
Journal of Inorganic Materials ›› 2023, Vol. 38 ›› Issue (11): 1245-1256.DOI: 10.15541/jim20230117
Special Issue: 【能源环境】污染物催化去除(202506)
• REVIEW • Next Articles
SUN Chen1,2( ), ZHAO Kunfeng2(
), ZHAO Kunfeng2( ), YI Zhiguo1,2(
), YI Zhiguo1,2( )
)
Received:2023-01-27
Revised:2023-04-25
Published:2023-11-20
Online:2023-05-04
Contact:
YI Zhiguo, professor. E-mail: zhiguo@mail.sic.ac.cn;About author:SUN Chen (1996-), male, Master candidate. E-mail: 895029730@qq.com
Supported by:CLC Number:
SUN Chen, ZHAO Kunfeng, YI Zhiguo. Research Progress in Catalytic Total Oxidation of Methane[J]. Journal of Inorganic Materials, 2023, 38(11): 1245-1256.
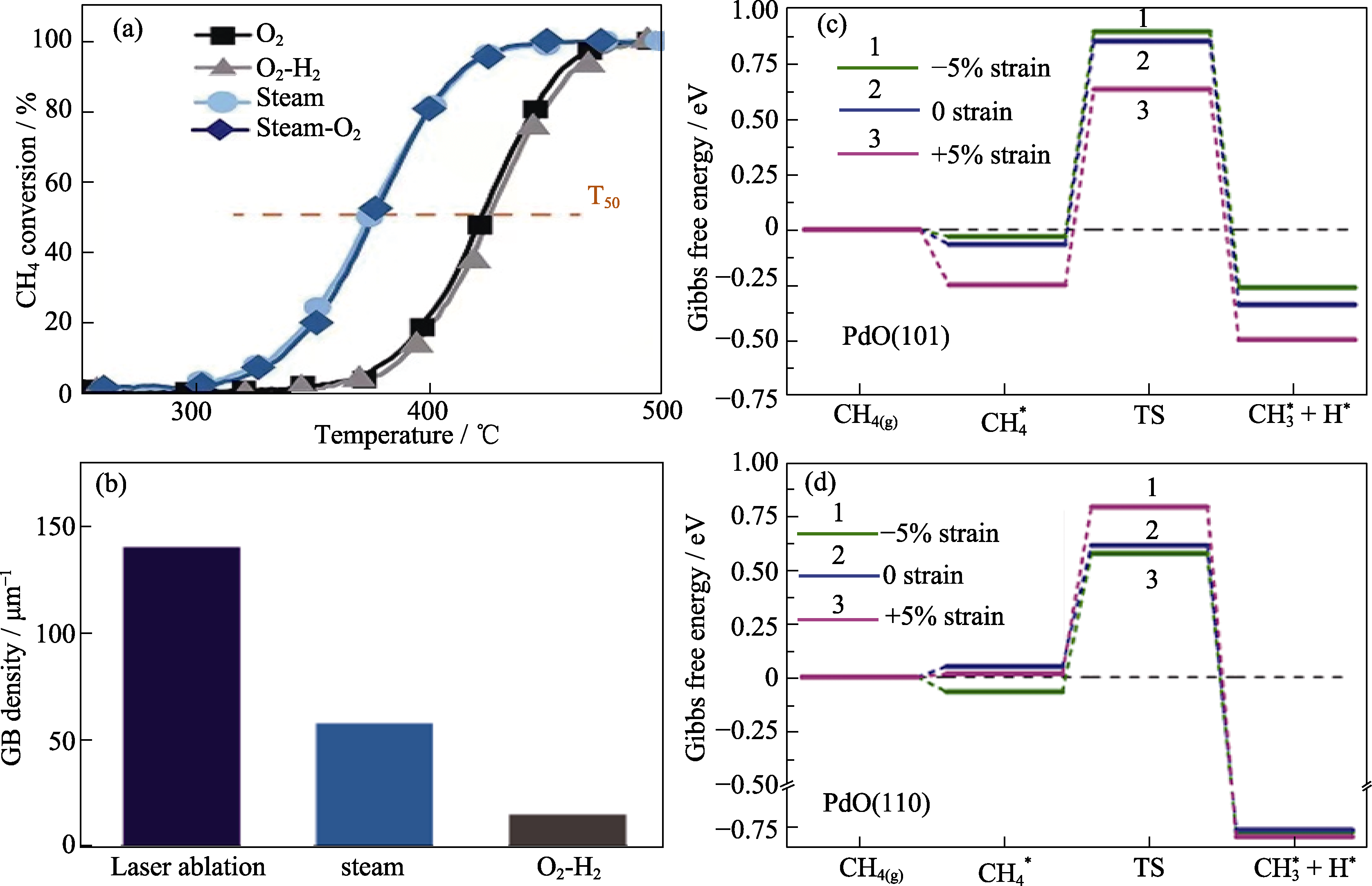
Fig. 2 Performance tests and theoretical calculations of catalysts[28] (a) Light-off curves and T50 values of Pd/Al2O3 after O2 (600 ℃), O2-H2, steam (600 ℃), and steam-O2 pretreatments; (b) GB density statistical histogram of laser-generated Pd/Al2O3 and Pd/Al2O3 after steam (600 ℃) and O2-H2 pretreatments; (c, d) Calculated free energy diagrams for breaking the first C-H bond in CH4 on PdO(101) and PdO(110), respectively. Reprinted from Ref. [28] with permission, Copyright 2021 AAAS
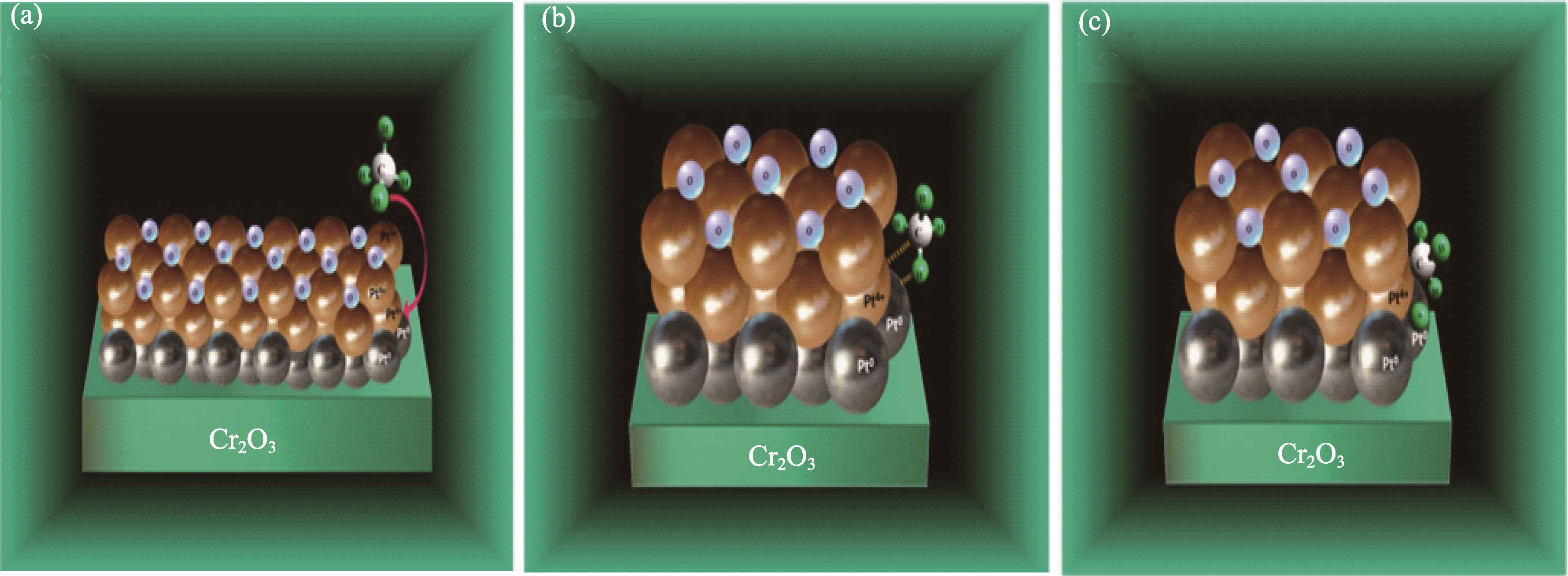
Fig. 3 Proposed model for the CH4 dissociative adsorption over Pt0−Pt4+ dipoles saturated with chemisorbtion oxygen atoms[30] (a) Reactants: CH4 in the gas phase and 1% Pt/Cr2O3; (b) CH4 polarization by Pt0−Pt4+ site and formation of a transition state; (c) Abstraction of the first hydrogen on the adsorbed CH4 molecule
| Catalyst | Tc* /℃ | Ea/(kJ·mol-1) | Feed gas | GHSV/(mL·g-1·h-1) | Stability | Ref. |
|---|---|---|---|---|---|---|
| Pd-Ce@SiO2 | T100=350 | 100.4 | 1% CH4, 21% O2, bal. N2 | 36000 | 25 h | [ |
| Pd/TiO2 | T99=370 | 83.1 | 1% CH4, 10% O2, bal. N2 | 30000 | 4 cycles | [ |
| Pd/Na-MOR | T50=335 | 75 | 1% CH4, 4% O2, bal. N2 | 70000 | 90 h | [ |
| Pd-Pt/CeO2 | T50=325 | 74 | 680 μg/mL CH4, 14% O2, 5% CO2, bal. N2 | 300000 | 12 h# | [ |
