无机材料学报 ›› 2022, Vol. 37 ›› Issue (9): 1030-1036.DOI: 10.15541/jim20210769 CSTR: 32189.14.10.15541/jim20210769
• 研究论文 • 上一篇
朱河圳1( ), 王选朋2,3(
), 王选朋2,3( ), 韩康1, 杨晨1, 万睿哲2, 吴黎明1, 麦立强1,3(
), 韩康1, 杨晨1, 万睿哲2, 吴黎明1, 麦立强1,3( )
)
收稿日期:2021-12-17
修回日期:2022-02-26
出版日期:2022-09-20
网络出版日期:2022-03-10
通讯作者:
王选朋, 讲师. E-mail: wxp122525691@whut.edu.cn;作者简介:朱河圳(1995-), 男, 硕士研究生. E-mail: 290761@whut.edu.cn
基金资助:
ZHU Hezhen1( ), WANG Xuanpeng2,3(
), WANG Xuanpeng2,3( ), HAN Kang1, YANG Chen1, WAN Ruizhe2, WU Liming1, MAI Liqiang1,3(
), HAN Kang1, YANG Chen1, WAN Ruizhe2, WU Liming1, MAI Liqiang1,3( )
)
Received:2021-12-17
Revised:2022-02-26
Published:2022-09-20
Online:2022-03-10
Contact:
WANG Xuanpeng, lecturer. E-mail: wxp122525691@whut.edu.cn;About author:ZHU Hezhen (1995-), male, Master candidate. E-mail: 290761@whut.edu.cn
Supported by:摘要:
