无机材料学报 ›› 2024, Vol. 39 ›› Issue (3): 299-305.DOI: 10.15541/jim20230312 CSTR: 32189.14.10.15541/jim20230312
所属专题: 【能源环境】储能电池(202506); 【能源环境】锂离子电池(202412)
程节( ), 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直(
), 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直( )
)
收稿日期:2023-07-11
修回日期:2023-09-01
出版日期:2024-03-20
网络出版日期:2023-09-12
通讯作者:
袁中直, 教授. E-mail: yuanzz@scnu.edu.cn作者简介:程节(1998-), 女, 硕士研究生. E-mail: 15007936259@163.com
CHENG Jie( ), ZHOU Yue, LUO Xintao, GAO Meiting, LUO Sifei, CAI Danmin, WU Xueyin, ZHU Licai, YUAN Zhongzhi(
), ZHOU Yue, LUO Xintao, GAO Meiting, LUO Sifei, CAI Danmin, WU Xueyin, ZHU Licai, YUAN Zhongzhi( )
)
Received:2023-07-11
Revised:2023-09-01
Published:2024-03-20
Online:2023-09-12
Contact:
YUAN Zhongzhi, professor. E-mail: yuanzz@scnu.edu.cnAbout author:CHENG Jie (1998-), female, Master candidate. E-mail: 15007936259@163.com
摘要:
FeF3∙0.33H2O具有理论容量和电压高的特点, 但其导电性差、氧化还原反应过程中体积变化严重导致电化学循环性能不佳, 应用受到限制。本研究采用多巴胺自组装包覆纳米立方Fe2O3颗粒, 再经过碳化、HCl刻蚀和HF氟化的策略, 合成了由N掺杂石墨烯外壳和纳米立方FeF3∙·0.33H2O内核所构成的蛋黄壳结构复合材料FeF3∙0.33H2O@CNBs, 粒径约250 nm, 碳壳厚度为30~40 nm。FeF3∙0.33H2O@CNBs在0.2C (1C=237 mA·g-1)电流密度下充放电初始容量为208 mAh·g-1, 循环50圈之后容量仍然有173 mAh·g-1, 每圈容量衰减率仅为0.3%; 而纯FeF3∙0.33H2O初始容量只有112 mAh·g-1, 循环50圈之后只有95 mAh·g-1。FeF3∙0.33H2O@CNBs的循环性能明显优于FeF3∙0.33H2O, 同时0.1C~1C充放电结果表明其倍率性能也明显优于FeF3∙0.33H2O。这是因为该策略制备的N掺杂石墨烯外壳提供了良好的电子/离子输运性能, 同时碳壳可缓冲和抑制内核FeF3∙0.33H2O的体积变化, 其空隙体积对电解液的储液保液性能缩短了离子迁移距离, 提升了Li+迁移速率, 从而得到了比文献报道更好的电化学性能。
中图分类号:
程节, 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直. 蛋黄壳结构FeF3·0.33H2O@N掺杂碳纳米笼正极材料的构筑及其电化学性能[J]. 无机材料学报, 2024, 39(3): 299-305.
CHENG Jie, ZHOU Yue, LUO Xintao, GAO Meiting, LUO Sifei, CAI Danmin, WU Xueyin, ZHU Licai, YUAN Zhongzhi. Construction and Electrochemical Properties of Yolk-shell Structured FeF3·0.33H2O@N-doped Graphene Nanoboxes[J]. Journal of Inorganic Materials, 2024, 39(3): 299-305.