| Au/Al2O3 | T50=480 | 73 | 0.8% CH4, 3.2% O2, bal. He, | 15000 | / | [ |
| Rh/ZrO2 | T50=400 | / | 1% CH4, 2% O2, bal. He | 15000 | / | [ |
| Ir/TiO2-H | T50=267 | 55.5 | 1% CH4, 20% O2, bal. N2 | 30000 | 50 h | [ |
| Ag/MnLaO3 | T50=580 | 74 | 2% CH4, 98% air | 12000 | / | [ |
| Pt/Cr2O3 | T50=350 | / | 0.2% CH4, 10% O2, bal. N2 | 30000 | / | [ |
| MgO | T50=225 | / | 1% CH4, 99% air | 6000 | 70 h | [ |
| LaCoO3 | T50=470 | / | 0.8% CH4, 5% O2, bal. N2 | 60000 | / | [ |
| NiCo2O4 | T100=350 | / | 5% CH4, 25% O2, bal. Ar | 24000 | 48 h# | [ |
| La0.6Sr0.4MnO3 | T50=566 | 56.6 | 2% CH4, 20% O2, bal. N2 | 30000 | / | [ |
| CoAlOx/CeO2 | T50=415 | 92.2 | 10% CH4, 25% O2, bal. Ar | 24000 | 50 h | [ |
Table 1 Comparison of properties of catalysts for total oxidation of methane by thermal catalysis
| Catalyst | Tc* /℃ | Ea/(kJ·mol-1) | Feed gas | GHSV/(mL·g-1·h-1) | Stability | Ref. |
|---|---|---|---|---|---|---|
| Pd-Ce@SiO2 | T100=350 | 100.4 | 1% CH4, 21% O2, bal. N2 | 36000 | 25 h | [ |
| Pd/TiO2 | T99=370 | 83.1 | 1% CH4, 10% O2, bal. N2 | 30000 | 4 cycles | [ |
| Pd/Na-MOR | T50=335 | 75 | 1% CH4, 4% O2, bal. N2 | 70000 | 90 h | [ |
| Pd-Pt/CeO2 | T50=325 | 74 | 680 μg/mL CH4, 14% O2, 5% CO2, bal. N2 | 300000 | 12 h# | [ |
| Au/Al2O3 | T50=480 | 73 | 0.8% CH4, 3.2% O2, bal. He, | 15000 | / | [ |
| Rh/ZrO2 | T50=400 | / | 1% CH4, 2% O2, bal. He | 15000 | / | [ |
| Ir/TiO2-H | T50=267 | 55.5 | 1% CH4, 20% O2, bal. N2 | 30000 | 50 h | [ |
| Ag/MnLaO3 | T50=580 | 74 | 2% CH4, 98% air | 12000 | / | [ |
| Pt/Cr2O3 | T50=350 | / | 0.2% CH4, 10% O2, bal. N2 | 30000 | / | [ |
| MgO | T50=225 | / | 1% CH4, 99% air | 6000 | 70 h | [ |
| LaCoO3 | T50=470 | / | 0.8% CH4, 5% O2, bal. N2 | 60000 | / | [ |
| NiCo2O4 | T100=350 | / | 5% CH4, 25% O2, bal. Ar | 24000 | 48 h# | [ |
| La0.6Sr0.4MnO3 | T50=566 | 56.6 | 2% CH4, 20% O2, bal. N2 | 30000 | / | [ |
| CoAlOx/CeO2 | T50=415 | 92.2 | 10% CH4, 25% O2, bal. Ar | 24000 | 50 h | [ |