超高镍正极材料具有高比能、高电压和低成本等特点, 在新一代锂离子电池中备受关注, 但在电池的长循环过程中会出现微裂纹、机械粉化和不可逆相变, 导致差的循环性能。本研究采用简便的湿化学法制备了一系列Ca3(PO4)2包覆的超高镍LiNi0.91Co0.06Al0.03O2材料(NCA@nCP)。其中, NCA@1CP在1C (1C=200 mA/g)、2.7~4.3 V下可获得204.8 mAh/g的放电比容量, 100圈循环后容量保持率为91.5%, 甚至在2C的倍率下循环300圈后仍保留153.4 mAh/g的放电比容量。表征结果证实该包覆层可抑制材料的Li/Ni混排、不可逆相变和机械粉化, 从而大幅提升了循环稳定性。本研究表明Ca3(PO4)2包覆策略在提升超高镍正极材料储锂稳定性方面具有较大的应用潜力。
中图分类号:
朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036.
ZHU Hezhen, WANG Xuanpeng, HAN Kang, YANG Chen, WAN Ruizhe, WU Liming, MAI Liqiang. Enhanced Lithium Storage Stability Mechanism of Ultra-high Nickel LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2 Cathode Materials[J]. Journal of Inorganic Materials, 2022, 37(9): 1030-1036.
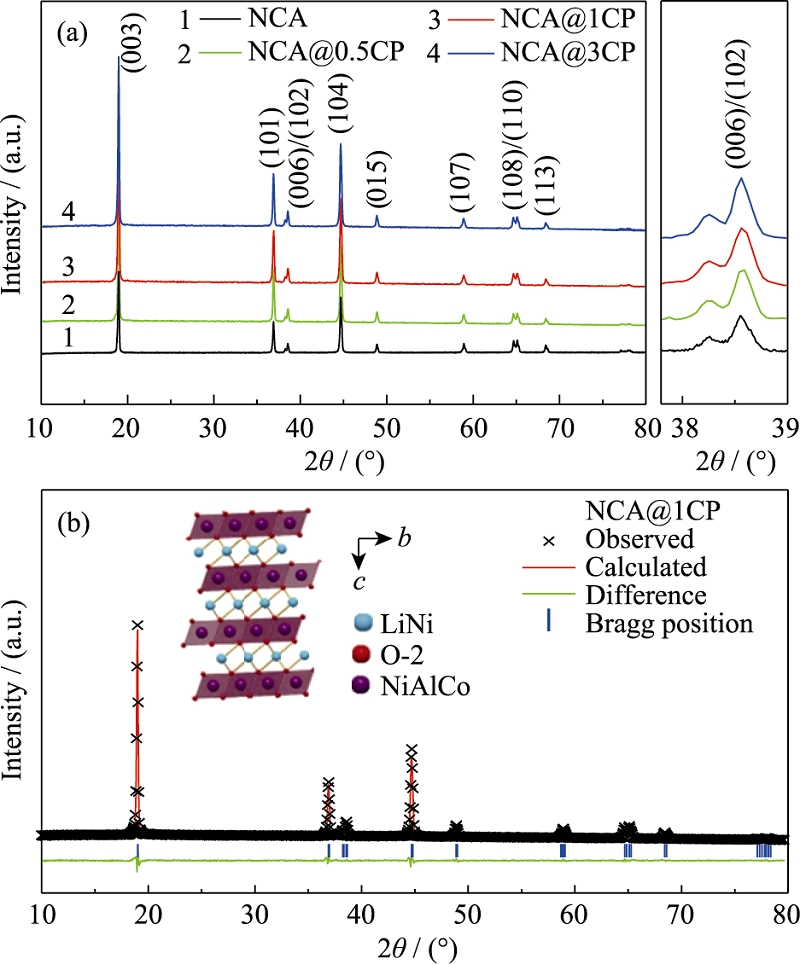
图1 (a)NCA和NCA@nCP(n=0.5, 1, 3)的XRD图谱; (b)NCA@1CP的精修结果
Fig. 1 (a) XRD patterns of NCA and NCA@nCP (n=0.5, 1, 3), and (b) Rietveld refinement results of NCA@1CP Colourful figures are available on website
