无机材料学报 ›› 2022, Vol. 37 ›› Issue (9): 1023-1029.DOI: 10.15541/jim20210757 CSTR: 32189.14.10.15541/jim20210757
王洋1,2( ), 范广新1,3(
), 范广新1,3( ), 刘培2, 尹金佩1, 刘宝忠2, 朱林剑3, 罗成果3
), 刘培2, 尹金佩1, 刘宝忠2, 朱林剑3, 罗成果3
收稿日期:2021-12-10
修回日期:2022-02-13
出版日期:2022-09-20
网络出版日期:2022-02-21
通讯作者:
范广新, 副教授. E-mail: fangx@hpu.edu.cn作者简介:王 洋(1997-), 男, 硕士研究生. E-mail: wangyang1857@126.com
基金资助:
WANG Yang1,2( ), FAN Guangxin1,3(
), FAN Guangxin1,3( ), LIU Pei2, YIN Jinpei1, LIU Baozhong2, ZHU Linjian3, LUO Chengguo3
), LIU Pei2, YIN Jinpei1, LIU Baozhong2, ZHU Linjian3, LUO Chengguo3
Received:2021-12-10
Revised:2022-02-13
Published:2022-09-20
Online:2022-02-21
Contact:
FAN Guangxin, associate professor. E-mail: fangx@hpu.edu.cnAbout author:WANG Yang (1997-), male, Master candidate. E-mail: wangyang1857@126.com
Supported by:摘要:
改善尖晶石锰酸锂的大倍率性能是目前锂离子电池的重点研究方向之一。本研究用高温固相法合成掺K+的尖晶石锰酸锂, 研究K+提高锰酸锂倍率性能的微观机制。结果表明, 尽管随着电流密度增大, 电极的放电比容量下降, 但掺K+提高材料的大倍率性能效果显著, 如最佳掺K+量(物质的量分数)1.0%时, 在10C (1C=150 mA·g-1)下比容量提高了一倍, 远高于0.5C下的1.9%。原因在于掺K+后, 首先, 锰酸锂的晶胞体积扩大, Li-O键变长, Li、Mn阳离子混排程度降低, 载流子(Mn3+)量增多; 其次, 电极极化和电荷迁移阻抗降低, 提高了材料的充放电可逆性、导电性及锂离子扩散能力; 再者, [Mn2]O4骨架更稳定, 减小了电化学过程中内应力变化, 抑制了晶体结构变化和颗粒破碎; 最后, 钾离子掺杂使制备过程中材料团聚, 从而减小电解液与电极的接触面积, 减轻电解液的侵蚀, 抑制锰的溶解。
中图分类号:
王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029.
WANG Yang, FAN Guangxin, LIU Pei, YIN Jinpei, LIU Baozhong, ZHU Linjian, LUO Chengguo. Microscopic Mechanism of K+ Doping on Performance of Lithium Manganese Cathode for Li-ion Battery[J]. Journal of Inorganic Materials, 2022, 37(9): 1023-1029.