Fig. 4 (a) Schematic diagram of methane activation over semiconductor-based photocatalysts[1]; (b) Schematic diagram of band structures of commonly used semiconductors and redox potentials of different reactants[55]
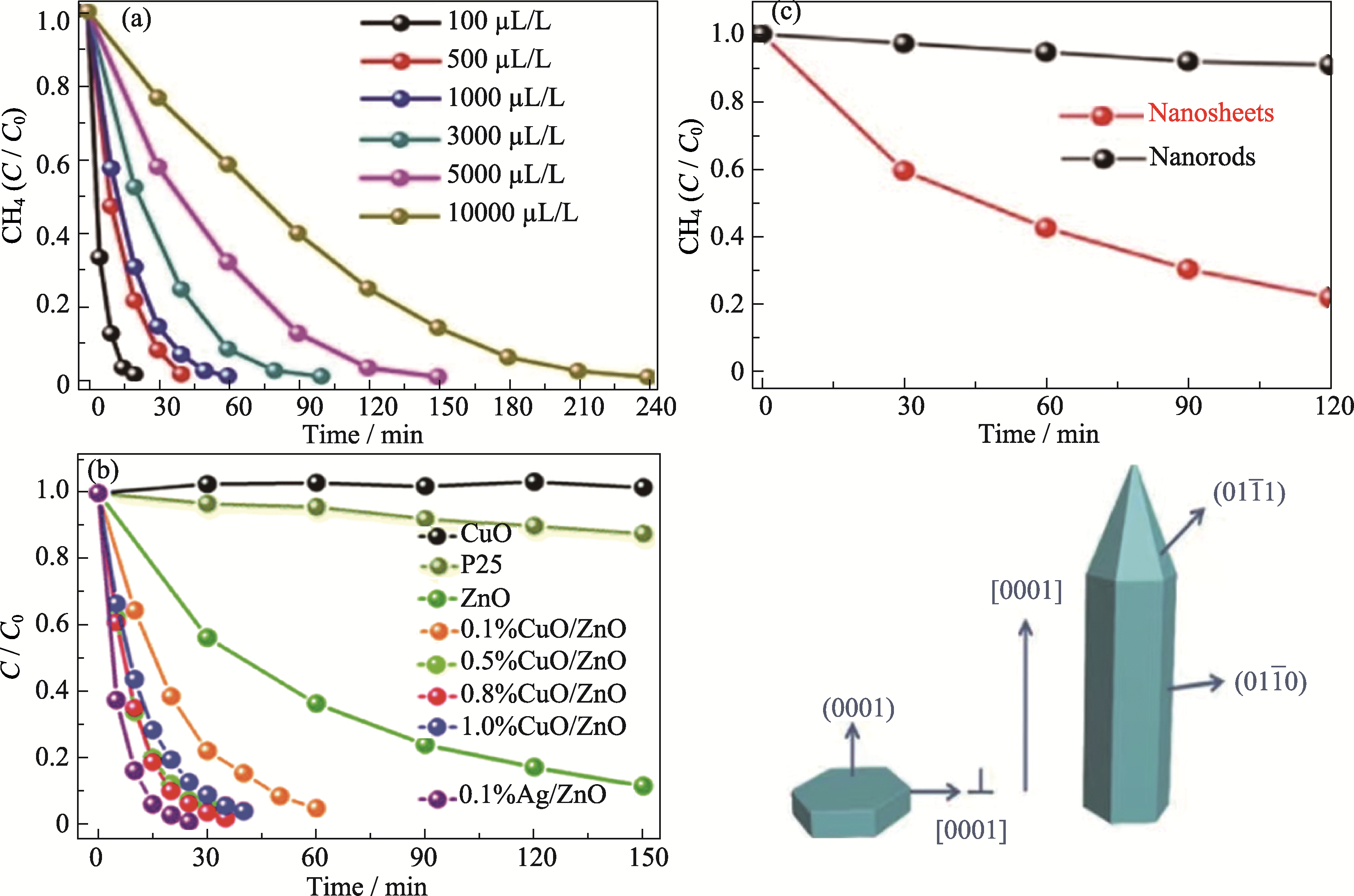
Fig. 5 Application of ZnO-based semiconductor in photocatalytic total oxidation of methane (a) Time evolution of photocatalytic total oxidation of methane over 0.1% Ag-decorated ZnO nanocatalysts at different CH4 concentrations[56] (Reprinted from Ref. [56] with permission, Copyright 2016 Springer Nature); (b) Time evolution of photocatalytic total oxidation of methaneover various catalysts with a CH4 input of 100 μL/L[58] (Reprinted from Ref. [58] with permission, Copyright 2019 Royal Society of Chemistry); (c) Catalytic activity of total oxidation of methane (top) and the crystal morphology (bottom) of a ZnO nanosheet and nanorod[59] (Reprinted from Ref. [59] with permission, Copyright 2019 American Chemical Society)
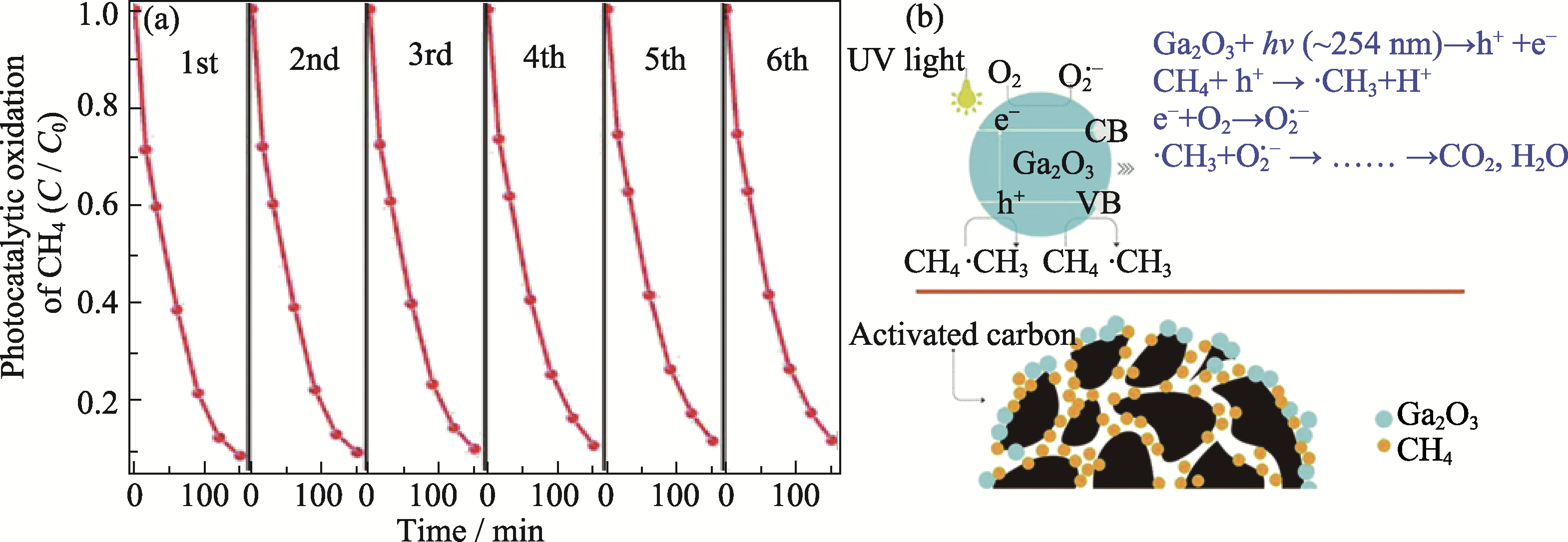
Fig. 6 Ga2O3/AC photocatalytic total oxidation of methane and schematic diagram of oxidation mechanism[60] (a) Recycled test of photocatalytic oxidation of CH4 over 15% Ga2O3/AC; (b) Proposed mechanism for photocatalytic oxidation of CH4 over Ga2O3/AC composites. Reprinted from Ref. [60] with permission, Copyright 2017 Royal Society of Chemistry
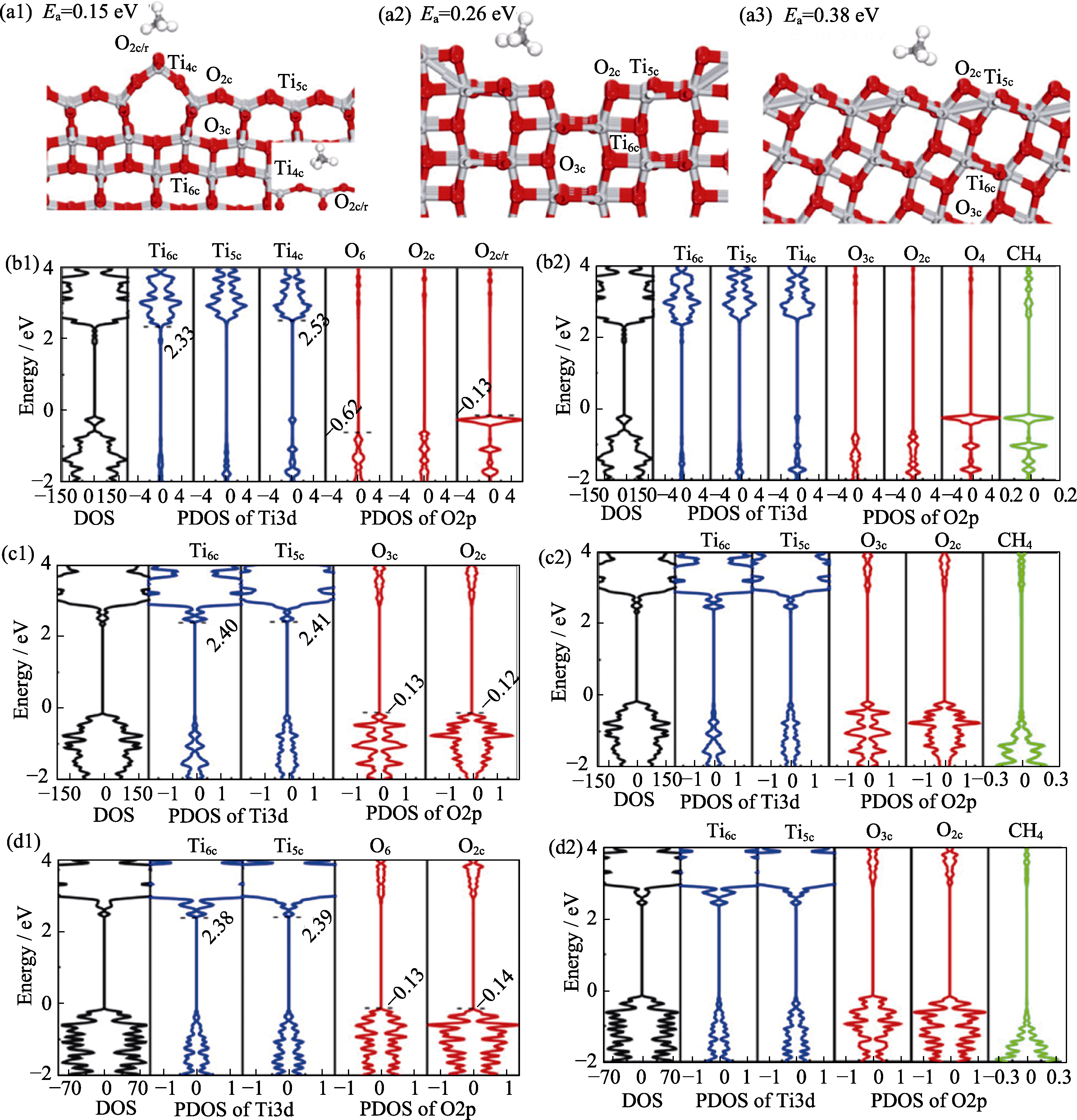
Fig. 7 Adsorption energy calculations of surface methane and DFT calculation of different TiO2 [61] (a1-a3) Most stable adsorption configurations of CH4 on (a1) anatase TiO2(001)-(1×4), (a2) anatase TiO2(100)-(1×2), and (a3) anatase TiO2(101) surfaces. Gray and red balls represent Ti and O atoms, respectively; (b1, b2, c1,c2, d1, d2) Calculated PDOS of (b1) bare and (b2) CH4-adsorbed anatase TiO2(001)-(1×4) surfaces, (c1) bare and (c2) CH4-adsorbed anatase TiO2(100)-(12) surfaces, and (d1) bare and (d2) CH4-adsorbed anatase TiO2-(101) surfaces. Reprinted from Ref. 61 with permission, Copyright 2022 American Chemical Society
