold. Chinese J. Orthop., 2006, 86(23):1633. |
| [23] | FORRESTAL D P, ALLENBY M C, SIMPSON B, et al. Personalized volumetric tissue generation by enhancing multiscale mass transport through 3D printed scaffolds in perfused bioreactors. Adv. Healthc. Mater., 2022, 11(24):e2200454. |
| [24] |
ENGEL N, FECHNER C, VOGES A, et al. An optimized 3D-printed perfusion bioreactor for homogeneous cell seeding in bone substitute scaffolds for future chairside applications. Sci. Rep., 2021, 11(1):22228.
DOI PMID |
| [25] |
DING M, KOROMA K E, WENDT D, et al. Efficacy of bioreactor-activated bone substitute with bone marrow nuclear cells on fusion rate and fusion mass microarchitecture in sheep. J. Biomed. Mater. Res. B Appl. Biomater., 2022, 110(8): 1862.
DOI URL |
| [26] |
KAZIMIERCZAK P, PRZEKORA A. Bioengineered living bone grafts: a concise review on bioreactors and production techniques in vitro. Int. J. Mol. Sci., 2022, 23(3):1765.
DOI URL |
| [27] | ZHANG Z, DU J, WEI Z, et al. Numerical simulation of dynamic seeding of mesenchymal stem cells in pore structure. Comput. & Mathemat. Appl., 2020, 79(1):88. |
| [28] | KUBOKI Y, JIN Q M, TAKITA H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. Bone and Joint Surg.-Am., 2001, 83A: S105. |
| [29] | MAHAPATRA C, KUMAR P, PAUL M K, et al. Angiogenic stimulation strategies in bone tissue regeneration. Tissue Cell, 2022, 79: 101908. |
| [30] |
GUERRERO J, MAEVSKAIA E, GHAYOR C, et al. Influence of scaffold microarchitecture on angiogenesis and regulation of cell differentiation during the early phase of bone healing: a transcriptomics and histological analysis. Int. J. Mol. Sci., 2023, 24(6):6000.
DOI URL |
| [31] |
BAI F, WANG Z, LU J, et al. The correlation between the internal structure and vascularization of controllable porous bioceramic materials in vivo: a quantitative study. Tissue Eng. Part A, 2010, 16(12):3791.
DOI URL |
| [32] |
CHOI S W, ZHANG Y, MACEWAN M R, et al. Neovascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes. Adv. Healthc. Mater., 2013, 2(1):145.
DOI URL |
| [33] | SHEN M, LI Y, LU F, et al. Bioceramic scaffolds with triply periodic minimal surface architectures guide early-stage bone regeneration. Bioact. Mater., 2023, 25: 374. |
| [34] |
LUTZWEILER G, NDREU HALILI A, ENGIN VRANA N. The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics, 2020, 12(7):602.
DOI URL |
| [35] | XIAO X, WANG W, LIU D, et al. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep., 2015, 5: 9409. |
| [36] |
NAMKOONG S, LEE S J, KIM C K, et al. Prostaglandin E2 stimulates angiogenesis by activating the nitric oxide/cGMP pathway in human umbilical vein endothelial cells. Exp. Mol. Med., 2005, 37(6):588.
DOI PMID |
| [37] |
DULAK J, JOZKOWICZ A. Nitric oxide and angiogenic activity of endothelial cells: direct or VEGF-dependent effect. Cardiovasc. Res., 2002, 56(3):489.
DOI URL |
| [38] |
LI J, HUANG H, XU T, et al. Effect of the interconnecting window diameter of hydroxyapatite scaffolds on vascularization and osteoinduction. Ceram. Int., 2022, 48(17):25070.
DOI URL |
| [39] | LU Y J, WANG Z, LU X, et al. Minimally invasive treatment for osteonecrosis of the femoral head in ARCO stage Ⅱ and Ⅲ with bioceramic system. Chin. J. Repar. Reconst. Surgery, 2019, 33(10):1291. |
| [40] |
MURAYAMA A, AJIKI T, HAYASHI Y, et al. A unidirectional porous beta-tricalcium phosphate promotes angiogenesis in a vascularized pedicle rat model. J. Orthop. Sci., 2019, 24(6):1118.
DOI PMID |
| [41] | KUNISADA T, HASEI J, FUJIWARA T, et al. Radiographic and clinical assessment of unidirectional porous hydroxyapatite to treat benign bone tumors. Sci. Rep., 2020, 10: 21578. |
| [42] |
KUMAGAI H, MAKIHARA T, FUNAYAMA T, et al. Angiogenesis and new bone formation in novel unidirectional porous beta-tricalcium phosphate: a histological study. J. Artif. Organs., 2019, 22(4):294.