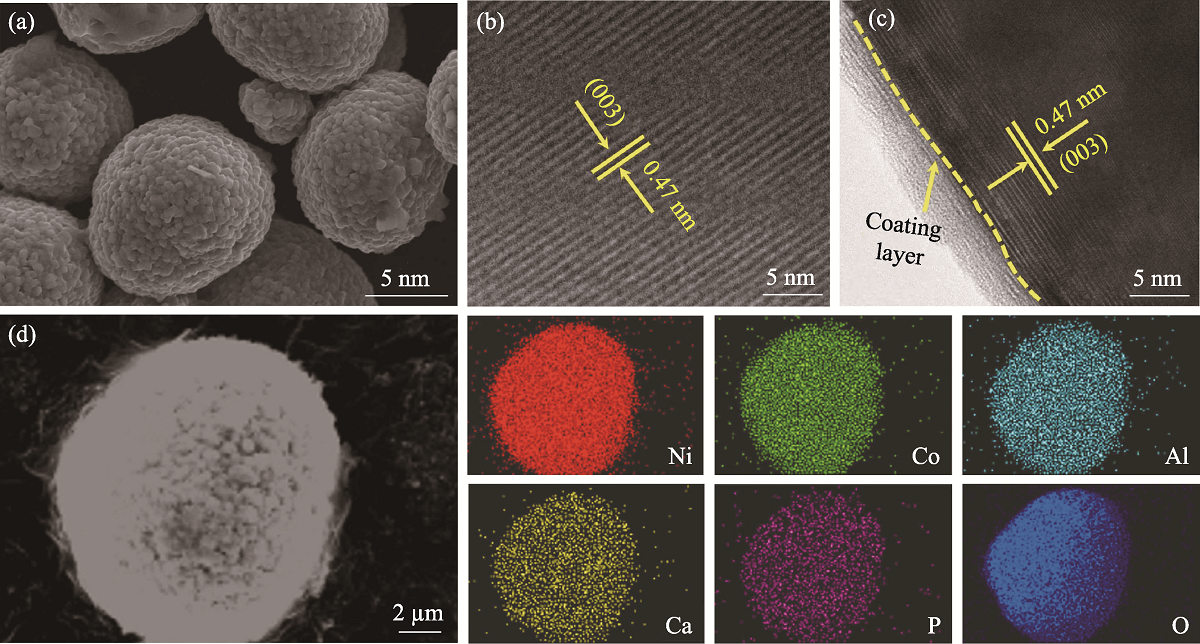
图2 (a)NCA@1CP的SEM照片; (b)NCA和(c)NCA@1CP的高分辨TEM照片; (d)NCA@1CP的EDS元素分布图
Fig. 2 (a) SEM image of NCA@1CP, high-resolution TEM images of (b) NCA and (c) NCA@1CP, and (d) EDS elemental mappings of NCA@1CP
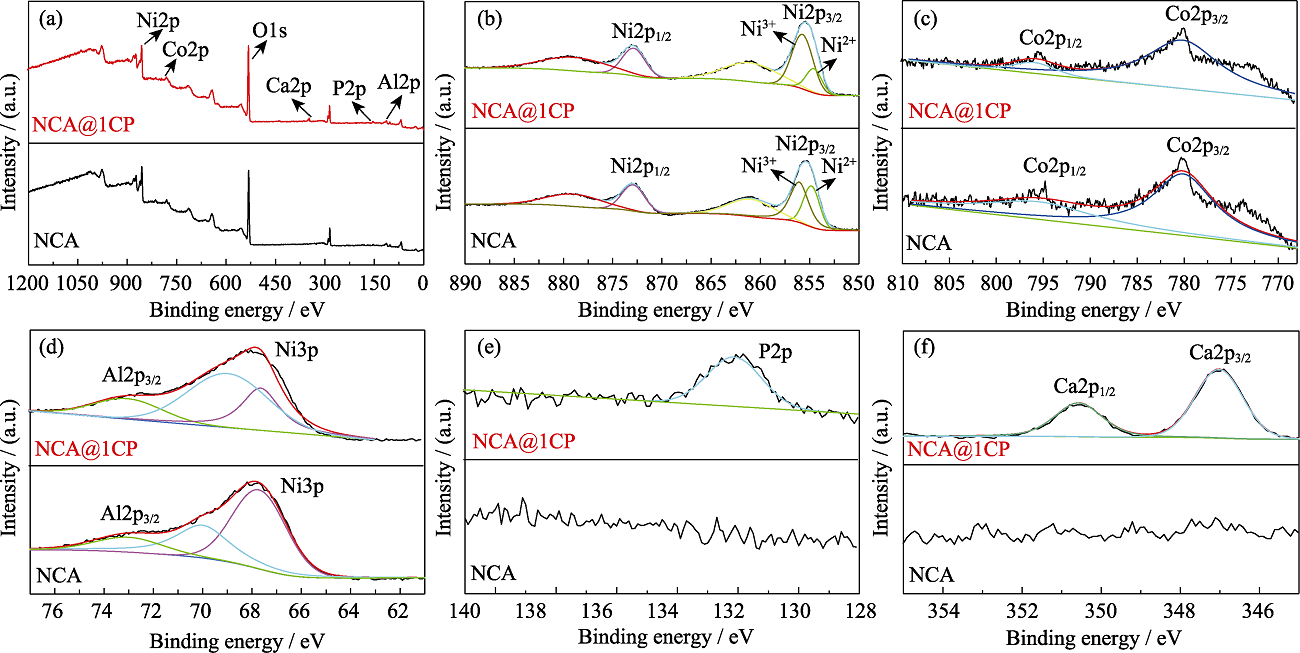
图3 NCA和NCA@1CP的(a)XPS全谱, (b)Ni2p、(c)Co2p、(d)Al2p、(e)P2p和(f)Ca2p XPS分谱图
Fig. 3 (a) Full survey, (b) Ni2p, (c) Co2p, (d) Al2p, (e) P2p and (f) Ca2p XPS spectra for NCA and NCA@1CP Colourful figures are available on website
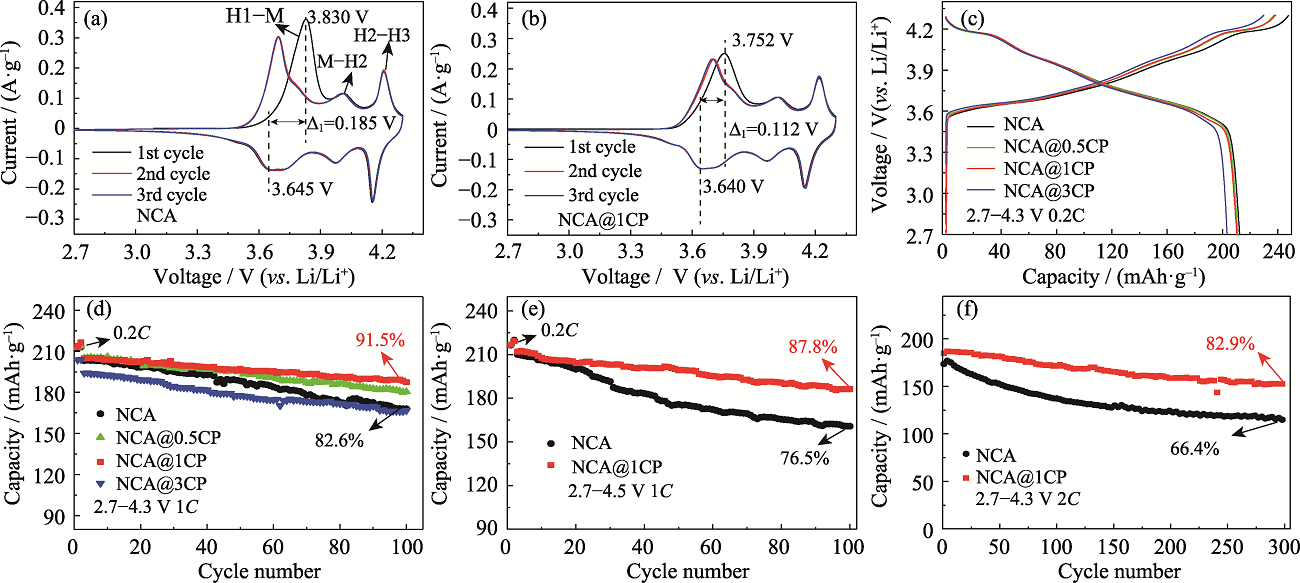
图4 (a)NCA和(b)NCA@1CP的CV曲线; NCA和NCA@nCP在(c)0.2C及2.7~4.3 V条件下的初始充电/放电曲线和(d)1C及2.7~4.3 V条件下的循环性能; NCA和NCA@1CP在(e)1C及2.7~4.5 V和(f)2C及2.7~4.3 V条件下的循环性能
Fig. 4 CV curves for (a) NCA and (b) NCA@1CP; (c) Initial charge-discharge curves under 2.7-4.3 V at 0.2C and (d) cycling properties under 2.7-4.3 V at 1C for NCA and NCA@nCP; Cycling properties for NCA and NCA@1CP (e) under 2.7-4.5 V at 1C and (f) under 2.7-4.3 V at 2C Colourful figures are available on website