| Sample | d(111)/nm | FWHM(111)/(°) | I(111)/(311) | a/nm | V/nm3 | d(Li-O)/nm | Mn/Li8a | Rwp | S |
|---|---|---|---|---|---|---|---|---|---|
| LKMO-0 | 0.474 | 0.151 | 1.529 | 0.822 | 0.556 | 0.187 | 2.53% | 10.23 | 1.13 |
| LKMO-1 | 0.476 | 0.166 | 1.585 | 0.824 | 0.561 | 0.189 | 2.29% | 10.97 | 1.13 |
| LKMO-2 | 0.476 | 0.177 | 1.876 | 0.825 | 0.563 | 0.193 | 2.27% | 10.53 | 1.07 |
| LKMO-3 | 0.477 | 0.179 | 1.678 | 0.826 | 0.564 | 0.191 | 2.23% | 10.91 | 1.15 |
表1 LKMO-n的详细晶体结构参数
Table 1 Detailed crystal structural parameters for LKMO-n
| Sample | d(111)/nm | FWHM(111)/(°) | I(111)/(311) | a/nm | V/nm3 | d(Li-O)/nm | Mn/Li8a | Rwp | S |
|---|---|---|---|---|---|---|---|---|---|
| LKMO-0 | 0.474 | 0.151 | 1.529 | 0.822 | 0.556 | 0.187 | 2.53% | 10.23 | 1.13 |
| LKMO-1 | 0.476 | 0.166 | 1.585 | 0.824 | 0.561 | 0.189 | 2.29% | 10.97 | 1.13 |
| LKMO-2 | 0.476 | 0.177 | 1.876 | 0.825 | 0.563 | 0.193 | 2.27% | 10.53 | 1.07 |
| LKMO-3 | 0.477 | 0.179 | 1.678 | 0.826 | 0.564 | 0.191 | 2.23% | 10.91 | 1.15 |

图2 LKMO-0(a, c)和LKMO-1(b, d)的SEM照片, LKMO-1的EDS分布图(e) (方框区域)
Fig. 2 SEM images of LKMO-0 (a, c) and LKMO-1 (b, d), EDS mappings for LKMO-1 (e) (rectangular area)
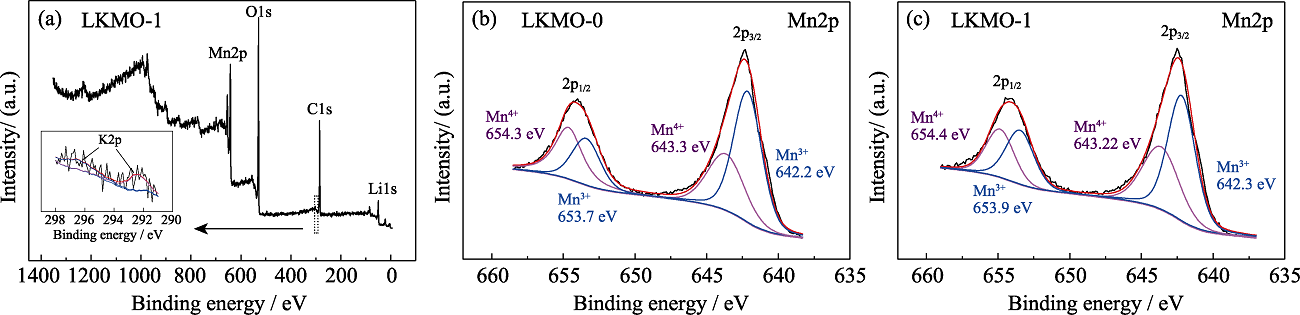
图3 LKMO-1的XPS全谱图(a), LKMO-0(b)和LKMO-1(c)的Mn2p XPS高分辨光谱图
Fig. 3 (a) XPS full spectrum of LKMO-1, and High-resolution Mn2p XPS spectra of LKMO-0 (b) and LKMO-1 (c)
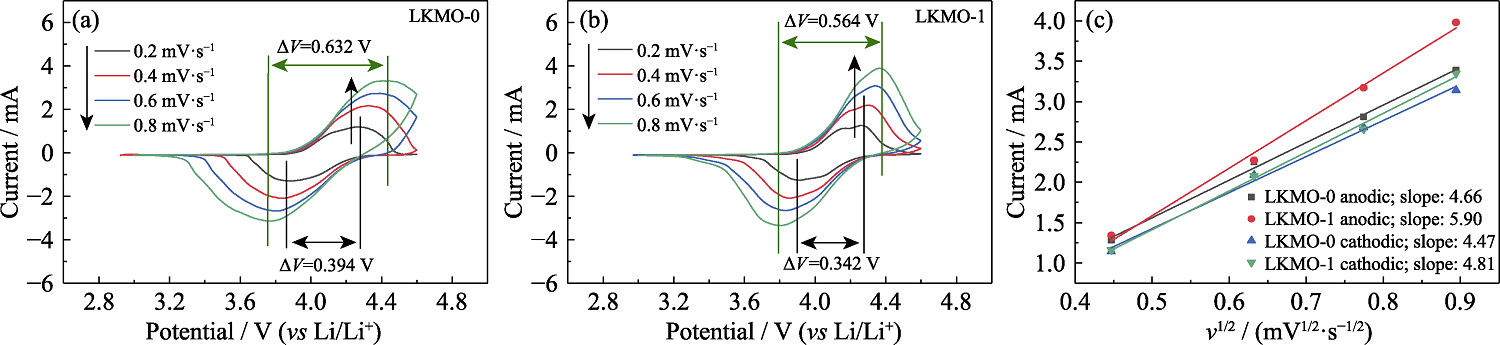
图5 LKMO-0(a)和LKMO-1(b)在不同扫描速率下的CV测试结果和氧化还原反应中ip随v1/2变化的拟合曲线(c)
Fig. 5 CV curves of LKMO-0 (a) and LKMO-1 (b) at different scan rates, and plots of ip versus v1/2 for redox reaction (c)
| Sample | Before charging | After charging/ discharging | DLi+ /(cm2·s-1) | ||
|---|---|---|---|---|---|
| Rs/Ω | Rct/Ω | Rs/Ω | Rct/Ω | ||
| LKMO-0 | 4.22 | 193.91 | 6.43 | 176.00 | 1.20×10-11 |
| LKMO-1 | 3.59 | 72.62 | 7.92 | 63.25 | 2.35×10-11 |
表2 LKMO-0和LKMO-1的EIS拟合结果和Li+扩散系数
Table 2 Fitting results of EIS and diffusion coefficients of Li-ions of LKMO-0 and LKMO-1
| Sample | Before charging | After charging/ discharging | DLi+ /(cm2·s-1) | ||
