无机材料学报 ›› 2022, Vol. 37 ›› Issue (7): 710-716.DOI: 10.15541/jim20210653 CSTR: 32189.14.10.15541/jim20210653
江依义1( ), 沈旻1, 宋半夏1, 李南1, 丁祥欢1, 郭乐毅2, 马国强1,2(
), 沈旻1, 宋半夏1, 李南1, 丁祥欢1, 郭乐毅2, 马国强1,2( )
)
收稿日期:2021-10-22
修回日期:2022-01-19
出版日期:2022-07-20
网络出版日期:2022-03-18
通讯作者:
马国强, 高级工程师. E-mail: erguo87@163.com; maguoqiang@sinochem.com作者简介:江依义(1988-), 女, 硕士. E-mail: jiangyiyi@sinochem.com
基金资助:
JIANG Yiyi1( ), SHEN Min1, SONG Banxia1, LI Nan1, DING Xianghuan1, GUO Leyi2, MA Guoqiang1,2(
), SHEN Min1, SONG Banxia1, LI Nan1, DING Xianghuan1, GUO Leyi2, MA Guoqiang1,2( )
)
Received:2021-10-22
Revised:2022-01-19
Published:2022-07-20
Online:2022-03-18
Contact:
MA Guoqiang, senior engineer. E-mail: erguo87@163.com; maguoqiang@sinochem.comAbout author:JIANG Yiyi(1988-), female, Master. E-mail: jiangyiyi@sinochem.com
Supported by:摘要:
三元锂离子动力电池的开发和应用受制于高温高电压条件下的容量衰减和电池产气鼓胀等技术难题。解决这些问题一方面要注重电极材料改性和电池设计, 另一方面还依赖于电解液的技术进步。本研究报道了四乙烯基硅烷(Tetravinylsilane, TVS)作为LiNi0.6Co0.2Mn0.2O2(NCM622)/石墨软包电池的电解液添加剂, 可以显著改善电池的高温(45~60 ℃) 高电压(4.4 V)性能, 包括存储和循环性能。结果表明, 电解液中含有质量分数0.5% TVS的电池在2.8~4.4 V区间, 1C (1C=1.1 Ah)倍率下循环400次后的容量保持率达到92%, 而电解液中未添加TVS的软包电池仅为82%。进一步研究表明, 一方面TVS高电压下优先被氧化, 可以在NCM622颗粒表面形成耐高温的CEI膜, 有效抑制NCM622颗粒内部裂纹和过渡金属离子溶出; 另一方面, TVS在低电位下还可以优先被还原, 在石墨负极表面聚合形成稳定的SEI膜, 抑制电解液与负极之间的副反应。
中图分类号:
江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716.
JIANG Yiyi, SHEN Min, SONG Banxia, LI Nan, DING Xianghuan, GUO Leyi, MA Guoqiang. Effect of Dual-functional Electrolyte Additive on High Temperature and High Voltage Performance of Li-ion Battery[J]. Journal of Inorganic Materials, 2022, 37(7): 710-716.
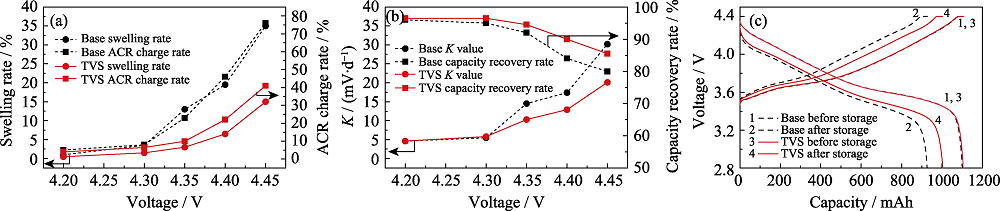
图3 对照组和实验组电池经60 ℃存储14 d后的(a)体积和内阻变化率、(b)自放电率和容量恢复率以及(c)充放电曲线
Fig. 3 (a) Gas swelling rates and ACR change rates, (b) K values and capacity recovery rates, (c) charge and discharge curves of pouch cells with and without TVS in electrolytes after storage at 60 ℃ for 14 d