DOI PMID |
| [43] | IKUTA K, NISHIDA Y, OTA T, et al. A clinical trial of a unidirectional porous tricalcium phosphate filling for defects after resection of benign bone lesions: a prospective multicenter study. Sci. Rep., 2022, 12: 16060. |
| [44] |
LU Y, LU X, LI M, et al. Minimally invasive treatment for osteonecrosis of the femoral head with angioconductive bioceramic rod. Int. Orthop., 2018, 42(7):1567.
DOI PMID |
| [45] |
LU J, DESCAMPS M, DEJOU J, et al. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res., 2002, 63(4):408.
DOI URL |
| [46] | ZHANG Y, SHU T, WANG S, et al. The osteoinductivity of calcium phosphate-based biomaterials: a tight interaction with bone healing. Front Bioeng. Biotechnol., 2022, 10: 911180. |
| [47] | STASTNY P, SEDLACEK R, SUCHY T, et al. Structure degradation and strength changes of sintered calcium phosphate bone scaffolds with different phase structures during simulated biodegradation in vitro. Mater. Sci. Eng.: C, 2019, 100: 544. |
| [48] |
CHAI Y C, CARLIER A, BOLANDER J, et al. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater., 2012, 8(11):3876.
DOI PMID |
| [49] |
SANTOS C P G, PRADO J P S, FERNANDES K R, et al. Different species of marine sponges diverge in osteogenic potential when therapeutically applied as natural scaffolds for bone regeneration in rats. J. Funct. Biomater., 2023, 14(3):112.
DOI URL |
| [50] |
AVERIANOV I, STEPANOVA M, SOLOMAKHA O, et al. 3D-printed composite scaffolds based on poly(ε-caprolactone) filled with poly(glutamic acid)‐modified cellulose nanocrystals for improved bone tissue regeneration. J. Biomed. Mater. Res. B: Appl. Biomater., 2022, 110(11):2422.
DOI URL |
| [51] |
CHENG X, YANG X, LIU C, et al. Stabilization of apatite coatings on PPENK surfaces by mechanical interlocking to promote bioactivity and osseointegration in vivo. ACS Appl. Mater. Interf., 2023, 15(1):697.
DOI URL |
| [52] |
KUMAR R, PATTANAYAK I, DASH P A, et al. Bioceramics: a review on design concepts toward tailor-made (multi)-functional materials for tissue engineering applications. J. Mater. Sci., 2023, 58(8):3460.
DOI |
| [53] | TAJVAR S, HADJIZADEH A, SAMANDARI S S. Scaffold degradation in bone tissue engineering: an overview. Int. Biodeter. & Biodegr. 2023, 180:105599. |
| [54] | BOHNER M, SANTONI B L G, DOBELIN N. Beta-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater., 2020, 113: 23. |
| [55] |
LU J X, BLARY M C, VAVASSEUR S, et al. Relationship between bioceramics sintering and micro-particles-induced cellular damages. J. Mater. Sci. Mater. Med., 2004, 15(4):361.
DOI URL |
| [56] |
SHEIKH Z, ABDALLAH M N, HANAFI A A, et al. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials (Basel), 2015, 8(11):7913.
DOI URL |
| [57] |
SIMON J L, ROY T D, PARSONS J R, et al. Engineered cellular response to scaffold architecture in a rabbit trephine defect. J. Biomed. Mater. Res. A, 2003, 66(2):275.
PMID |
| [58] |
LU J X, GALLUR A, FLAUTRE B, et al. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J. Biomed. Mater. Res., 1998, 42(3):357.
DOI URL |
| [59] |
GALOIS L, MAINARD D, DELAGOUTTE J P. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int. Orthop., 2002, 26(2):109.
DOI PMID |
| [60] |
WANG Z, LU B, CHEN L, et al. Evaluation of an osteostimulative putty in the sheep spine. J. Mater. Sci. Mater. Med., 2011, 22(1):185.
DOI URL |
| [61] | ZHI W, WANG X, SUN D, et al. Optimal regenerative repair of large segmental bone defect in a goat model with osteoinductive calcium phosphate bioceramic implants. Bioact. Mater., 2022, 11: 240. |
| [62] |
HAMPP C, ANGRISANI N, REIFENRATH J, et al. Evaluation of the biocompatibility of two magnesium alloys as degradable implant materials in comparison to titanium as non-resorbable material in the rabbit. Mater. Sci. Eng. C Mater. Biol. Appl., 2013, 33(1):317.