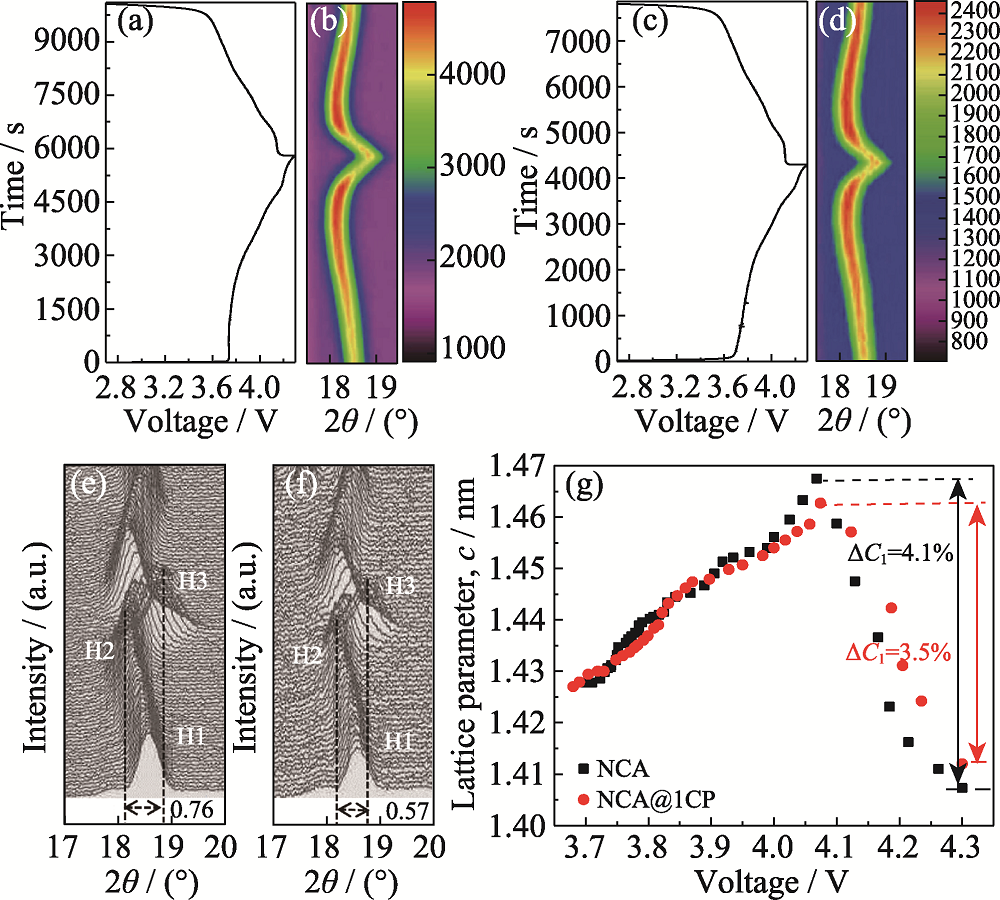
图5 (a)NCA和(c)NCA@1CP在原位XRD测试时的电压-时间变化曲线; (b, e)NCA和(d, f)NCA@1CP的原位XRD图谱; (g)NCA和NCA@1CP充电过程中晶格参数c的变化曲线
Fig. 5 Voltage-time variation curves during in-situ XRD for (a) NCA and (c) NCA@1CP; In-situ XRD patterns for (b, e) NCA and (d, f) NCA@1CP; (g) Variation curves of the lattice parameter c during charging for NCA and NCA@1CP
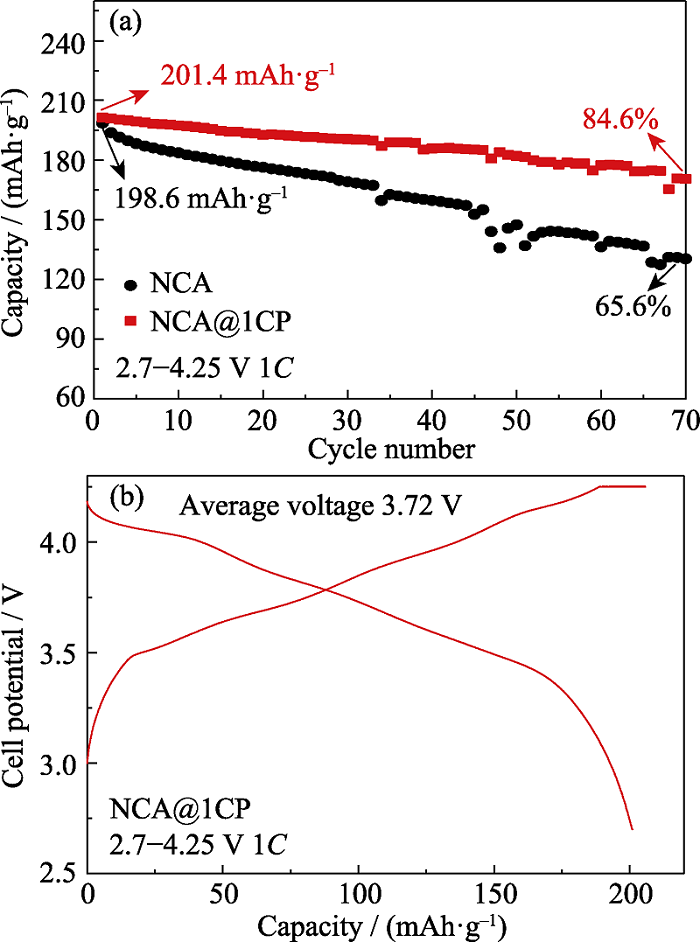
图6 (a)在1C及2.7~4.25 V条件下NCA和NCA@1CP为正极、石墨为负极的全电池循环性能; (b)NCA@1CP全电池第二圈充放电曲线
Fig. 6 (a) Cycling properties of full cells both NCA and NCA@1CP as cathode, graphite as anode under 2.7-4.25 V at 1C, and (b) charge-discharge curves of the second cycle for NCA@1CP in full cell
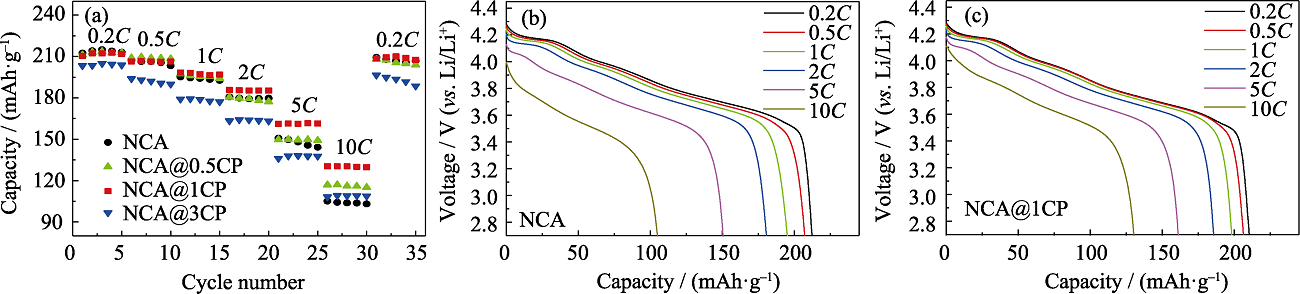
图S5 (a)NCA、NCA@0.5CP、NCA@1CP和NCA@3CP的倍率性能; (b)NCA和(c)NCA@1CP倍率测试中相应的放电曲线