| Sample | Ni/(×10-4, %) | Co/(×10-4, %) | Mn/(×10-4, %) |
|---|---|---|---|
| Base | 90 | 21 | 130 |
| TVS | 35 | 4 | 69 |
表1 对照组和实验组电池经4.4 V、60 ℃存储14 d后负极上沉积的过渡金属离子含量(质量分数)
Table 1 Uess fractions of Ni, Co and Mn ions deposited on anodes from pouch cell with and without TVS in electrolyte stored at cutoff potential of 4.4 V and temperature of 60 ℃ for 14 d
| Sample | Ni/(×10-4, %) | Co/(×10-4, %) | Mn/(×10-4, %) |
|---|---|---|---|
| Base | 90 | 21 | 130 |
| TVS | 35 | 4 | 69 |
| Sample | CO/µL | CH4/µL | CO2/µL | C2H4/µL | H2/µL |
|---|---|---|---|---|---|
| Base | 393.3 | 253.2 | 209.3 | 67.0 | 17.6 |
| TVS | 9.2 | 4.8 | 2.2 | 1.1 | 0.2 |
表S1 实验组和对照组电池经4.4 V、60 ℃存储14 d后的气体成分及含量
Table S1 Gas composition and contents of pouch cells with and without TVS in electrolytes stored at cutoff potential of 4.4 V and temperature of 60 ℃ for 14 d
| Sample | CO/µL | CH4/µL | CO2/µL | C2H4/µL | H2/µL |
|---|---|---|---|---|---|
| Base | 393.3 | 253.2 | 209.3 | 67.0 | 17.6 |
| TVS | 9.2 | 4.8 | 2.2 | 1.1 | 0.2 |
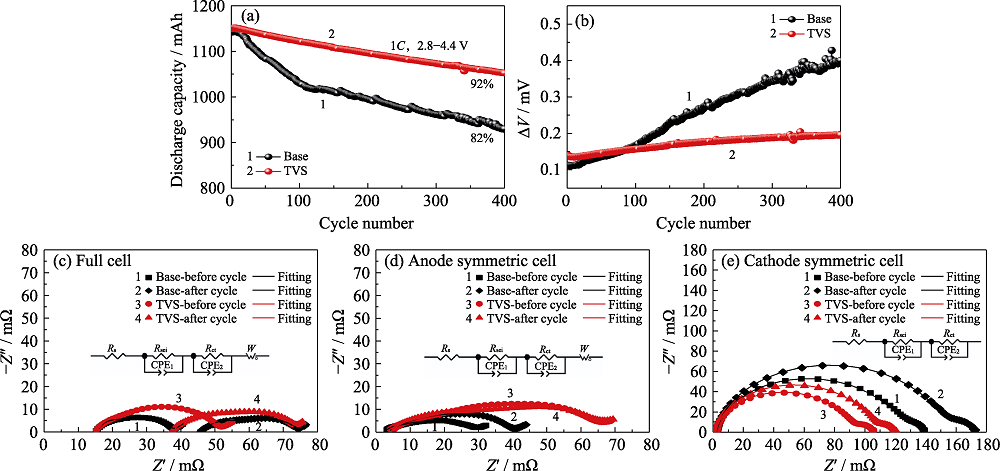
图4 对照组和实验组电池在45 ℃的循环性能
Fig. 4 Cycling performance of pouch cells with and without TVS in electrolytes at 45 ℃ (a) Discharge capacity and (b) ΔV vs cycle number; EIS plots of (c) pouch full cells, (d) graphite/graphite symmetric cells and (e) NCM622/NCM622 symmetric cells before and after 100 cycles
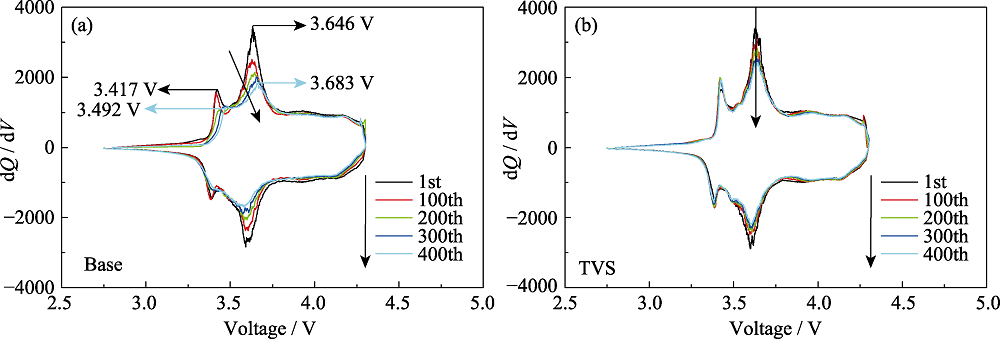
图S2 对照组和实验组电池在45 ℃的循环性能
Fig. S2 Cycling performance of pouch cells with and without TVS in electrolytes at 45 ℃ (a) Differential capacity (dQ/dV) versus potential of Base; (b) Differential capacity (dQ/dV) versus potential of TVS
| Sample | Full cell | Anode symmetric cell | Cathode symmetric cell | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs/mΩ | Rsei/mΩ | Rct/mΩ | Rs/mΩ | Rsei/mΩ | Rct/mΩ | Rs/mΩ | Rsei/mΩ | Rct/mΩ | |
| Base-before cycle | 16.2 | 7.5 | 14.2 | 3.4 | 4.7 | 23.2 | 2.6 | 116.1 | 21.2 |
| Base-after cycle | 46.7 | 9.1 | 19.3 | 4.2 | 17.1 | 26.5 | 2.7 | 144.3 | 26.7 |