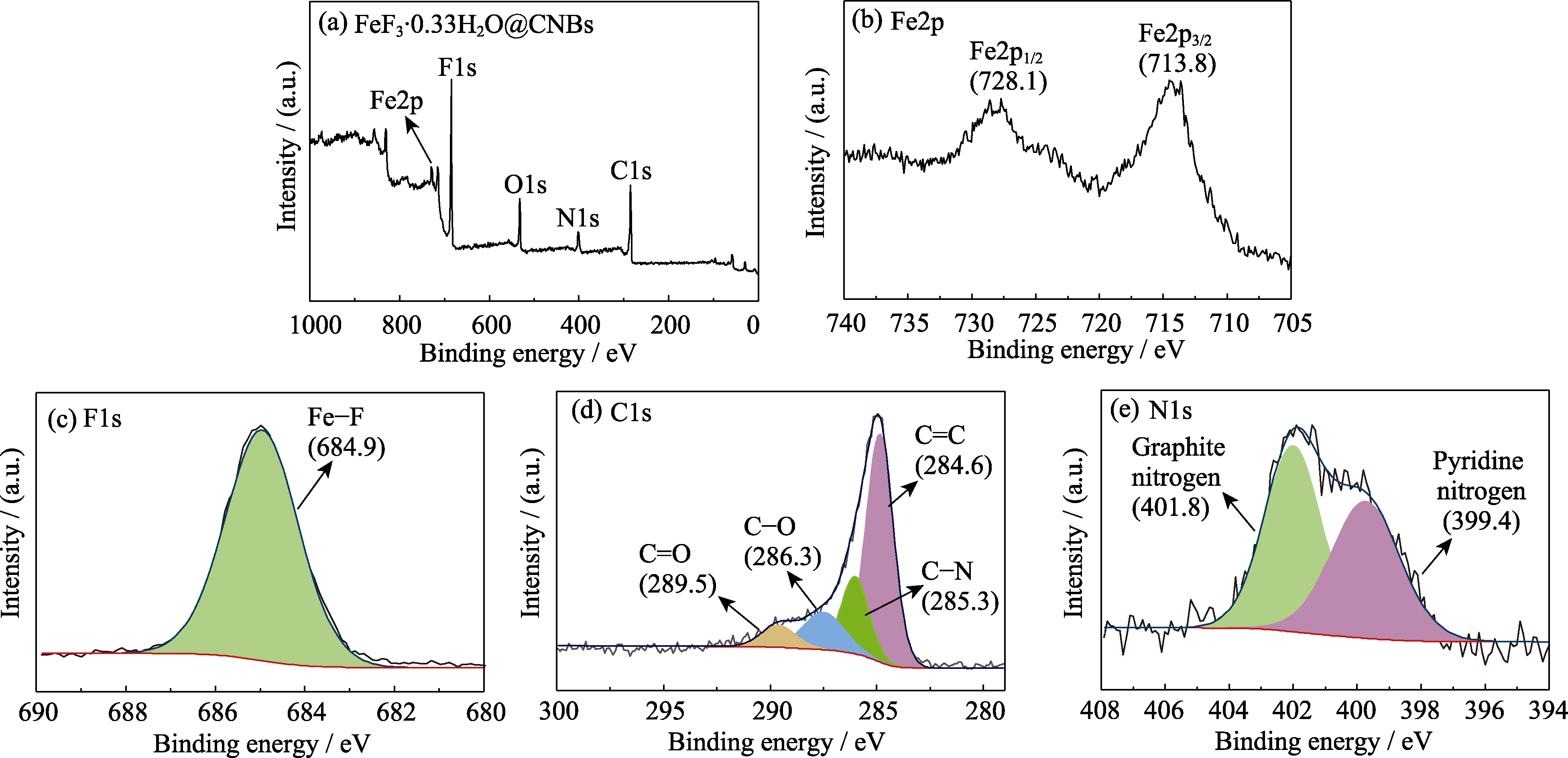
图4 FeF3·0.33H2O@CNBs的(a)XPS总谱图, 以及高分辨(b)Fe2p, (c) F1s, (d)C1s, (e)N1s XPS谱图
Fig. 4 (a) XPS survey spectrum and high-resolution (b) Fe2p, (c) F1s, (d) C1s, (e) N1s XPS spectra of FeF3·0.33H2O@CNBs
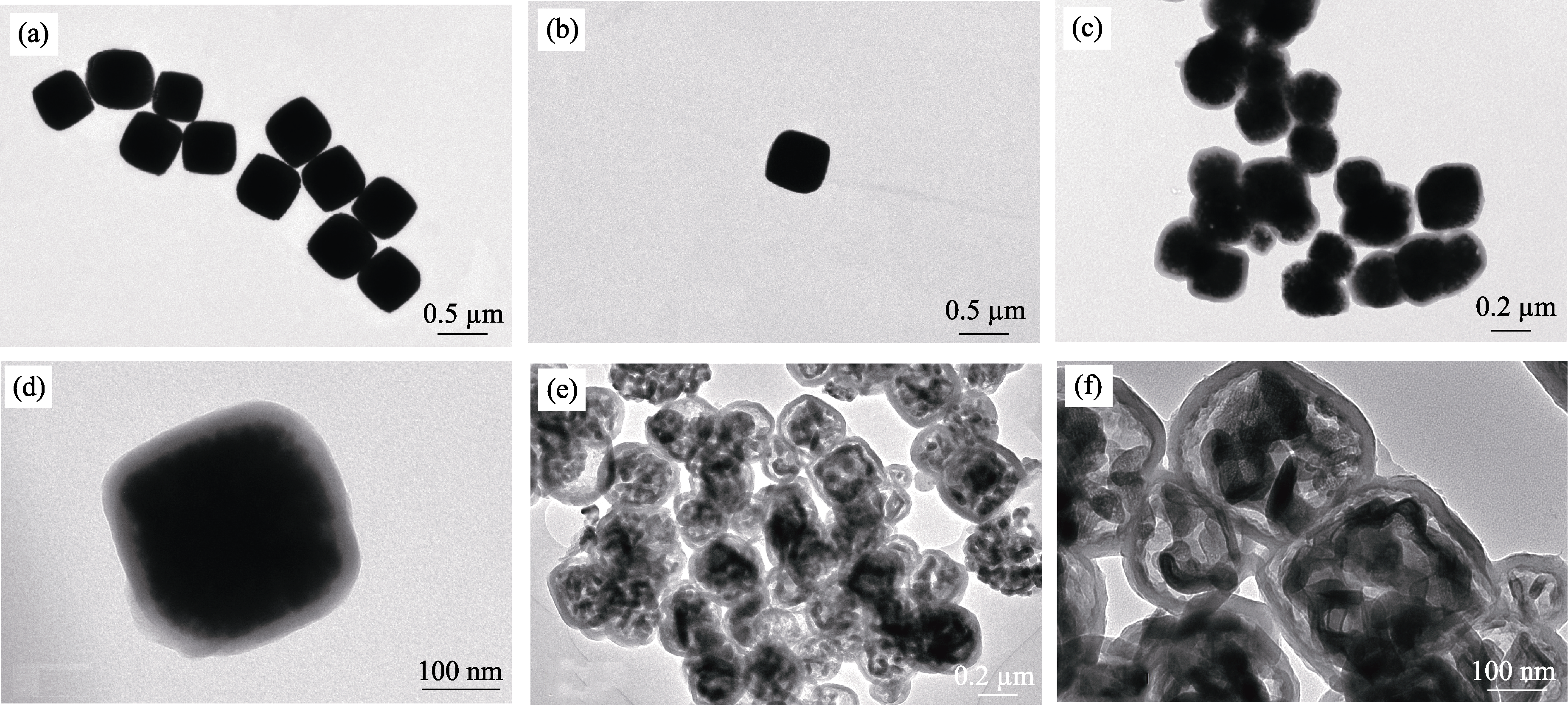
图5 (a, b)Fe2O3纳米立方体, (c, d)核壳结构Fe2O3@C以及(e, f)蛋黄壳结构FeF3·0.33H2O@CNBs的TEM照片
Fig. 5 TEM images of (a, b) cubic Fe2O3 nano-particle, (c, d) core-shell Fe2O3@C and (e, f) yolk-shell FeF3·0.33H2O@CNB
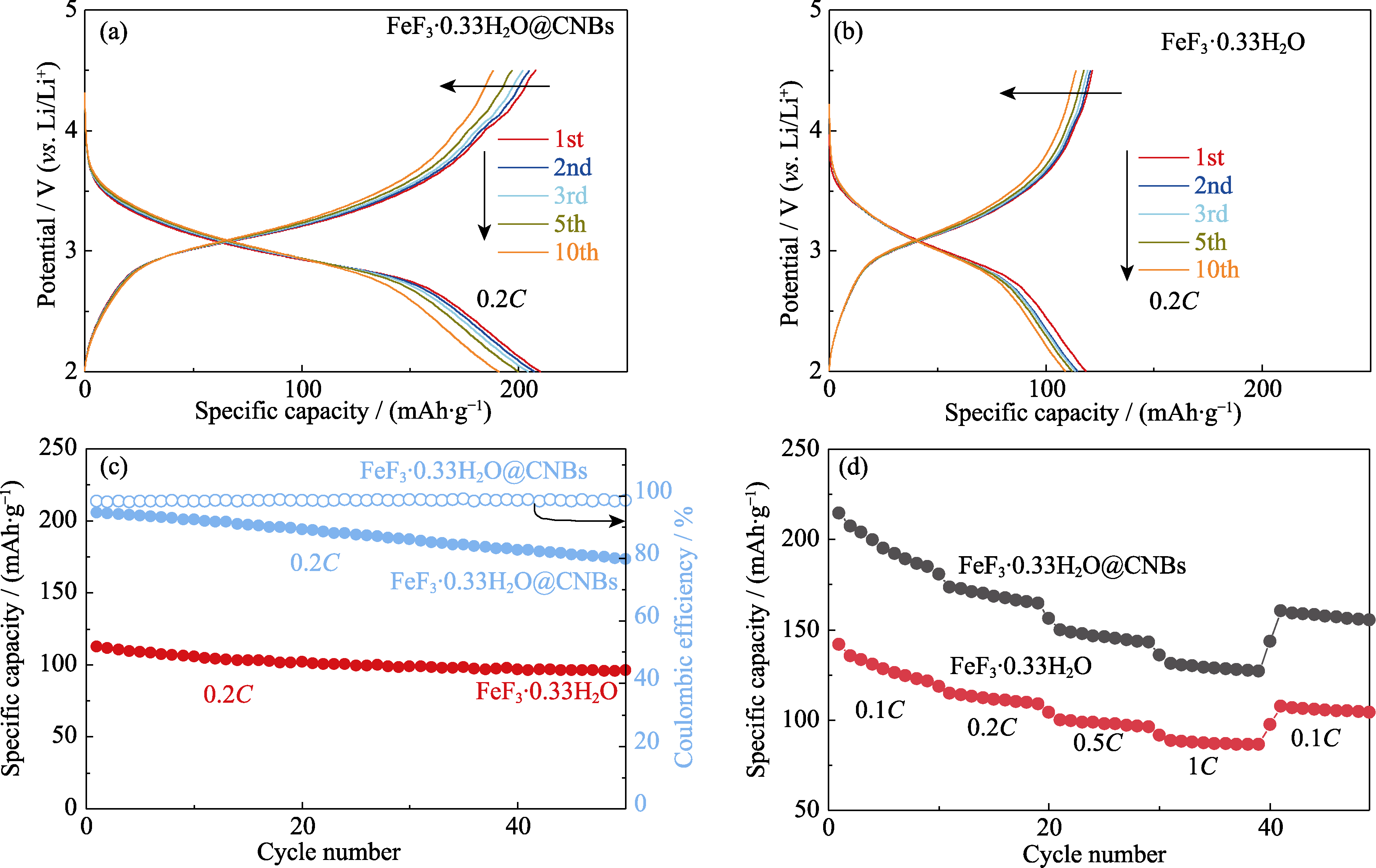
图7 FeF3·0.33H2O@CNBs和纯FeF3·0.33H2O作为正极材料的锂离子电池的电化学性能
Fig. 7 Electrochemical performances of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O as cathodes of lithium ion cell (a) Voltage-capacity curves of FeF3·0.33H2O@CNBs at 0.2C; (b) Voltage-capacity curves of bare FeF3·0.33H2O at 0.2C; (c) Cycling and (d) rate performances of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O. Colorful figures are available on website
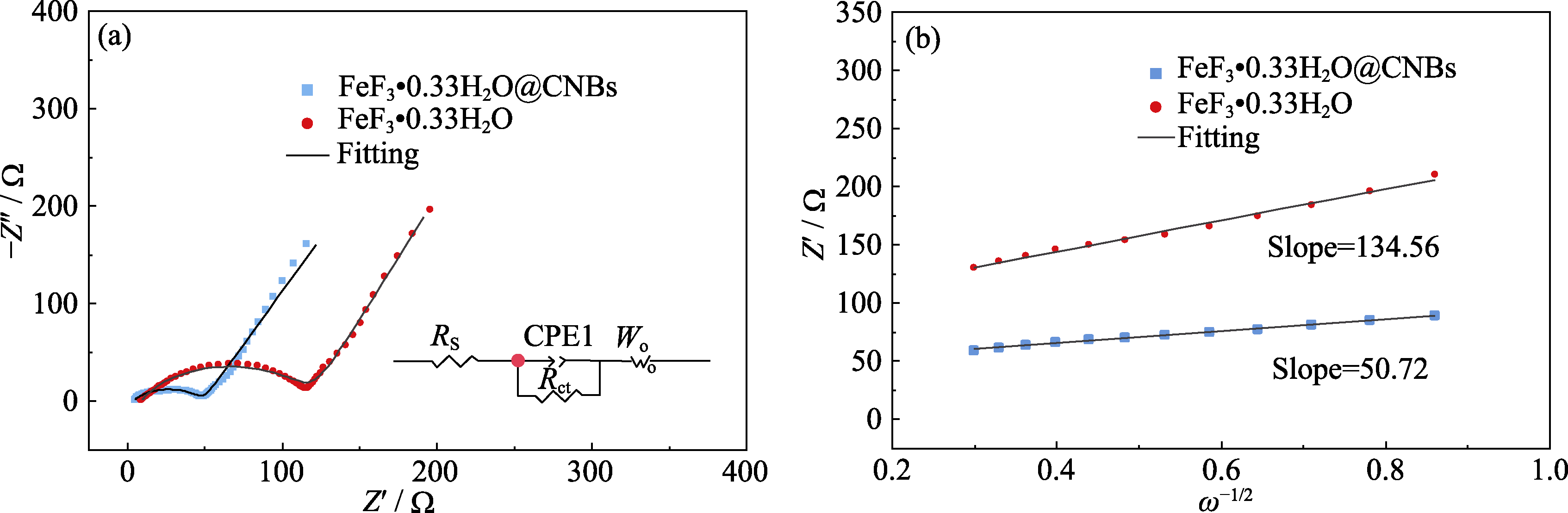