| Catalyst | Reaction conditions | Yield/(μmol·h-1) | Ref. |
|---|---|---|---|
| TiO2 | Batch reactor, 3×105 Pa CH4, Xe lamp, RT | 1.1 | [ |
| TiO2 | Batch reactor, 2×106 Pa CH4, 5 bar O2, Xe lamp, RT | 23 | [ |
| ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4 in air, Xe lamp, RT | 2 | [ |
| Ag/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 22 | [ |
| CuO/ZnO | Batch reactor, 1×105 Pa, 100 μg/mL CH4, Xe lamp, RT | 4 | [ |
| Au-CeO2/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 0.6 | [ |
| Ag/AgCl | Batch reactor, 1×105 Pa, 500 μg/mL CH4, Xe lamp, RT | 5.4 | [ |
| SrCO3/SrTiO3 | Batch reactor, 1×105 Pa, 200 μg/mL CH4, Xe lamp, RT | 0.8 | [ |
| BiVO4 | Batch reactor, 1×105 Pa, 20 μg/mL CH4, visible light, RT | 0.05 | [ |
Table 2 Comparison of performances of photocatalysts for total oxidation of methane by photocatalysis
| Catalyst | Reaction conditions | Yield/(μmol·h-1) | Ref. |
|---|---|---|---|
| TiO2 | Batch reactor, 3×105 Pa CH4, Xe lamp, RT | 1.1 | [ |
| TiO2 | Batch reactor, 2×106 Pa CH4, 5 bar O2, Xe lamp, RT | 23 | [ |
| ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4 in air, Xe lamp, RT | 2 | [ |
| Ag/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 22 | [ |
| CuO/ZnO | Batch reactor, 1×105 Pa, 100 μg/mL CH4, Xe lamp, RT | 4 | [ |
| Au-CeO2/ZnO | Batch reactor, 1×105 Pa, 250 μg/mL CH4, Xe lamp, RT | 0.6 | [ |
| Ag/AgCl | Batch reactor, 1×105 Pa, 500 μg/mL CH4, Xe lamp, RT | 5.4 | [ |
| SrCO3/SrTiO3 | Batch reactor, 1×105 Pa, 200 μg/mL CH4, Xe lamp, RT | 0.8 | [ |
| BiVO4 | Batch reactor, 1×105 Pa, 20 μg/mL CH4, visible light, RT | 0.05 | [ |
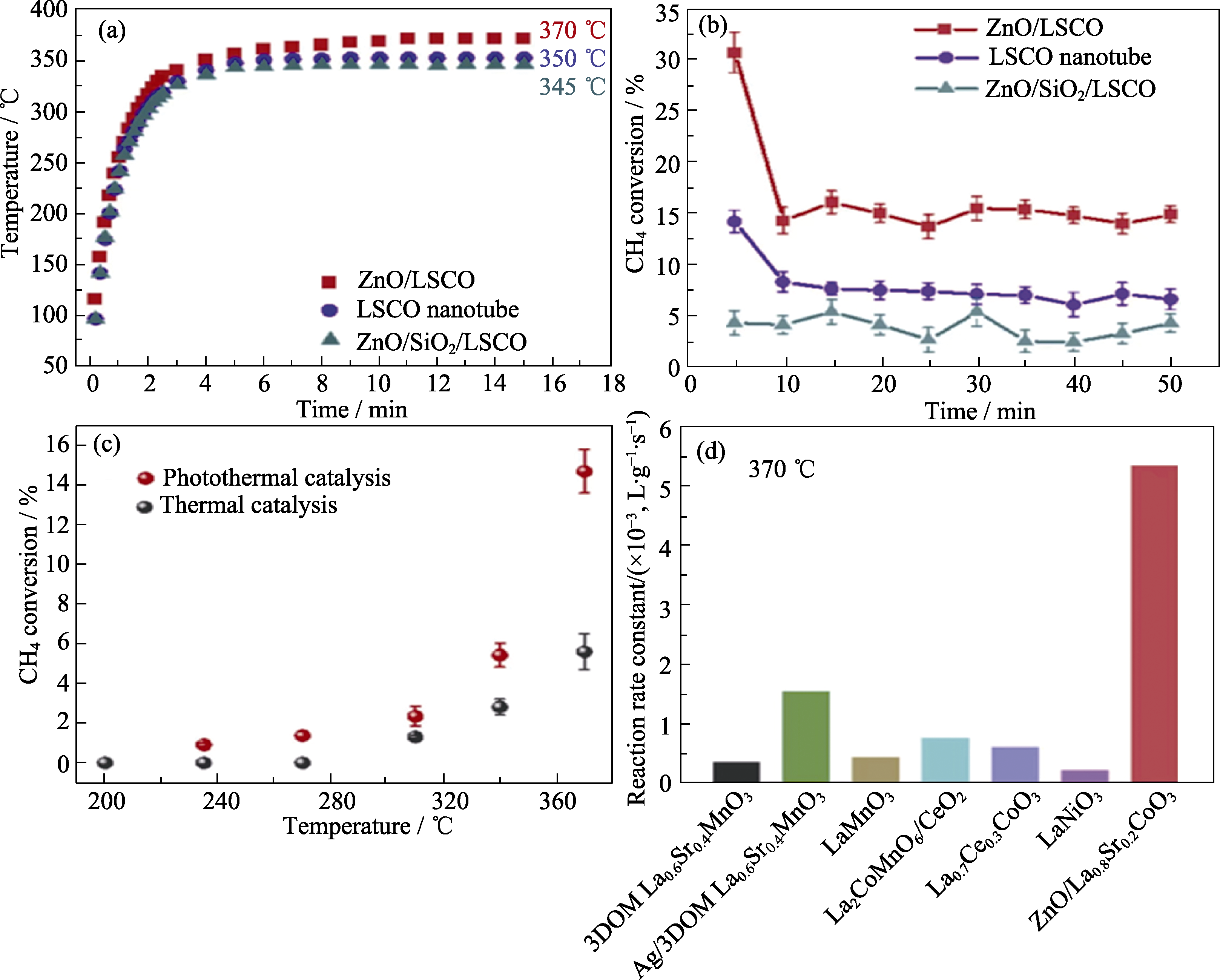