| TVS-before cycle | 16.4 | 8.9 | 25.8 | 4.5 | 20.5 | 43.3 | 2.1 | 86.1 | 18.2 |
| TVS-after cycle | 38.5 | 10.2 | 25.4 | 4.8 | 22.6 | 43.8 | 2.2 | 100.4 | 18.1 |
表S2 对照组和实验组电池循环100周前后及相应的对称电池的EIS拟合参数
Table S2 Fitted EIS results before and after 100 cycles of pouch full cells and corresponding symmetric cells with and without TVS in electrolytes
| Sample | Full cell | Anode symmetric cell | Cathode symmetric cell | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs/mΩ | Rsei/mΩ | Rct/mΩ | Rs/mΩ | Rsei/mΩ | Rct/mΩ | Rs/mΩ | Rsei/mΩ | Rct/mΩ | |
| Base-before cycle | 16.2 | 7.5 | 14.2 | 3.4 | 4.7 | 23.2 | 2.6 | 116.1 | 21.2 |
| Base-after cycle | 46.7 | 9.1 | 19.3 | 4.2 | 17.1 | 26.5 | 2.7 | 144.3 | 26.7 |
| TVS-before cycle | 16.4 | 8.9 | 25.8 | 4.5 | 20.5 | 43.3 | 2.1 | 86.1 | 18.2 |
| TVS-after cycle | 38.5 | 10.2 | 25.4 | 4.8 | 22.6 | 43.8 | 2.2 | 100.4 | 18.1 |
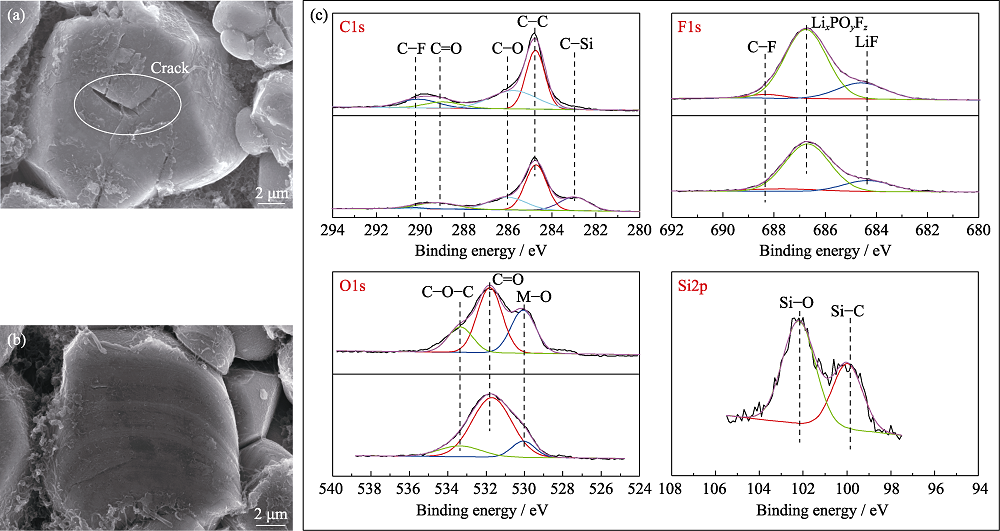
图5 (a)对照组和(b)实验组电池循环100周后正极SEM照片, (c)对照组和实验组电池循环10周后正极表面XPS分谱
Fig. 5 SEM images of cathodes from pouch cells (a) without and (b) with TVS in electrolytes after 100 cycles; (c) XPS spectra of cathodes from pouch cells without and with TVS in electrolytes after 10 cycles
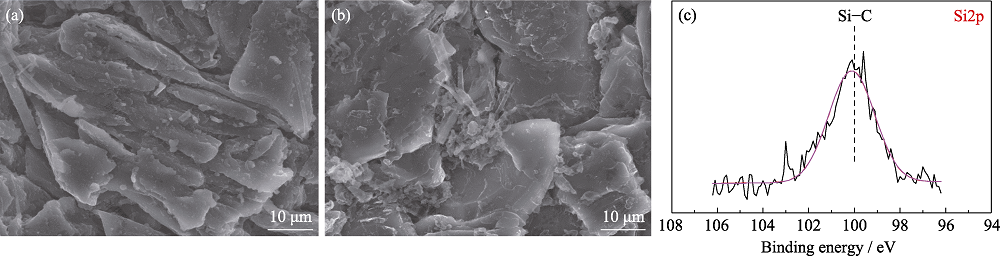
图S3 (a)对照组和(b)实验组电池循环100周后负极SEM照片; (c)实验组电池循环10周后负极表面Si2p XPS分谱
Fig. S3 SEM images of anodes from pouch cells (a) without and (b) with TVS in electrolytes after 100 cycles; (c) Si2p XPS spectra of anode from pouch cell with TVS in electrolyte after 10 cycles
| [1] | ZENG X, LI M, EL-HADY D A, et al. Commercialization of lithium battery technologies for electric vehicles. Advanced Energy Materials, 2019, 9(27): 1900161. |
| [2] |
WANG C, OUYANG L, FAN W, et al. Citraconic anhydride as an electrolyte additive to improve the high temperature performance of LiNi0.6Co0.2Mn0.2O2/graphite pouch batteries. Journal of Alloys and Compounds, 2019, 805: 757-766.
DOI URL |