|---|---|---|---|---|---|
| Rs/Ω | Rct/Ω | Rs/Ω | Rct/Ω | ||
| LKMO-0 | 4.22 | 193.91 | 6.43 | 176.00 | 1.20×10-11 |
| LKMO-1 | 3.59 | 72.62 | 7.92 | 63.25 | 2.35×10-11 |
| Sample | Condition | a/nm | d(111)/nm | 2θ(111)/(°) | FWHM(111)/(°) | I(111)/(311) | L/nm | Strain/% | ΔStrain/% |
|---|---|---|---|---|---|---|---|---|---|
| LKMO-0 | 0.2C, 5th | 0.822 | 0.474 | 18.661 | 0.148 | 1.385 | 62.7 | 0.153 | 0.139 |
| 10C, 5th | 0.813 | 0.469 | 18.823 | 0.155 | 1.663 | 68.0 | 0.292 | ||
| LKMO-1 | 0.2C, 5th | 0.824 | 0.476 | 18.679 | 0.171 | 1.394 | 51.0 | 0.338 | 0.020 |
| 10C, 5th | 0.822 | 0.474 | 18.679 | 0.165 | 1.393 | 51.5 | 0.358 |
表3 LKMO-0和LKMO-1在0.2C和10C循环5周后的晶体结构参数
Table 3 Crystal structural parameters of LKMO-0 and LKMO-1 after 5 cycles at 0.2C and 10C
| Sample | Condition | a/nm | d(111)/nm | 2θ(111)/(°) | FWHM(111)/(°) | I(111)/(311) | L/nm | Strain/% | ΔStrain/% |
|---|---|---|---|---|---|---|---|---|---|
| LKMO-0 | 0.2C, 5th | 0.822 | 0.474 | 18.661 | 0.148 | 1.385 | 62.7 | 0.153 | 0.139 |
| 10C, 5th | 0.813 | 0.469 | 18.823 | 0.155 | 1.663 | 68.0 | 0.292 | ||
| LKMO-1 | 0.2C, 5th | 0.824 | 0.476 | 18.679 | 0.171 | 1.394 | 51.0 | 0.338 | 0.020 |
| 10C, 5th | 0.822 | 0.474 | 18.679 | 0.165 | 1.393 | 51.5 | 0.358 |
| Sample | Particle size distribution/μm | SBET/(m2·g-1) | ||
|---|---|---|---|---|
| d10 | d50 | d90 | ||
| LKMO-0 | 1.49 | 9.23 | 26.1 | 1.60 |
| LKMO-1 | 1.71 | 8.27 | 24.9 | 1.34 |
表S1 LKMO-0和LKMO-1的粒度分布和比表面积
Table S1 Particle size distributions and specific surface areas of LKMO-0 and LKMO-1
| Sample | Particle size distribution/μm | SBET/(m2·g-1) | ||
|---|---|---|---|---|
| d10 | d50 | d90 | ||
| LKMO-0 | 1.49 | 9.23 | 26.1 | 1.60 |
| LKMO-1 | 1.71 | 8.27 | 24.9 | 1.34 |
| Sample | Average specific discharge capacity/ (mAh·g-1) | ||||||
|---|---|---|---|---|---|---|---|
| 0.2C | 0.5C | 1C | 2C | 5C | 10C | 0.2C | |
| LKMO-0 | 106.33 | 97.27 | 90.39 | 77.54 | 48.86 | 27.90 | 102.40 |
| LKMO-1 | 100.11 | 99.15 | 97.21 | 93.37 | 82.66 | 56.59 | 96.83 |