图8 FeF3·0.33H2O@CNBs和纯FeF3·0.33H2O电极的电化学阻抗
Fig. 8 Electrochemical impedance spectra of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O (a) Nyquist plots; (b) Relationship between Z′ and ω−1/2 in low-frequency region
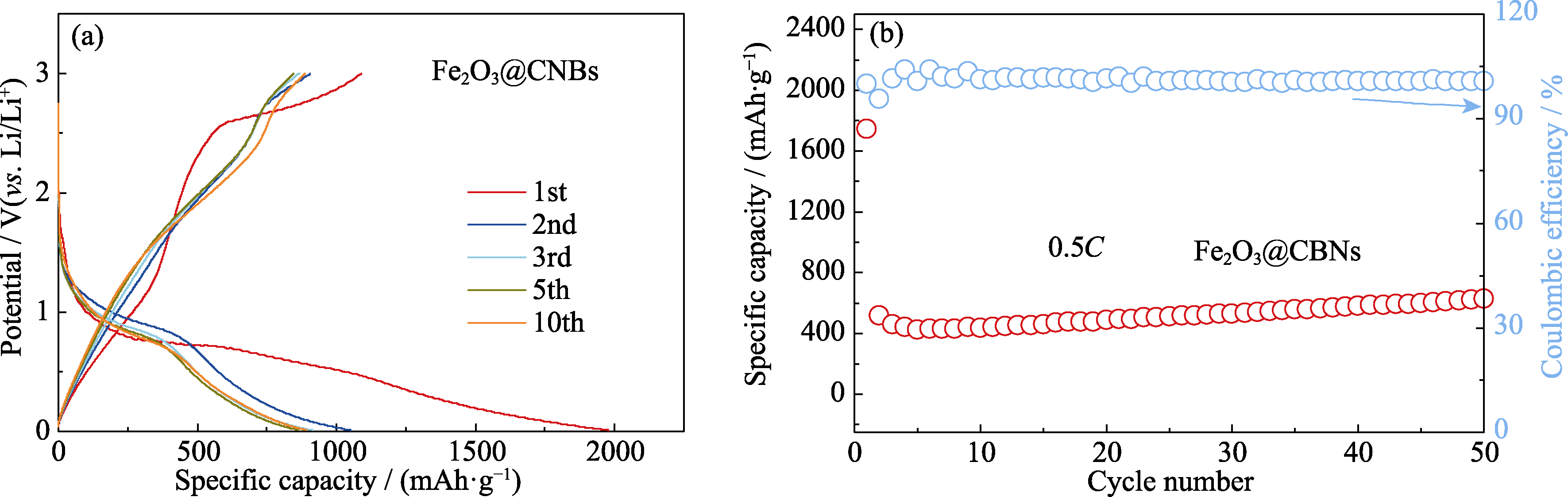
图S2 Li/Fe2O3@CNBs电池的电化学性能
Fig. S2 Electrochemical performance of the Li/Fe2O3@CNBs cell(a) Voltage-capacity curves at 0.1C; (b) Cycling performance at 0.5C
| Material | Particle size | Voltage range/V | Discharge density | Initial discharge capacity/(mAh·g-1) | (Reversible capacity/ (mAh·g-1))/cycle number | Ref. |
|---|---|---|---|---|---|---|
| FeF3·0.33H2O@CNBs | 250 nm | 2.0-4.5 | 0.2C | 208 | 173.4/50 | This work |
| FeF3·0.33H2O/C | 1-1.7 µm | 2.0-4.5 | 1C | 187.1 | 172.3/50 | [ |
| FeF3·0.33H2O@CNHs | 80-100 nm | 1.5-4.5 | 1C | 155 | 154/50 | [ |
| FeF3·0.33H2O/MWCNTs | 30 nm | 2.0-4.3 | 0.1C | 186 | 116/50 | [ |
| FeF3·0.33H2O@rGO | 400 nm | 2.0-4.5 | 0.1C | 205 | 183.8/60 | [ |