| [3] |
WANG C, YU L, FAN W, et al. Lithium difluorophosphate as a promising electrolyte lithium additive for high-voltage lithium-ion batteries. ACS Applied Energy Materials, 2018, 1(6): 2647-2656.
DOI URL |
| [4] | ZHAO W, ZHENG B, LIU H, et al. Toward a durable solid electrolyte film on the electrodes for Li-ion batteries with high performance. Nano Energy, 2019, 63: 103815. |
| [5] |
JUNG R, METZGER M, MAGLIA F, et al. Chemical versus electrochemical electrolyte oxidation on NMC111, NMC622, NMC811, LNMO, and conductive carbon. Journal of Physical Chemistry Letters, 2017, 8(19): 4820-4825.
DOI URL |
| [6] |
XIE K, ZHENG C, LI Y, et al. Storage aging mechanism of LiNi0.8Co0.15Al0.05O2/graphite Li-ion batteries at high state of charge. Journal of Inorganic Materials, 2021, 36(2): 175-180.
DOI URL |
| [7] | JUNG R, METZGER M, MAGLIA F, et al. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2(NMC) cathode materials for Li-ion batteries. Journal of The Electrochemical Society, 2017, 164(7): A1361-A1377. |
| [8] |
FAN X, WANG C. High-voltage liquid electrolytes for Li batteries progress and perspectives. Chemical Society Reviews, 2021, 50(18): 10486-10566.
DOI URL |
| [9] | ZHAO D, WANG J, LU H, et al. Tailoring interfacial architecture of high-voltage cathode with lithium difluoro(bisoxalato) phosphate for high energy density battery. Journal of Power Sources, 2020, 456: 228006. |
| [10] |
LEE W J, PRASANNA K, JO Y N, et al. Depth profile studies on nickel rich cathode material surfaces after cycling with an electrolyte containing vinylene carbonate at elevated temperature. Physical Chemistry Chemical Physics, 2014, 16(32): 17062-17071.
DOI URL |
| [11] |
MICHAN A L, PARIMALAM B S, LESKES M, et al. Fluoroethylene carbonate and vinylene carbonate reduction: understanding lithium-ion battery electrolyte additives and solid electrolyte interphase formation. Chemistry of Materials, 2016, 28(22): 8149-8159.