| LKMO-2 | 106.09 | 101.47 | 90.60 | 72.37 | 44.12 | 24.11 | 103.69 |
| LKMO-3 | 102.89 | 100.32 | 91.89 | 65.63 | 37.16 | 16.98 | 98.14 |
表S2 LKMO-n的倍率性能
Table S3 Rate performances of LKMO-n
| Sample | Average specific discharge capacity/ (mAh·g-1) | ||||||
|---|---|---|---|---|---|---|---|
| 0.2C | 0.5C | 1C | 2C | 5C | 10C | 0.2C | |
| LKMO-0 | 106.33 | 97.27 | 90.39 | 77.54 | 48.86 | 27.90 | 102.40 |
| LKMO-1 | 100.11 | 99.15 | 97.21 | 93.37 | 82.66 | 56.59 | 96.83 |
| LKMO-2 | 106.09 | 101.47 | 90.60 | 72.37 | 44.12 | 24.11 | 103.69 |
| LKMO-3 | 102.89 | 100.32 | 91.89 | 65.63 | 37.16 | 16.98 | 98.14 |
| Sample | Charge-discharge efficiency/% | |||||
|---|---|---|---|---|---|---|
| 0.2C | 0.5C | 1C | 2C | 5C | 10C | |
| LKMO-0 | 87.88 | 90.86 | 90.97 | 88.81 | 76.81 | 56.76 |
| LKMO-1 | 94.54 | 98.46 | 96.65 | 94.79 | 85.18 | 63.53 |
表S3 LKMO-0和LKMO-1在不同倍率下的充放电效率
Table S4 Charge-discharge efficiency at different rates for LKMO-0 and LKMO-1
| Sample | Charge-discharge efficiency/% | |||||
|---|---|---|---|---|---|---|
| 0.2C | 0.5C | 1C | 2C | 5C | 10C | |
| LKMO-0 | 87.88 | 90.86 | 90.97 | 88.81 | 76.81 | 56.76 |
| LKMO-1 | 94.54 | 98.46 | 96.65 | 94.79 | 85.18 | 63.53 |
| Sample | Molar concentration/(mmol·L-1) | Molar ratio | ||
|---|---|---|---|---|
| Li | K | Mn | Li/K/Mn | |
| LKMO-0 | 5.895 | - | 10.452 | 1.128/-/2 |
| LKMO-1 | 5.914 | 0.055 | 10.775 | 1.097/0.010/2 |
| LKMO-2 | 5.805 | 0.101 | 10.668 | 1.088/0.019/2 |
| LKMO-3 | 5.716 | 0.154 | 10.546 | 1.084/0.029/2 |
表S4 LKMO-n的元素含量
Table S2 Elemental contents of LKMO-n
| Sample | Molar concentration/(mmol·L-1) | Molar ratio | ||
|---|---|---|---|---|
| Li | K | Mn | Li/K/Mn | |
| LKMO-0 | 5.895 | - | 10.452 | 1.128/-/2 |
| LKMO-1 | 5.914 | 0.055 | 10.775 | 1.097/0.010/2 |
| LKMO-2 | 5.805 | 0.101 | 10.668 | 1.088/0.019/2 |
| LKMO-3 | 5.716 | 0.154 | 10.546 | 1.084/0.029/2 |
| [1] |
DURMUS Y E, ZHANG H, BAAKES F, et al. Side by side battery technologies with lithium-ion based batteries. Advanced Energy Materials, 2020, 10(24): 2000089.