Fig. 8 Tests of ZnO/LSCO photocatalysis and photothermal cocatalysis for methane total oxidation[70] (a) Temperature profiles on these monolithic catalysts under irradiation of Xe lamp; (b) CH4 photothermal conversions over these monolithic catalysts under Xe lamp irradiation; (c) Comparison of CH4 conversion for ZnO/LSCO under Xe lamp irradiation and direct thermal heating (furnace) at the same temperature; (d) Activity comparison of methane oxidation with previous studies by normalized reaction rate constant. Reprinted from Ref. [70] with permission, Copyright 2008 American Chemical Society
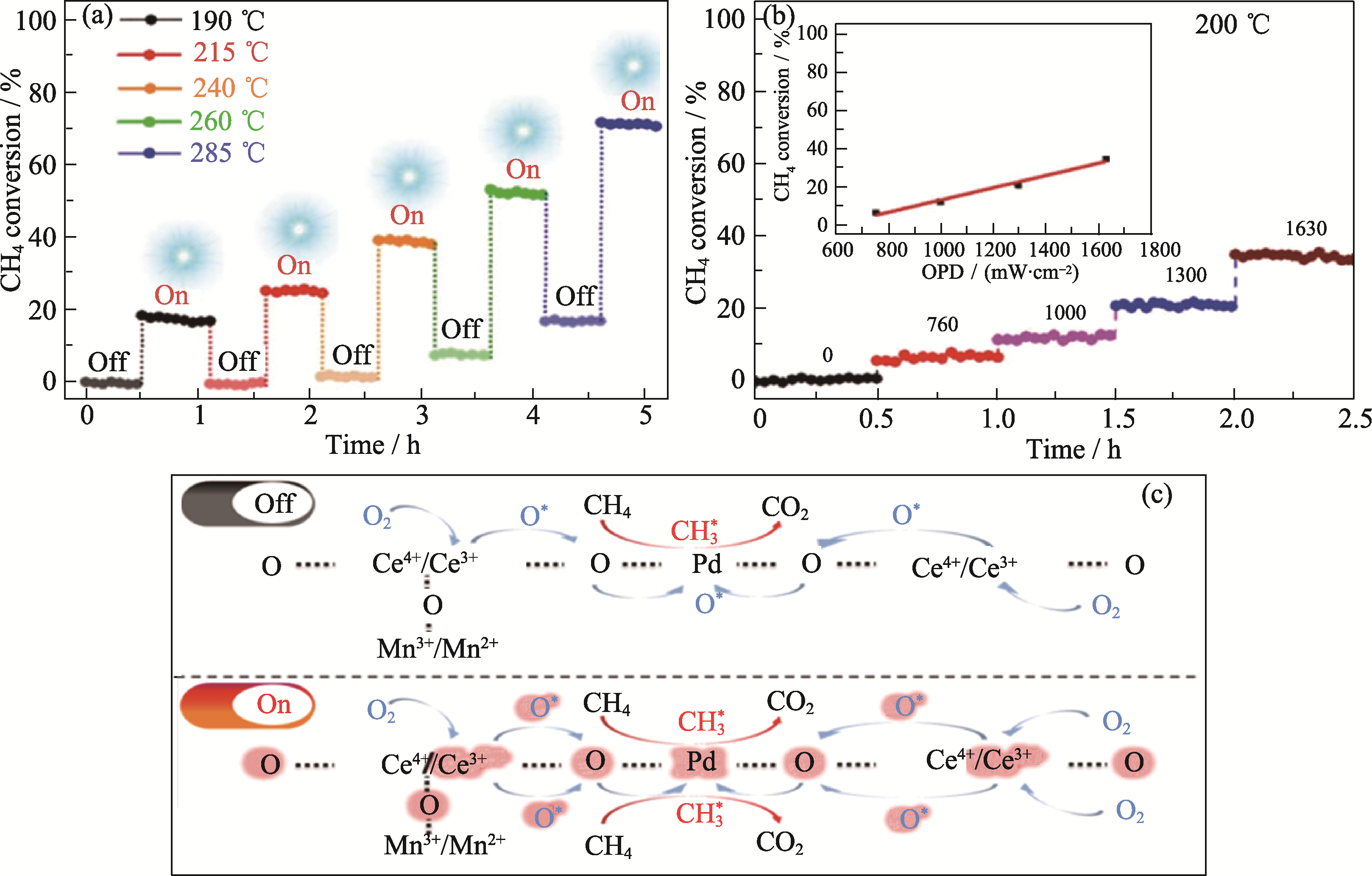
Fig. 9 HPMC photothermal co-catalyzed methane total oxidation performance (a, b) and its mechanism (c)[71] (a) Cycling stability test of HPMC; (b) Methane conversion measured at 200 ℃ with different optical power (OPD); (c) Reaction mechanism of HPMC catalyzed methane combustion. Reprinted from Ref. [71] with permission, Copyright 2021 Wiley
| [1] |
SONG H, MENG X, WANG Z J, et al. Solar-energy-mediated methane conversion. Joule, 2019, 3(7): 1606.
DOI |
| [2] |
NEWTON M A, KNORPP A J, SUSHKEVICH V L, et al. Active sites and mechanisms in the direct conversion of methane to methanol using Cu in zeolitic hosts: a critical examination. Chemical Society Reviews, 2020, 49(5): 1449.
DOI PMID |
| [3] |
SCHWACH P, PAN X, BAO X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chemical Reviews, 2017, 117(13): 8497.
DOI PMID |
| [4] |
VOOSE P. Ominous feedback loop may be accelerating methane emissions. Science, 2022, 377(6603): 250.
DOI PMID |
| [5] |
PENG S, LIN X, THOMPSON R L, et al. Wetland emission and atmospheric sink changes explain methane growth in 2020. Nature, 2022, 612(7940): 477.
DOI |
| [6] |
LASHOF D A, AHUJA D. R. Relative contributions of greenhouse gas emissions to global warming. Nature, 1990, 344(5): 529.
DOI |
| [7] |
ALLEN G H, Cause of the 2020 rise in atmospheric methane. Nature, 2022, 612(15): 13.
DOI |
| [8] |
HE L, FAN Y, BELLETTRE J, et al. A review on catalytic methane combustion at low temperatures: catalysts, mechanisms, reaction conditions and reactor designs. Renewable and Sustainable Energy Reviews, 2020, 119: 109589.