Fig. S5 (a) Rate performance for NCA, NCA@0.5CP, NCA@1CP and NCA@3CP; Corresponding discharge curves during rate tests of (b) NCA and (c) NCA@1CP
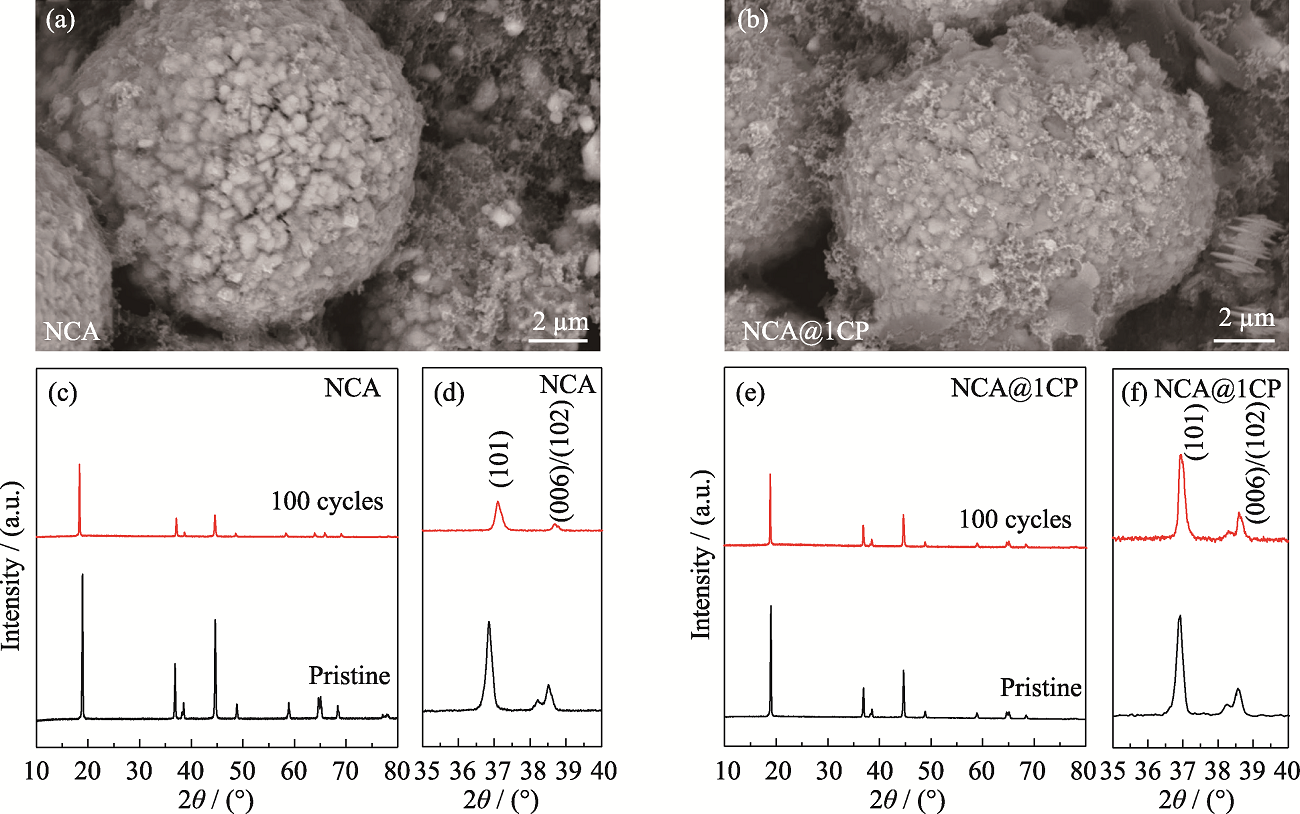
图S6 NCA和NCA@1CP在1C及2.7~4.3 V条件下循环100圈后的(a, b)SEM照片与(c~f)XRD图谱
Fig. S6 (a, b) SEM images and (c-f) XRD patterns of NCA and NCA@1CP after 100 cycles under 2.7-4.3 V at 1C
| Sample | NCA | NCA@0.5CP | NCA@1CP | NCA@3CP |
|---|---|---|---|---|
| I(003)/I(104) | 1.426 | 2.120 | 2.261 | 1.981 |
表S1 NCA、NCA@0.5CP、NCA@1CP和NCA@3CP的I(003)/I(104)值
Table S1 I(003)/I(104) values for NCA, NCA@0.5CP, NCA@1CP and NCA@3CP
| Sample | NCA | NCA@0.5CP | NCA@1CP | NCA@3CP |
|---|---|---|---|---|
| I(003)/I(104) | 1.426 | 2.120 | 2.261 | 1.981 |
| Sample | a/nm | c/nm | V/nm3 | c/a | Rwp | Rp |
|---|---|---|---|---|---|---|
| NCA | 0.287398 | 1.421071 | 0.101652 | 4.944609 | 7.13 | 4.18 |
| NCA@0.5CP | 0.287296 | 1.420733 | 0.101556 | 4.945188 | 6.63 | 4.73 |