DOI URL |
| [2] |
LI L L, LI S Y, LU Y Y. Suppression of dendritic lithium growth in lithium metal-based batteries. Chemical Communications, 2018, 54(50): 6648-6661.
DOI URL |
| [3] | DING Y L, CANO Z P, YU A P, et al. Automotive Li-ion batteries: current status and future perspectives. Electrochemical Energy Reviews, 2019, 2(1): 1-28. |
| [4] | MU C H, LOU S A, ALI R, et al. Carbon-decorated LiMn2O4 nanorods with enhanced performance for supercapacitors. Journal of Alloys and Compounds, 2019, 805: 624-630. |
| [5] | ZHU Z X, WANG M M, MENG Y H, et al. A high-rate lithium manganese oxide-hydrogen battery. Nano Letters, 2020, 20(5): 3278-3283. |
| [6] | LI S Y, ZHU K L, LIU J L, et al. Porous LiMn2O4 microspheres with different pore size: preparation and application as cathode materials for lithium ion batteries. Journal of Electrochemical Energy Conversion and Storage, 2019, 16(1): 1-8. |
| [7] | XIANG M W, ZHOU X Y, ZHANG Z F, et al. LiMn2O4 prepared by liquid phase flameless combustion with F-Doped for lithium- ion battery cathode materials. Advanced Materials Research, 2013, 652-654: 825-830. |
| [8] | JIANG Q Q, LIU D D, ZHANG H, et al. Plasma-assisted sulfur doping of LiMn2O4 for high-performance lithium-ion batteries. Journal of Physical Chemistry C, 2015, 119(52): 28776-28782. |
| [9] | LIU J T, LI G, YU Y, et al. Synthesis and electrochemical performance evaluations of polyhedra spinel LiAlxMn2-xO4 (x≤0.20) cathode materials prepared by a solution combustion technique. Journal of Alloys and Compounds, 2017, 728: 1315-1328. |
| [10] | CHANDA P, VIVEK BANSALA, SUKRITIA V S. Investigations of spinel LiZnxMn2-xO4(x≤0.03) cathode materials for a lithium ion battery application. Materials Science & Engineering B, 2018, 238-239(DEC): 93-99. |
| [11] | XIONG L L, XU Y L, LEI P, et al. The electrochemical performance of sodium-ion-modified spinel LiMn2O4 used for lithium-ion batteries. Journal of Solid State Electrochemistry, 2014, 18(3): 713-719. |
| [12] | XIONG L L, XU Y L, XIAO X, et al. The effect of K-ion on the electrochemical performance of spinel LiMn2O4. Electronic Materials Letters, 2015, 11(1): 138-142. |
| [13] | CHUDZIK K, ŚWIĘTOSŁAWSKI M, BAKIERSKA M, et al. Electrochemical properties of K and S doped LiMn2O4 studied by GITT and EIS. Electrochimica Acta, 2021, 373: 137901. |
| [14] | CHUDZIK K, ŚWIĘTOSŁAWSKI M, BAKIERSKA M, et al. Surface modification and carbon coating effect on a high-performance K and S doped LiMn2O4. Applied Surface Science, 2020, 531:147138. |
| [15] | BAKIERSKA M, ŚWIĘTOSŁAWSKI M, CHUDZIK K, et al. Enhancing the lithium ion diffusivity in LiMn2O4-ySy cathode materials through potassium doping. Solid State Ionics, 2018, 317(January): 190-193. |
| [16] | RODRÍGUEZ R A, PÉREZ-CAPPE E L, LAFFITA Y M, et al. Structural defects in LiMn2O4 induced by gamma radiation and its influence on the Jahn-Teller effect. Solid State Ionics, 2018, 324(June): 77-86. |
| [17] | YU Z M, ZHAO L C. Structure and electrochemical properties of LiMn2O4. Transactions of Nonferrous Metals Society of China, 2007, 17(3): 659-664. |