| FeF3·0.33H2O/C | 1-5 µm | 1.5-4.5 | 1C | 276.4 | 193.5/50 | [ |
| Co/Ni dual-doped FeF3·0.33H2O | 200 nm | 1.5-4.5 | 5C | 200.1 | 177.8/400 | [ |
| FeF3·0.33H2O/rGO | 150 nm | 1.8-4.5 | 0.5C | 208.3 | 133.1/100 | [ |
表S1 本工作与文献报道FeF3·0.33H2O类正极材料的性能对比
Table S1 Performance comparison of FeF3·0.33H2O based cathode materials of lithium ion battery in this work and literature
| Material | Particle size | Voltage range/V | Discharge density | Initial discharge capacity/(mAh·g-1) | (Reversible capacity/ (mAh·g-1))/cycle number | Ref. |
|---|---|---|---|---|---|---|
| FeF3·0.33H2O@CNBs | 250 nm | 2.0-4.5 | 0.2C | 208 | 173.4/50 | This work |
| FeF3·0.33H2O/C | 1-1.7 µm | 2.0-4.5 | 1C | 187.1 | 172.3/50 | [ |
| FeF3·0.33H2O@CNHs | 80-100 nm | 1.5-4.5 | 1C | 155 | 154/50 | [ |
| FeF3·0.33H2O/MWCNTs | 30 nm | 2.0-4.3 | 0.1C | 186 | 116/50 | [ |
| FeF3·0.33H2O@rGO | 400 nm | 2.0-4.5 | 0.1C | 205 | 183.8/60 | [ |
| FeF3·0.33H2O/C | 1-5 µm | 1.5-4.5 | 1C | 276.4 | 193.5/50 | [ |