| NCA@1CP | 0.287286 | 1.420440 | 0.101527 | 4.944341 | 5.86 | 4.39 |
| NCA@3CP | 0.287305 | 1.420646 | 0.101556 | 4.944731 | 6.74 | 4.82 |
表S2 NCA、NCA@0.5CP、NCA@1CP和NCA@3CP的晶格参数
Table S2 Lattice parameters of the NCA, NCA@0.5CP, NCA@1CP and NCA@3CP calculated from XRD Rietveld refinement
| Sample | a/nm | c/nm | V/nm3 | c/a | Rwp | Rp |
|---|---|---|---|---|---|---|
| NCA | 0.287398 | 1.421071 | 0.101652 | 4.944609 | 7.13 | 4.18 |
| NCA@0.5CP | 0.287296 | 1.420733 | 0.101556 | 4.945188 | 6.63 | 4.73 |
| NCA@1CP | 0.287286 | 1.420440 | 0.101527 | 4.944341 | 5.86 | 4.39 |
| NCA@3CP | 0.287305 | 1.420646 | 0.101556 | 4.944731 | 6.74 | 4.82 |
| [1] |
SCROSATI B, GARCHE J. Lithium batteries: status, prospects and future. Journal of Power Sources, 2010, 195(9): 2419-2430.
DOI URL |
| [2] |
MANTHIRAM A. An outlook on lithium ion battery technology. ACS Central Science, 2017, 3(10): 1063-1069.