DOI URL |
| [9] |
RAVI M, RANOCCHIARI M, VAN BOKHOVEN J A. The direct catalytic oxidation of methane to methanol-a critical assessment. Angewandte Chemie International Edition, 2017, 56(52): 16464.
DOI URL |
| [10] |
IGLESIAS-JUEZ, FRESNO A F, CORONADO J M, et al. Emerging high-prospect applications in photothermal catalysis. Current Opinion in Green and Sustainable Chemistry, 2022, 37: 100652.
DOI URL |
| [11] | LUO Y R. Comprehensive handbook of chemical bond energies. Boca Raton: CRC Press, 2007, 19. |
| [12] | DIETL N, ENGESER M, SCHWARZ H. Competitive hydrogen- atom abstraction versus oxygen-atom and electron transfers in gas-phase reactions of [X4O10] (X = P, V) with C2H4. Chemistry Europe, 2010, 16(15): 4452. |
| [13] |
DIETL N, SCHLANGEN M, SCHWARZ H. Thermal hydrogen- atom transfer from methane: the role of radicals and spin states in oxo-cluster chemistry. Angewandte Chemie International Edition, 2012, 51(23): 5544.
DOI URL |
| [14] |
YULIATI L, YOSHIDA H. Photocatalytic conversion of methane. Chemical Society Reviews, 2008, 37(8): 1592.
DOI PMID |
| [15] |
CHEN J, ARANDIYAN H, GAO X, et al. Recent advances in catalysts for methane combustion. Catalysis Surveys from Asia, 2015, 19(3): 140.
DOI URL |
| [16] |
WANG C, XU Y, TANG J. Catalytic methane removal to mitigate its environmental effect. Science China Chemistry, 2023, 66: 1032.
DOI |
| [17] |
FENG X, JIANG L, LI D, et al. Progress and key challenges in catalytic combustion of lean methane. Journal of Energy Chemistry, 2022, 75: 173.
DOI |
| [18] |
SCHWARTZ W R, CIUPARU D, PFEFFERLE L D. Combustion of methane over palladium-based catalysts: catalytic deactivation and role of the support. The Journal of Physical Chemistry C, 2012, 116(15): 8587.
DOI URL |
| [19] |
GARETTO T F, APESTEGUIA C R. Oxidative catalytic removal of hydrocarbons over Pt/Al2O3 catalysts. Catalysis Today, 2000, 62: 189.