| Co/Ni dual-doped FeF3·0.33H2O | 200 nm | 1.5-4.5 | 5C | 200.1 | 177.8/400 | [ |
| FeF3·0.33H2O/rGO | 150 nm | 1.8-4.5 | 0.5C | 208.3 | 133.1/100 | [ |
| Sample | Rs/Ω | Rct/Ω | DLi+ /(m2·s-1) |
|---|---|---|---|
| FeF3·0.33H2O@CNBs | 3.39 | 46.0 | 1.84×10-14 |
| FeF3·0.33H2O | 8.78 | 112.6 | 2.74×10-15 |
表S2 FeF3·0.33H2O@CNBs和纯FeF3·0.33H2O的Li+扩散系数
Table S2 Li-ion diffusion coefficients of FeF3·0.33H2O@CNBs and bare FeF3·0.33H2O
| Sample | Rs/Ω | Rct/Ω | DLi+ /(m2·s-1) |
|---|---|---|---|
| FeF3·0.33H2O@CNBs | 3.39 | 46.0 | 1.84×10-14 |
| FeF3·0.33H2O | 8.78 | 112.6 | 2.74×10-15 |
| [1] |
DUFFNER F, KRONEMEYER N, TUBKE J, et al. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nature Energy, 2021, 6(2): 123.
DOI |
| [2] |
MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry. Nature Communications, 2020, 11: 1550.
DOI PMID |
| [3] | SUN L D, LI Y, FENG W. Metal fluoride cathode materials for lithium rechargeable batteries: focus on iron fluorides. Small Methods, 2023, 7: 202201152. |