DOI URL |
| [3] |
YU T, KE B Y, LI H Y, et al. Recent advances in sulfide electrolytes toward high specific energy solid-state lithium batteries. Materials Chemistry Frontiers, 2021, 5(13): 4892-4911.
DOI URL |
| [4] |
YE Z C, QIU L, YANG W, et al. Recent progress of nickel-rich layered cathode materials for lithium-ion batteries. Chemistry-A European Journal, 2021, 27(13): 4249-4269.
DOI URL |
| [5] |
KIM J, LEE H, CHA H, et al. Prospect and reality of Ni-rich cathode for commercialization. Advanced Energy Materials, 2018, 8(6): 1702028.
DOI URL |
| [6] |
SUN Y K. High-capacity layered cathodes for next-generation electric vehicles. ACS Energy Letters, 2019, 4(5): 1042-1044.
DOI URL |
| [7] |
GANNETT C N, MELECIO-ZAMBRANO L, THEIBAULT M J, et al. Organic electrode materials for fast-rate, high-power battery applications. Materials Reports: Energy, 2021, 1(1): 100008.
DOI URL |
| [8] |
PAN J X, YE Y J, ZHOU M Z, et al. Improving the activity and stability of Ni-based electrodes for solid oxide cells through surface engineering: recent progress and future perspectives. Materials Reports: Energy, 2021, 1(2): 100025.
DOI URL |
| [9] |
SUN Y K, MYUNG S T, PARK B C, et al. High-energy cathode material for long-life and safe lithium batteries. Nature Materials, 2009, 8(4): 320-324.
DOI URL |
| [10] |
WANG X X, DING Y L, DENG Y P, et al. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: promises and challenges. Advanced Energy Materials, 2020, 10(12): 1903864.
DOI URL |
| [11] | MANTHIRAM A, SONG B H, LI W D. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Materials, 2017, 6: 125-139. |
| [12] |
KIM U H, KUO L Y, KAGHAZCHI P, et al. Quaternary layered Ni-rich NCMA cathode for lithium-ion batteries. ACS Energy Letters, 2019, 4(2): 576-582.
DOI URL |
| [13] |
RYU H H, PARK K J, YOON D R, et al. Li[Ni0.9Co0.09W0.01]O2: a new type of layered oxide cathode with high cycling stability. Advanced Energy Materials, 2019, 9(44): 1902698.
DOI URL |
| [14] | LIU L H, LI M C, CHU L H, et al. Layered ternary metal oxides: performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Progress in Materials Science, 2020, 111: 100655. |
| [15] |
HOU P Y, YIN J M, DING M, et al. Surface/interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: advances and perspectives. Small, 2017, 13(45): 1701802.
DOI URL |
| [16] | NOH H J, YOUN S, YOON C S, et al. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. Journal of Power Sources, 2013, 233: 121-130. |
| [17] | GUAN P Y, ZHOU L, YU Z L, et al. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. Journal of Energy Chemistry, 2020, 43: 220-235. |
| [18] |
TAN X R, ZHANG M L, LI J, et al. Recent progress in coatings and methods of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials: a short review. Ceramics International, 2020, 46(14): 21888-21901.
DOI URL |
| [19] |
HERZOG M J, GAUQUELIN N, ESKEN D, et al. Facile dry coating method of high-nickel cathode material by nanostructured fumed alumina (Al2O3) improving the performance of lithium-ion batteries. Energy Technology, 2021, 9(4): 2100028.
DOI URL |
| [20] | ZHAO S Y, ZHU Y T, QIAN Y C, et al. Annealing effects of TiO2 coating on cycling performance of Ni-rich cathode material LiNi0.8Co0.1Mn0.1O2 for lithium-ion battery. Materials Letters, 2020, 265: 127418. |
| [21] |
ZHOU P F, ZHANG Z, MENG H J, et al. SiO2-coated LiNi0.915Co0.075Al0.01O2 cathode material for rechargeable Li-ion batteries. Nanoscale, 2016, 8(46): 19263-19269.
DOI URL |
| [22] | HO V C, JEONG S, YIM T, et al. Crucial role of thioacetamide for ZrO2 coating on the fragile surface of Ni-rich layered cathode in lithium ion batteries. Journal of Power Sources, 2020, 450: 227625. |
| [23] | HUANG W, ZHUANG W D, LI N, et al. Nanoscale Y-doped ZrO2 modified LiNi0.88Co0.09Al0.03O2 cathode material with enhanced electrochemical properties for lithium-ion batteries. Solid State Ionics, 2019, 343: 115087. |
| [24] |
XIAO Y H, MIARA L J, WANG Y, et al. Computational screening of cathode coatings for solid-state batteries. Joule, 2019, 3(5): 1252-1275.