DOI URL |
| [20] |
NEUBERG S, PENNEMANN H, SHANMUGAM V, et al. Promoting effect of Rh on the activity and stability of Pt-based methane combustion catalyst in microreactors. Catalysis Communications, 2021, 149: 106202.
DOI URL |
| [21] |
CHOUDHARY R V, PATIL V P, JANA P, et al. Nano-gold supported on Fe2O3: a highly active catalyst for low temperature oxidative destruction of methane green house gas from exhaust/waste gases. Applied Catalysis A: General, 2008, 350(2): 186.
DOI URL |
| [22] |
DING Y, WU Q, LIN B, et al. Superior catalytic activity of a Pd catalyst in methane combustion by fine-tuning the phase of ceria-zirconia support. Applied Catalysis B: Environmental, 2020, 266: 118631.
DOI URL |
| [23] |
XIAO L H, SUN K P, XU X L, et al. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition- precipitation method. Catalysis Communications, 2005, 6(12): 796.
DOI URL |
| [24] |
KINNUNEN N M, SUVANTO M, MORENO M A, et al. Methane oxidation on alumina supported palladium catalysts: effect of Pd precursor and solvent. Applied Catalysis A: General, 2009, 370(1/2): 78.
DOI URL |
| [25] |
HONG E, KIM C, LIM D H, et al. Catalytic methane combustion over Pd/ZrO2 catalysts: effects of crystalline structure and textural properties. Applied Catalysis B: Environmental, 2018, 232: 544.
DOI URL |
| [26] |
VENEZIA A M, DI CARLO G, PANTALEO G, et al. Oxidation of CH4 over Pd supported on TiO2-doped SiO2: effect of Ti(IV) loading and influence of SO2. Applied Catalysis B: Environmental, 2009, 88(3/4): 430.
DOI URL |
| [27] |
SEKIZAWA KOSHI, WIDJIAJA H, SHINGO MAEDA, et al. Low temperature oxidation of methane over Pd catalyst supported on metal oxides. Catalysis Today, 2000, 59: 69.
DOI URL |
| [28] |
HUANG W X, JOHNSTON-PECK A C, WOLTER T, et al. Steam-created grain boundaries for methane C-H activation in palladium catalysts. Science, 2021, 373(6562): 1518.
DOI PMID |
| [29] |
LUO L, WANG S, FAN C, et al. Promoting effect of alkali metal cations on the catalytic performance of Pd/H-ZSM-5 in the combustion of lean methane. Applied Catalysis A: General, 2020, 602: 117678.
DOI URL |
| [30] | CORRO G, TORRALBA R, PAL U, et al. Total oxidation of methane over Pt/Cr2O3 catalyst at low temperature: effect of Pt0-Ptx+ dipoles at the metal-support interface. The Journal of Physical Chemistry C, 2019, 123(5): 28823. |
| [31] | PECCHIA G, REYES P, GOÂMEZB R, et al. Methane combustion on Rh/ZrO2 catalysts. Applied Catalysis B: Environmental, 1998, 17: L13. |
| [32] |
GRISEL R J H, KOOYMAN P J, NIEUWENHUYS B E. Influence of the preparation of Au/Al2O3 on CH4oxidation activity. Journal of Catalysis, 2000, 191(2): 430.
DOI URL |
| [33] |
ZHANG Y, WANG X, ZHU Y, et al. Thermal evolution crystal structure and Fe crystallographic sites in LaFexAl12-xO19 hexaaluminates. The Journal of Physical Chemistry C, 2014, 118(20): 10792.
DOI URL |
| [34] |
WANG Z, HAO Z, SHI F, et al. Boosting the oxygen evolution reaction through migrating active sites from the bulk to surface of perovskite oxides. Journal of Energy Chemistry, 2022, 69: 434.
DOI |
| [35] |
YU Q, LIU C, LI X, et al. N-doping activated defective Co3O4 as an efficient catalyst for low-temperature methane oxidation. Applied Catalysis B: Environmental, 2020, 269: 118757.
DOI URL |
| [36] | HUANG F, WANG X, WANG A, et al. A two-step synthesis of Fe-substituted hexaaluminates with enhanced surface area and activity in methane catalytic combustion. Catalysis Science & Technology, 2016, 6(13): 4962. |
| [37] |
MIAO F, WANG F, MAO D, et al. Effect of different reaction conditions on catalytic activity of La(Mn, Fe)O3+λ catalyst for methane combustion. Materials Research Express, 2019, 6(5): 055001.
DOI URL |
| [38] |
JIANG L, LI D, DENG G, et al. Design of hybrid La1-xCexCoO3- catalysts for lean methane combustion via creating active Co and Ce species. Chemical Engineering Journal, 2023, 456: 141054.
DOI URL |
| [39] |
PENG H, RAO C, ZHANG N, et al. Confined ultrathin Pd-Ce nanowires with outstanding moisture and SO2 tolerance in methane combustion. Angewandte Chemie International Edition, 2018, 57(29): 8953.
DOI URL |
| [40] | XIAO Y, LI J, WANG C, et al. Construction and evolution of active palladium species on phase-regulated reducible TiO2 for methane combustion. Catalysis Science & Technology, 2021, 11(3): 836. |
| [41] | PETROV A W, FERRI D, KRUMEICH F, et al. Stable complete methane oxidation over palladium based zeolite catalysts. Nature Communcations, 2018, 9(1): 2545. |
| [42] |
XIONG H, KUNWAR D, JIANG D, et al. Engineering catalyst supports to stabilize PdOx two-dimensional rafts for water-tolerant methane oxidation. Nature Catalysis, 2021, 4(10): 830.
DOI |
| [43] |
CHEN J, WANG X, ZHANG L, et al. Strong metal-support interaction assisted redispersion strategy for obtaining ultrafine and stable IrO2/Ir active sites with exceptional methane oxidation activity. Applied Catalysis B: Environmental, 2021, 297: 120410.
DOI URL |
| [44] |
MACHOCKI A, IOANNIDES T, STASINKSA B, et al. Manganese- lanthanum oxides modified with silver for the catalytic combustion of methane. Journal of Catalysis, 2004, 227(2): 282.
DOI URL |
| [45] |
HAO Y J, TIAN L G, DUAN E, et al. Low-temperature methane oxidation triggered by peroxide radicals over noble-metal-free MgO Catalyst. ACS Applied Materials Interfaces, 2020, 12(19): 21761.
DOI URL |
| [46] |
WANG Y G, REN J R, WANG Y Q, et al. Nanocasted synthesis of mesoporous LaCoO3 perovskite with extremely high surface area and excellent activity in methane combustion. The Journal of Physical Chemistry C, 2008, 112: 15293.
DOI URL |
| [47] | TAO F F, SHAN J J, NGUYEN L, et al. Understanding complete oxidation of methane on spinel oxides at a molecular level. Nature Communcations, 2015, 6: 7798. |
| [48] |
ARANDIYAN H, DAI H, DENG J, et al. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 with high surface areas: active catalysts for the combustion of methane. Journal of Catalysis, 2013, 307: 327.