DOI URL |
| [63] |
WANG Z, GUO Z, BAI H, et al. Clinical evaluation of beta-TCP in the treatment of lacunar bone defects: a prospective, randomized controlled study. Mater. Sci. Eng. C Mater. Biol. Appl., 2013, 33(4): 1894.
DOI URL |
| [64] |
DEHOUX E, MADI K, FOURATI E, et al. High tibial open-wedge osteotomy using a tricalcium phosphate substitute: 70 cases with 18 months mean follow-up. Rev. Chir. Orthop. Reparatrice. Appar. Mot., 2005, 91(2):143.
DOI URL |
| [65] |
HERNIGOU P, ROUSSIGNOL X, FLOUZAT-LACHANIETTE C H, et al. Opening wedge tibial osteotomy for large varus deformity with ceraver resorbable beta-tricalcium phosphate wedges. Int. Orthop., 2010, 34(2):191.
DOI URL |
| [66] |
KRAAL T, MULLENDER M, DE BRUINE J H, et al. Resorbability of rigid beta-tricalcium phosphate wedges in open-wedge high tibial osteotomy: a retrospective radiological study. Knee, 2008, 15(3):201.
DOI PMID |
| [67] | TANAKA T, KUMAGAE Y, SAITO M, et al. Bone formation and resorption in patients after implantation of beta-tricalcium phosphate blocks with 60% and 75% porosity in opening-wedge high tibial osteotomy. J. Biomed. Mater. Res. B Appl. Biomater., 2008, 86(2):453. |
| [68] | OGOSE A, HOTTA T, KAWASHIMA H, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J. Biomed. Mater. Res. B Appl. Biomater., 2005, 72(1):94. |
| [69] |
HIRATA M, MURATA H, TAKESHITA H, et al. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int. Orthop., 2006, 30(6):510.
DOI PMID |
| [70] |
JAVAHERI B, PITSILLIDES A A. Aging and mechanoadaptive responsiveness of bone. Curr. Osteoporos. Rep., 2019, 17(6):560.
DOI PMID |
| [71] | ZHANG Y, ZHANG Q, HE F, et al. Fabrication of cancellous- bone-mimicking β-tricalcium phosphate bioceramic scaffolds with tunable architecture and mechanical strength by stereolithography 3D printing. J. Europ. Ceram. Soci., 2022, 42(14):6713. |
| [72] |
ZHANG F, CHANG J, LU J, et al. Bioinspired structure of bioceramics for bone regeneration in load-bearing sites. Acta Biomater., 2007, 3(6):896.
DOI PMID |
| [73] |
LIN KAILI, WANG XIAOHONG, STEVE G S, et al. Research progress on functional modifications and applications of bioceramic scaffolds. J. Inorg. Mater., 2020, 35(8):867.
DOI |
| [74] |
MIAO X, SUN D. Graded/gradient porous biomaterials. Materials, 2009, 3(1):26.
DOI URL |
| [75] |
HENDERSON E R, GROUNDLAND J S, PALA E, et al. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J. Bone Joint. Surg. Am., 2011, 93(5):418.
DOI PMID |
| [76] |
KENDAL J K, HAMAD C D, ABBOTT A G, et al. What are the indications and survivorship of tumor endoprosthetic reconstructions for patients with extremity metastatic bone disease. J. Surg. Oncol., 2023, 127(7):1196.
DOI URL |
| [77] | LU Y, CHEN G, LONG Z, et al. Novel 3D-printed prosthetic composite for reconstruction of massive bone defects in lower extremities after malignant tumor resection. J. Bone Oncol., 2019, 16: 100220. |
| [78] | KELLY C N, WANG T, CROWLEY J, et al. High-strength, porous additively manufactured implants with optimized mechanical osseointegration. Biomaterials, 2021, 279: 121206. |
| [79] |
HAIJIE L, DASEN L, TAO J, et al. Implant survival and complication profiles of endoprostheses for treating tumor around the knee in adults: a systematic review of the literature over the past 30 years. J. Arthroplasty, 2018, 33(4):1275.
DOI PMID |
| [80] |
VIJAYAVENKATARAMAN S, GOPINATH A, LU W F. A new design of 3D-printed orthopedic bone plates with auxetic structures to mitigate stress shielding and improve intra-operative bending. Bio-Design Manufact., 2020, 3(2):98.