DOI URL |
| [12] |
MARKEVICH E, SALITRA G, FRIDMAN K, et al. Fluoroethylene carbonate as an important component in electrolyte solutions for high-voltage lithium batteries: role of surface chemistry on the cathode. Langmuir, 2014, 30(25): 7414-7424.
DOI URL |
| [13] |
LI J, XING L, ZHANG R, et al. Tris(trimethylsilyl)borate as an electrolyte additive for improving interfacial stability of high voltage layered lithium-rich oxide cathode/carbonate-based electrolyte. Journal of Power Sources, 2015, 285: 360-376.
DOI URL |
| [14] |
WANG K, XING L, ZHU Y, et al. A comparative study of Si-containing electrolyte additives for lithium ion battery: which one is better and why is it better. Journal of Power Sources, 2017, 342: 677-684.
DOI URL |
| [15] |
DENG B, SUN D, WAN Q, et al. Review of electrolyte additives for ternary cathode lithium-ion battery. Acta Chimica Sinica, 2018, 76(4): 259.
DOI URL |
| [16] |
LIAO X, ZHENG X, CHEN J, et al. Tris(trimethylsilyl)-phosphate as electrolyte additive for self-discharge suppression of layered nickel cobalt manganese oxide. Electrochimica Acta, 2016, 212: 352-359.
DOI URL |
| [17] |
KIM K, PARK I, HA S Y, et al. Understanding the thermal instability of fluoroethylene carbonate in LiPF6-based electrolytes for lithium ion batteries. Electrochimica Acta, 2017, 225: 358-368.
DOI URL |
| [18] |
QI X, TAO L, HAHN, H, et al. Lifetime limit of tris(trimethylsilyl) phosphite as electrolyte additive for high voltage lithium ion batteries. RSC Advance, 2016, 6(44): 38342-38349.
DOI URL |
| [19] | OH J, KIM J, LEE Y M, et al. Effects of vinylene carbonate and 1,3-propane sultone on high-rate cycle performance and surface properties of high-nickel layered oxide cathodes. Materials Research Bulletin, 2020, 132: 111008. |
| [20] |
XU M, LI W, LUCHT B L. Effect of propane sultone on elevated temperature performance of anode and cathode materials in lithium- ion batteries. Journal of Power Sources, 2009, 193(2): 804-809.
DOI URL |
| [21] |
WANG Y, NAKAMURA S, UE M, et al. Theoretical studies to understand surface chemistry on carbon anodes for lithium-ion batteries: reduction mechanisms of ethylene carbonate. Journal of the American Chemical Society, 123: 11708-11718.
DOI URL |
| [22] | LI J, LIU H, XIA J, et al. The impact of electrolyte additives and upper cut-off voltage on the formation of a rocksalt surface layer in LiNi0.8Mn0.1Co0.1O2 electrodes. Journal of The Electrochemical Society, 2017, 164(4): A655-A665. |
| [23] |
HONG P, XU M, ZHENG X, et al. Effect of ethylene glycol bis (propionitrile) ether (EGBE) on the performance and interfacial chemistry of lithium-rich layered oxide cathode. Journal of Power Sources, 2016, 329: 216-224.
DOI URL |
| [24] | YAN X, CHEN C, ZHU X, et al. Aminoalkyldisiloxane as effective electrolyte additive for improving high temperature cycle life of nickel-rich LiNi0.6Co0.2Mn0.2O2/graphite batteries. Journal of Power Sources, 2020, 461: 228099. |
| [25] | BUBERGER I, SEIDLMAYER S, GASTEIGER H A, et al. Aging analysis of graphite/LiNi1/3Mn1/3Co1/3O2 cells using XRD, PGAA, and AC Impedance. Journal of The Electrochemical Society, 2015, 162(14): A2737-A2746. |
| [26] |
GILBERT J A, SHKROB I A, ABRAHAM D P. Transition metal dissolution, ion migration, electrocatalytic reduction and capacity loss in lithium-ion full cells. Journal of The Electrochemical Society, 2017, 164(2): A389.