DOI URL |
| [25] |
MIN K, PARK K, PARK S Y, et al. Improved electrochemical properties of LiNi0.91Co0.06Mn0.03O2 cathode material via Li-reactive coating with metal phosphates. Scientific Reports, 2017, 7(1): 7151.
DOI URL |
| [26] |
JAMIL S, WANG G, YANG L, et al. Suppressing H2-H3 phase transition in high Ni-low Co layered oxide cathode material by dual modification. Journal of Materials Chemistry A, 2020, 8(40): 21306-21316.
DOI URL |
| [27] |
HU G R, DENG X R, PENG Z D, et al. Comparison of AlPO4- and Co3(PO4)2-coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery. Electrochimica Acta, 2008, 53(5): 2567-2573.
DOI URL |
| [28] |
YAN P F, ZHENG J M, LIU J, et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nature Energy, 2018, 3(7): 600-605.
DOI URL |
| [29] | FENG Z, RAJAGOPALAN R, SUN D, et al. In-situ formation of hybrid Li3PO4-AlPO4-Al(PO3)3 coating layer on LiNi0.8Co0.1Mn0.1O2 cathode with enhanced electrochemical properties for lithium-ion battery. Chemical Engineering Journal, 2020, 382: 122959. |
| [30] |
JO C H, JO J H, YASHIRO H, et al. Bioinspired surface layer for the cathode material of high-energy-density sodium-ion batteries. Advanced Energy Materials, 2018, 8(13): 1702942.
DOI URL |
| [31] |
WEIGEL T, SCHIPPER F, ERICKSON E M, et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2cathode materials doped by various cations. ACS Energy Letters, 2019, 4(2): 508-516.
DOI URL |
| [32] |
HU S K, CHENG G H, CHENG M Y, et al. Cycle life improvement of ZrO2-coated spherical LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries. Journal of Power Sources, 2009, 188(2): 564-569.
DOI URL |
| [33] |
ZHOU P F, MENG H J, ZHANG Z, et al. Stable layered Ni-rich LiNi0.9Co0.07Al0.03O2 microspheres assembled with nanoparticles as high-performance cathode materials for lithium-ion batteries. Journal of Materials Chemistry A, 2017, 5(6): 2724-2731.
DOI URL |
| [34] |
YANG X Q, SUN X, MCBREEN J. New findings on the phase transitions in Li1-xNiO2: in situ synchrotron X-ray diffraction studies. Electrochemistry Communications, 1999, 1(6): 227-232.
DOI URL |
| [35] | DUAN J G, HU G R, CAO Y B, et al. Enhanced electrochemical performance and storage property of LiNi0.815Co0.15Al0.035O2 via Al gradient doping. Journal of Power Sources, 2016, 326: 322-330. |
| [36] | LIANG H M, WANG Z X, GUO H J, et al. Improvement in the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by Li2ZrO3 coating. Applied Surface Science, 2017, 423: 1045-1053. |
| [37] | LI H Y, GUO S H, ZHOU H S. In-situ/operando characterization techniques in lithium-ion batteries and beyond. Journal of Energy Chemistry, 2021, 59: 191-211. |
| [38] |
CROGUENNEC L, POUILLERIE C, MANSOUR A N, et al. Structural characterisation of the highly deintercalated LixNi1.02O2 phases (with ≤0.30). Journal of Materials Chemistry, 2001, 11(1): 131-141.
DOI URL |
| [39] |
CROGUENNEC L, POUILLERIE C, DELMAS C. NiO2obtained by electrochemical lithium deintercalation from lithium nickelate: structural modifications. Journal of The Electrochemical Society, 2000, 147(4): 1314.
DOI URL |
| [1] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [2] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [3] | 程节, 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直. 蛋黄壳结构FeF3·0.33H2O@N掺杂碳纳米笼正极材料的构筑及其电化学性能[J]. 无机材料学报, 2024, 39(3): 299-305. |
| [4] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| [5] | 苏楠, 邱介山, 王治宇. 高容量氟掺杂碳包覆纳米硅负极材料: 气相氟化法制备及其储锂性能[J]. 无机材料学报, 2023, 38(8): 947-953. |
| [6] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [7] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [8] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [9] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [10] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [11] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [12] | 苏东良, 崔锦, 翟朋博, 郭向欣. 石榴石型Li6.4La3Zr1.4Ta0.6O12对Si/C负极表面固体电解质中间相的调控机制研究[J]. 无机材料学报, 2022, 37(7): 802-808. |
| [13] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [14] | 王禹桐, 张非凡, 许乃才, 王春霞, 崔立山, 黄国勇. 水系锂离子电池负极材料LiTi2(PO4)3的研究进展[J]. 无机材料学报, 2022, 37(5): 481-492. |
| [15] | 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安. 源于溪木贼的高性能锂离子电池三维多孔生物质硅/碳复合负极材料[J]. 无机材料学报, 2021, 36(9): 929-935. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||