DOI |
| [81] | POBLOTH A M, CHECA S, RAZI H, et al. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med., 2018, 10(423):8828. |
| [82] |
WU N, LI S, ZHANG B, et al. The advances of topology optimization techniques in orthopedic implants: a review. Med. Biol. Eng. Comput., 2021, 59(9):1673.
DOI PMID |
| [83] |
SAFAVI S, YU Y, ROBINSON D L, et al. Additively manufactured controlled porous orthopedic joint replacement designs to reduce bone stress shielding: a systematic review. J. Orthop. Surg. Res., 2023, 18(1):42.
DOI PMID |
| [84] | YUAN J, WANG B, HAN C, et al. Nanosized-Ag-doped porous beta-tricalcium phosphate for biological applications. Mater. Sci. Eng. C Mater. Biol. Appl., 2020, 114: 111037. |
| [85] |
YUAN J, WANG B, HAN C, et al. In vitro comparison of three rifampicin loading methods in a reinforced porous beta-tricalcium phosphate scaffold. J. Mater. Sci. Mater. Med., 2015, 26(4):174.
DOI URL |
| [86] |
GUO X M, WANG C Y, DUAN C M, et al. Repair of osteochondral defects with autologous chondrocytes seeded onto bioceramic scaffold in sheep. Tissue Eng., 2004, 10(11/12): 1830.
DOI PMID |
| [87] |
GUO X M, WANG C Y, ZHANG Y F, et al. Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into beta-tricalcium phosphate in a sheep model. Tissue Eng., 2004, 10(11/12): 1818.
DOI URL |
| [88] |
LI D, LI M, LIU P, et al. Tissue-engineered bone constructed in a bioreactor for repairing critical-sized bone defects in sheep. Int. Orthop., 2014, 38(11):2399.
DOI PMID |
| [89] |
SPONER P, KUCERA T, BRTKOVA J, et al. Comparative study on the application of mesenchymal stromal cells combined with tricalcium phosphate scaffold into femoral bone defects. Cell Transplant., 2018, 27(10):1459.
DOI PMID |
| [90] |
FATALE V, PAGNONI S, PAGNONI A E, et al. Histomorphometric comparison of new bone formed after maxillary sinus lift with lateral and crestal approaches using periostal mesenchymal stem cells and beta-tricalcium phosphate: a controlled clinical trial. J. Craniofac. Surg., 2022, 33(5):1607.
DOI PMID |
| [91] |
CHU W, WANG X, GAN Y, et al. Screen-enrich-combine circulating system to prepare MSC/beta-TCP for bone repair in fractures with depressed tibial plateau. Regen. Med., 2019, 14(6):555.
DOI URL |
| [92] |
BLANCO J F, VILLARON E M, PESCADOR D, et al. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: results of a prospective phase I/II clinical trial with long-term follow-up. Stem Cell Res. Ther., 2019, 10(1):63.
DOI PMID |
| [93] |
LYU J, MA T, HUANG X, et al. Core decompression with β-tri-calcium phosphate grafts in combination with platelet-rich plasma for the treatment of avascular necrosis of femoral head. BMC Musculoskelet. Disord., 2023, 24(1):40.
DOI |
| [94] | GAO Y, CHENG J, LONG Z, et al. Repair of segmental ulnar bone defect in juvenile caused by osteomyelitis with induced membrane combined with tissue-engineered bone:a case report with 4-year follow-up. Int. J. Surg. Case Rep., 2022, 99: 107569. |
| [95] |
GUPTA S, MALHOTRA A, JINDAL R, et al. Role of beta tri-calcium phosphate-based composite ceramic as bone-graft expander in masquelet’s-induced membrane technique. Indian J. Orthop., 2019, 53(1):63.
DOI |
| [96] |
SASAKI G, WATANABE Y, MIYAMOTO W, et al. Induced membrane technique using beta-tricalcium phosphate for reconstruction of femoral and tibial segmental bone loss due to infection: technical tips and preliminary clinical results. Int. Orthop., 2018, 42(1):17.
DOI PMID |
| [97] |
RAVEN T F, MOGHADDAM A, ERMISCH C, et al. Use of Masquelet technique in treatment of septic and atrophic fracture nonunion. Injury, 2019, 50(Suppl 3):40.
DOI URL |
| [98] | JUN F, ZHENG G, HONGBIN F, et al. Aplication of 3D-printed prosthesis on construction of long segmental bone defect after tumor resection. Chin. J. Orthop., 2017, 37(7):433. |
| [99] |
HU X, KENAN S, CHENG M, et al. 3D-printed patient-customized artificial vertebral body for spinal reconstruction after total En Bloc spondylectomy of complex multi-level spinal tumors. Int. J. Bioprint., 2022, 8(3):576.