DOI URL |
| [49] |
FAN X, LI L, JING F, et al. Effects of preparation methods on CoAlOx/CeO2catalysts for methane catalytic combustion. Fuel, 2018, 225: 588.
DOI URL |
| [50] |
COLUSSI S, FORNASIERO P, TROVARELLI A. Structure- activity relationship in Pd/CeO2 methane oxidation catalysts. Chinese Journal of Catalysis, 2020, 41(6): 938.
DOI URL |
| [51] |
CHEN J, WU Y, HU W, et al. New insights into the role of Pd-Ce interface for methane activation on monolithic supported Pd catalysts: a step forward the development of novel PGM three-way catalysts for natural gas fueled engines. Applied Catalysis B: Environmental, 2020, 264: 118475.
DOI URL |
| [52] |
EISWIRTH M, BURGER P, STRASSER P, et al. Oscillating langmuir-hinshelwood mechanisms. Journal of Physical Chemistry, 1996, 100: 19118.
DOI URL |
| [53] |
YANG J, HU S, SHI L, et al. Oxygen vacancies and lewis acid sites synergistically promoted catalytic methane combustion over perovskite oxides. Environmental Science & Technology, 2021, 55(13): 9243.
DOI URL |
| [54] |
KWON Y, KIM T Y, KWON G, et al. Selective activation of methane on single-atom catalyst of rhodium dispersed on zirconia for direct conversion. Journal of the American Chemical Society, 2017, 139(48): 17694.
DOI PMID |
| [55] |
LI Q, OUYANG Y, LI H, et al. Photocatalytic conversion of methane: recent advancements and pospects. Angewandte Chemie International Edition, 2022, 61(2): e202108069.
DOI URL |
| [56] | CHEN X, LI Y, PAN X, et al. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nature Communcations, 2016, 7: 12273. |
| [57] |
LIANG X, WANG P, GAO Y, et al. Design and synthesis of porous M-ZnO/CeO2 microspheres as efficient plasmonic photocatalysts for nonpolar gaseous molecules oxidation: insight into the role of oxygen vacancy defects and M=Ag, Au nanoparticles. Applied Catalysis B: Environmental, 2020, 260: 118151.
DOI URL |
| [58] |
LI Z, PAN X, YI Z. Photocatalytic oxidation of methane over CuO-decorated ZnO nanocatalysts. Journal of Materials Chemistry A, 2019, 7(2): 469.
DOI |
| [59] | LI Z, BODA M A, PAN X, et al. Photocatalytic oxidation of small molecular hydrocarbons over ZnO nanostructures: the difference between methane and ethylene and the impact of polar and nonpolar facets. ACS Sustainable Chemistry & Engineering, 2019, 7(23): 19042. |
| [60] |
WEI J, YANG J, WEN Z, et al. Efficient photocatalytic oxidation of methane over β-Ga2O3/activated carbon composites. RSC Advances, 2017, 7(60): 37508.
DOI URL |
| [61] |
FU C, LI F, YANG J, et al. Spontaneous bulk-surface charge separation of TiO2-{001} nanocrystals leads to high activity in photocatalytic methane combustion. ACS Catalysis, 2022, 12(11): 6457.
DOI URL |
| [62] |
YU X, ZHOLOBENKE V L, OLDOVAN S, et al. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nature Energy, 2020, 5(7): 511.
DOI |
| [63] |
JIANG Y, ZHAO W, LI S, et al. Elevating photooxidation of methane to formaldehyde via TiO2 crystal phase engineering. Journal of the American Chemical, 2022, 144(35): 15977.
DOI URL |
| [64] |
WANG F, LIANG X, WANG P, et al. Ag/AgCl as an efficient plasmonic photocatalyst for greenhouse gaseous methane oxidation. Journal of Environmental Chemical Engineering, 2021, 9(6): 106435.
DOI URL |
| [65] |
PAN X, CHEN X, YI Z. Photocatalytic oxidation of methane over SrCO3 decorated SrTiO3 nanocatalysts via a synergistic effect. Physical Chemistry Chemical Physics, 2016, 18(46): 31400.
DOI URL |
| [66] |
WANG J, XU R, XIA Y, et al. Ti2CTx MXene: a novel p-type sensing material for visible light-enhanced room temperature methane detection. Ceramics International, 2021, 47(24): 34437.
DOI URL |
| [67] |
YANG Z, ZHANG Q, REN L, et al. Efficient photocatalytic conversion of CH4 into ethanol with O2 over nitrogen vacancy-rich carbon nitride at room temperature. Chemical Communication, 2021, 57(7): 871.
DOI URL |
| [68] |
ZHANG W, FU C, LOW J, et al. High-performance photocatalytic nonoxidative conversion of methane to ethane and hydrogen by heteroatoms-engineered TiO2. Nature Communications, 2022, 13(1): 2806.
DOI PMID |
| [69] |
MAYERNICK A D, JANIK M J. Methane oxidation on Pd-ceria: a DFT study of the mechanism over PdxCe1-xO2, Pd, and PdO. Journal of Catalysis, 2011, 278(1): 16.
DOI URL |
| [70] |
YANG J, XIAO W, CHI X, et al. Solar-driven efficient methane catalytic oxidation over epitaxial ZnO/La0.8Sr0.2CoO3 heterojunctions. Applied Catalysis B: Environmental, 2020, 265: 118469.
DOI URL |
| [71] |
FENG X, LIU D, YAN B, et al. Highly active PdO/Mn3O4/CeO2 nanocomposites supported on one dimensional halloysite nanotubes for photoassisted thermal catalytic methane combustion. Angewandte Chemie International Edition, 2021, 60(34): 18552.
DOI URL |
| [72] |
KANG L, LIU X Y, WANG A, et al. Photo-thermo catalytic oxidation over a TiO2-WO3-supported platinum catalyst. Angewandte Chemie International Edition, 2020, 59(31): 12909.
DOI URL |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | FAN Xiaoxuan, ZHENG Yonggui, XU Lirong, YAO Zimin, CAO Shuo, WANG Kexin, WANG Jiwei. Organic Pollutant Fenton Degradation Driven by Self-activated Afterglow from Oxygen-vacancy-rich LiYScGeO4: Bi3+ Long Afterglow Phosphor [J]. Journal of Inorganic Materials, 2025, 40(5): 481-488. |
| [8] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [9] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [10] | JIA Xianghua, ZHANG Huixia, LIU Yanfeng, ZUO Guihong. Cu2O/Cu Hollow Spherical Heterojunction Photocatalysts Prepared by Wet Chemical Approach [J]. Journal of Inorganic Materials, 2025, 40(4): 397-404. |
| [11] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [12] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [13] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [14] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [15] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||