| [4] |
LIU L, GUO H, ZHOU M, et al. A comparison among FeF3·3H2O, FeF3·0.33H2O and FeF3 cathode materials for lithium ion batteries: structural, electrochemical, and mechanism studies. Journal of Power Sources, 2013, 238: 501.
DOI URL |
| [5] | XIAO A W, LEE H J, CAPONE I, et al. Understanding the conversion mechanism and performance of monodisperse FeF2 nanocrystal cathodes. Nature Materials, 2020, 644(2): 644. |
| [6] |
LI C, GU L, TSUKIMOTO S, et al. Low-temperature ionic-liquid- based synthesis of nanostructured iron-based fluoride cathodes for lithium batteries. Advanced Materials, 2010, 22(33): 3650.
DOI URL |
| [7] |
TAN J, LIU J, HU H, et al. Iron fluoride with excellent cycle performance synthesized by solvothermal method as cathodes for lithium ion batteries. Journal of Power Sources, 2014, 251: 75.
DOI URL |
| [8] |
BADWAY F, COSANDEY F, PEREIRA N, et al. Carbon metal fluoride nanocomposites: high-capacity reversible metal fluoride conversion materials as rechargeable positive electrodes for Li batteries. Journal of The Electrochemical Society, 2003, 150(10): A1318.
DOI URL |
| [9] |
ZENG C, CHEN F, YE Q, et al. Facile preparation of hierarchical micro-nano FeF3·0.33H2O by a one-pot method with dual surfactants. Nanotechnology, 2021, 32(15): 155402.
DOI |
| [10] |
LIN J, CHEN S, ZHU L, et al. Soft-template fabrication of hierarchical nanoparticle iron fluoride as high-capacity cathode materials for Li-ion batteries. Electrochimica Acta, 2020, 364: 137293.
DOI URL |
| [11] |
SHI Q, ZHOU Y, CHENG J, et al. Turning carbon black into hollow carbon nanospheres to encapsulate Fe2O3 as high-performance lithium-ion batteries anode. Microporous and Mesoporous Materials, 2022, 332: 111681.
DOI URL |
| [12] |
CHEN S, LIN J, SHI Q, et al. Nanoscale iron fluoride supported by three-dimensional porous graphene as long-life cathodes for lithium-ion batteries. Journal of The Electrochemical Society, 2020, 167: 080506.