DOI URL |
| [100] | BROWN T S, SALIB C G, ROSE P S, et al. Reconstruction of the hip after resection of periacetabular oncological lesions: a systematic review. Bone Joint J., 2018, 100-B(1 Supple A): 22. |
| [101] | THOMAS D, SINGH D. 3D-printing for engineering the next generation of artificial trabecular bone structures. Int. J. Surg., 2017, 46: 195. |
| [102] | GAO P, ZHANG H, LIU Y, et al. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci. Rep., 2016, 6: 23367. |
| [1] | DENG Shungui, ZHANG Chuanfang. MXene Multifunctional Inks: a New Perspective toward Printable Energy-related Electronic Devices [J]. Journal of Inorganic Materials, 2024, 39(2): 195-203. |
| [2] | LIU Yanyan, XIE Xi, LIU Zengqian, ZHANG Zhefeng. Metal Matrix Composites Reinforced by MAX Phase Ceramics: Fabrication, Properties and Bioinspired Designs [J]. Journal of Inorganic Materials, 2024, 39(2): 145-152. |
| [3] | YIN Jianyu, LIU Nishuang, GAO Yihua. Recent Progress of MXene in Pressure Sensing [J]. Journal of Inorganic Materials, 2024, 39(2): 179-185. |
| [4] | BA Kun, WANG Jianlu, HAN Meikang. Perspectives for Infrared Properties and Applications of MXene [J]. Journal of Inorganic Materials, 2024, 39(2): 162-170. |
| [5] | LI La, SHEN Guozhen. 2D MXenes Based Flexible Photodetectors: Progress and Prospects [J]. Journal of Inorganic Materials, 2024, 39(2): 186-194. |
| [6] | XU Xiangming, Husam N ALSHAREEF. Perspective of MXetronics [J]. Journal of Inorganic Materials, 2024, 39(2): 171-178. |
| [7] | LI Lei, CHENG Qunfeng. Recent Advances in the High Performance MXenes Nanocomposites [J]. Journal of Inorganic Materials, 2024, 39(2): 153-161. |
| [8] | CHEN Ze, ZHI Chunyi. MXene Based Zinc Ion Batteries: Recent Development and Prospects [J]. Journal of Inorganic Materials, 2024, 39(2): 204-214. |
| [9] | DING Haoming, CHEN Ke, LI Mian, LI Youbing, CHAI Zhifang, HUANG Qing. Chemical Scissor-mediated Structural Editing of Inorganic Materials [J]. Journal of Inorganic Materials, 2024, 39(2): 115-128. |
| [10] | WAN Hujie, XIAO Xu. Terahertz Electromagnetic Shielding and Absorbing of MXenes and Their Composites [J]. Journal of Inorganic Materials, 2024, 39(2): 129-144. |
| [11] | FEI Ling, LEI Lei, WANG Degao. Progress of Two-dimensional MXene in New-type Thin-film Solar Cells [J]. Journal of Inorganic Materials, 2024, 39(2): 215-224. |
| [12] | CHEN Mengjie, WANG Qianqian, WU Chengtie, HUANG Jian. Predicting the Degradability of Bioceramics through a DFT-based Descriptor [J]. Journal of Inorganic Materials, 2024, 39(10): 1175-1181. |
| [13] | TAO Shunyan, YANG Jiasheng, SHAO Fang, WU Yingchen, ZHAO Huayu, DONG Shaoming, ZHANG Xiangyu, XIONG Ying. Thermal Spray Coatings for Aircraft CMC Hot-end Components: Opportunities and Challenges [J]. Journal of Inorganic Materials, 2024, 39(10): 1077-1083. |
| [14] | SHI Zhe, LIU Weiye, ZHAI Dong, XIE Jianjun, ZHU Yufang. Akermanite Scaffolds for Bone Tissue Engineering: 3D Printing Using Polymer Precursor and Scaffold Properties [J]. Journal of Inorganic Materials, 2023, 38(7): 763-770. |
| [15] | XIAO Wen, LIU Yu-Mei, REN Kai-Ge, SHI Feng, LI Yan, ZHI Wei, WENG Jie, QU Shu-Xin. Evaluation of Vascularization of Porous Calcium Phosphate by Chick Chorioallantoic Membrane Model ex vivo [J]. Journal of Inorganic Materials, 2017, 32(6): 649-654. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||