DOI URL |
| [27] | TENG X, BAI Y, MA L, et al. In-situ analysis of gas generation in lithium ion batteries with different carbonate-based electrolytes. ACS Applied Materials & Interfaces, 2015, 7(41): 22751-22755. |
| [28] |
YAO W, ZHANG H, ZHONG S, et al. Microwave-assisted synthesis and Co, Al co-modification of Ni-rich LiNi0.8Mn0.2O2 materials for Li-ion battery electrode. Journal of Inorganic Materials, 2021, 36(7): 718-732.
DOI URL |
| [29] | KEEFE A S, BUTEAU S, HILL I G, et al. Temperature dependent EIS studies separating charge transfer impedance from contact impedance in lithium-ion symmetric cells. Journal of The Electrochemical Society, 2019, 166(14): A3272-A3279. |
| [30] | LI Y K, CHENG B, JIAO F P, et al. The roles and working mechanism of salt-type additives on the performance of high-voltage lithium-ion batteries. ACS Applied Materials & Interfaces, 2020, 12: 16298-16307. |
| [1] | 周阳阳, 张艳艳, 于子怡, 傅正钱, 许钫钫, 梁瑞虹, 周志勇. 通过Bi3+自掺杂增强CaBi4Ti4O15基陶瓷压电性能[J]. 无机材料学报, 2025, 40(6): 719-728. |
| [2] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [3] | 周帆, 田志林, 李斌. 热防护系统用碳化物超高温陶瓷抗烧蚀涂层研究进展[J]. 无机材料学报, 2025, 40(1): 1-16. |
| [4] | 王文婷, 徐敬军, 马科, 李美栓, 李兴超, 李同起. 原位反应/热压合成Ti2AlC-20TiB2复合材料在1000~1300 ℃空气中的高温氧化行为[J]. 无机材料学报, 2025, 40(1): 31-38. |
| [5] | 全文心, 余艺平, 方冰, 李伟, 王松. 管状C/SiC复合材料高温空气氧化行为与宏细观建模研究[J]. 无机材料学报, 2024, 39(8): 920-928. |
| [6] | 谭敏, 陈小武, 杨金山, 张翔宇, 阚艳梅, 周海军, 薛玉冬, 董绍明. 流延成型结合反应熔渗制备ZrB2-SiC陶瓷及其微观结构与氧化行为研究[J]. 无机材料学报, 2024, 39(8): 955-964. |
| [7] | 黄建锋, 梁瑞虹, 周志勇. W/Cr共掺杂对CaBi2Nb2O9陶瓷晶体结构及电学性能的影响[J]. 无机材料学报, 2024, 39(8): 887-894. |
| [8] | 陈乾, 苏海军, 姜浩, 申仲琳, 余明辉, 张卓. 超高温氧化物陶瓷激光增材制造及组织性能调控研究进展[J]. 无机材料学报, 2024, 39(7): 741-753. |
| [9] | 张育育, 吴轶城, 孙佳, 付前刚. 聚合物转化SiHfCN陶瓷的制备及其吸波性能[J]. 无机材料学报, 2024, 39(6): 681-690. |
| [10] | 蔡飞燕, 倪德伟, 董绍明. 高熵碳化物超高温陶瓷的研究进展[J]. 无机材料学报, 2024, 39(6): 591-608. |
| [11] | 张幸红, 王义铭, 程源, 董顺, 胡平. 超高温陶瓷复合材料研究进展[J]. 无机材料学报, 2024, 39(6): 571-590. |
| [12] | 苟燕子, 康伟峰, 张庆雨. 由聚钛碳硅烷制备高结晶近化学计量比SiC(Ti)纤维[J]. 无机材料学报, 2024, 39(12): 1377-1383. |
| [13] | 邹凯, 张文斌, 关胜, 孙海轶, 彭凯伦, 邹家杰, 李学红, 王成, 冷雨欣, 梁瑞虹, 周志勇. 压电式高温液滴喷射元件研制[J]. 无机材料学报, 2023, 38(8): 987-988. |
| [14] | 姚磊, 杨东旺, 鄢永高, 唐新峰. 激光诱导方钴矿自蔓延高温合成过程研究[J]. 无机材料学报, 2023, 38(7): 815-822. |
| [15] | 吴爽, 苟燕子, 王永寿, 宋曲之, 张庆雨, 王应德. 高温热处理对国产KD-SA型SiC纤维组成结构与力学性能的影响[J]. 无机材料学报, 2023, 38(5): 569-576. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||