DOI URL |
| [13] | ZHOU Y, CHENG J, WU X, et al. Octahedral FeF3·0.33H2O nanocrystalline fixed on carbon fibers as the cathode of lithium-ion battery based on the “gravel and glue” strategy. Elecchimica Acta, 2022, 435: 141363. |
| [14] |
WANG Y, XIE K, ZHU Y, et al. Prussian blue microcubes-derived FeF3 cathodes for high-energy and ultra-stable lithium and lithium- ion batteries. Journal of Power Sources, 2023, 577: 233234.
DOI URL |
| [15] |
ZHANG L, YU L, LI O L, et al. FeF3·0.33H2O@carbon nanosheets with honeycomb architectures for high-capacity lithium-ion cathode storage by enhanced pseudocapacitance. Journal of Materials Chemistry A, 2021, 9(30): 16370.
DOI URL |
| [16] |
CHEN S, SHI Q, LIN J, et al. Growth behavior and influence factors of three-dimensional hierarchical flower-like FeF3·0.33H2O. CrystEngComm, 2020, 22(33): 5550.
DOI URL |
| [17] |
ZHOU H, SUN H, WANG T, et al. Low temperature nanotailoring of hydrated compound by alcohols: FeF3·3H2O as an example. preparation of nanosized FeF3·0.33H2O cathode material for Li-ion batteries. Inorganic Chemistry, 2019, 58: 6765.
DOI URL |
| [18] | MURATA Y, MINAMI R, TAKADA S, et al. A fundamental study on carbon composites of FeF3·0.33H2O as open-framework cathode materials for calcium-ion batteries. AIP Conference Proceedings, 2017, 1807: 020005. |
| [19] |
PACHFULE P, SHINDE D, MAJUMDER M, et al. Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework. Nature Chemistry, 2016, 8(7): 718.
DOI PMID |
| [20] |
WANG J, HU Q, HU W, et al. Preparation of hollow core-shell Fe3O4/nitrogen-doped carbon nanocomposites for lithium-ion batteries. Molecules, 2022, 27(2): 396.
DOI URL |
| [21] |
YANG F, GAO H, HAO J, et al. Yolk-shell structured FeP@C nanoboxes as advanced anode materials for rechargeable lithium-/potassium-ion batteries. Advanced Functional Materials, 2019, 29(16): 1808291.
DOI URL |
| [22] |
LIAN P, ZHU X, LIANG S, et al. High reversible capacity of SnO2/graphene nanocomposite as an anode material for lithium-ion batteries. Electrochimica Acta, 2011, 56(12): 4532.
DOI URL |
| [23] |
MANTIA F L, HUGGINS R A, CUI Y. Oxidation processes on conducting carbon additives for lithium-ion batteries. Journal of Applied Electrochemistry, 2013, 43: 1.
DOI URL |
| [24] |
LI J, FU L, ZHU J, et al. Improved electrochemical performance of FeF3 by inlaying in a nitrogen-doped carbon matrix. ChemElectroChem, 2019, 6(20): 5203.
DOI URL |
| [25] | QIU D, FU L, ZHAN C, et al. Seeding iron trifluoride nanoparticles on reduced graphite oxide for lithium-ion batteries with enhanced loading and stability. ACS Applied Materials & Interfaces, 2018, 10(35): 29505. |
| [1] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [2] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [3] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| [4] | 苏楠, 邱介山, 王治宇. 高容量氟掺杂碳包覆纳米硅负极材料: 气相氟化法制备及其储锂性能[J]. 无机材料学报, 2023, 38(8): 947-953. |
| [5] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [6] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [7] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [8] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [9] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [10] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [11] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [12] | 苏东良, 崔锦, 翟朋博, 郭向欣. 石榴石型Li6.4La3Zr1.4Ta0.6O12对Si/C负极表面固体电解质中间相的调控机制研究[J]. 无机材料学报, 2022, 37(7): 802-808. |
| [13] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [14] | 王禹桐, 张非凡, 许乃才, 王春霞, 崔立山, 黄国勇. 水系锂离子电池负极材料LiTi2(PO4)3的研究进展[J]. 无机材料学报, 2022, 37(5): 481-492. |
| [15] | 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安. 源于溪木贼的高性能锂离子电池三维多孔生物质硅/碳复合负极材料[J]. 无机材料学报, 2021, 36(9): 929-935. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||