| [18] | MARCHINI F, CALVO E J, WILLIAMS F J. Effect of the electrode potential on the surface composition and crystal structure of LiMn2O4 in aqueous solutions. Electrochimica Acta, 2018, 269: 706-713. |
| [19] | ZHAO H Y, LI F, BAI X Z, et al. Enhanced cycling stability of LiCuxMn1.95-xSi0.05O4 cathode material obtained by solid-state method. Materials, 2018, 11(8): 1-10. |
| [20] | RAGAVENDRAN K, CHOU H L, LU L, et al. Crystal habits of LiMn2O4 and their influence on the electrochemical performance. Materials Science and Engineering B: Solid-State Materials for Advanced Technology, 2011, 176(16): 1257-1263. |
| [21] | WANG X Y, HAO H, LIU J L, et al. A novel method for preparation of macroposous lithium nickel manganese oxygen as cathode material for lithium ion batteries. Electrochimica Acta, 2011, 56(11): 4065-4069. |
| [22] | CHEN S, CHEN Z, CAO C B. Mesoporous spinel LiMn2O4 cathode material by a soft-templating route. Electrochimica Acta, 2016, 199: 51-58. |
| [23] | ZHOU S, WANG G X, XIAO Y, et al. Influence of charge status on the stress safety properties of Li(Ni1/3Co1/3Mn1/3)O2 cells. RSC Advances, 2016, 6(68): 63378-63389. |
| [24] | SETHURAMAN V A, VAN WINKLE N, ABRAHAM D P, et al. Real-time stress measurements in lithium-ion battery negative- electrodes. Journal of Power Sources, 2012, 206: 334-342. |
| [25] |
FAN G X, WEN Y, LIU B Z, et al. An insight into the influence of crystallite size on the performances of microsized spherical Li(Ni0.5Co0.2Mn0.3)O2 cathode material composed of aggregated nanosized particles. Journal of Nanoparticle Research, 2018, 20(2): 43.
DOI URL |
| [26] | KIZILTAŞ-YAVUZ N, HERKLOTZ M, HASHEM A M, et al. Synthesis, structural, magnetic and electrochemical properties of LiNi1/3Mn1/3Co1/3O2 prepared by a Sol-Gel method using table sugar as chelating agent. Electrochimica Acta, 2013, 113: 313-321. |
| [27] | GREELEY J, WARBURTON R E, CASTRO F C, et al. Oriented LiMn2O4 particle fracture from delithiation-driven surface stress. ACS Applied Materials and Interfaces, 2020, 12(43): 49182-49191. |
| [28] | THACKERAY M M. Exploiting the spinel structure for Li-ion battery applications: a tribute to John B. Goodenough. Advanced Energy Materials, 2021, 11(2): 1-8. |
| [1] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [2] | 朱志杰, 申明远, 吴涛, 李文翠. Cu和Mg协同取代抑制钠离子电池正极材料P2-Na2/3Ni1/3Mn2/3O2的P2-O2相变[J]. 无机材料学报, 2025, 40(2): 184-195. |
| [3] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [4] | 程节, 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直. 蛋黄壳结构FeF3·0.33H2O@N掺杂碳纳米笼正极材料的构筑及其电化学性能[J]. 无机材料学报, 2024, 39(3): 299-305. |
| [5] | 周靖渝, 李兴宇, 赵晓琳, 王有伟, 宋二红, 刘建军. Ti和Cu掺杂β-NaMnO2正极材料:钠离子电池的倍率和循环性能[J]. 无机材料学报, 2024, 39(12): 1404-1412. |
| [6] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| [7] | 苏楠, 邱介山, 王治宇. 高容量氟掺杂碳包覆纳米硅负极材料: 气相氟化法制备及其储锂性能[J]. 无机材料学报, 2023, 38(8): 947-953. |
| [8] | 孔国强, 冷明哲, 周战荣, 夏池, 沈晓芳. Sb掺杂O3型Na0.9Ni0.5Mn0.3Ti0.2O2钠离子电池正极材料[J]. 无机材料学报, 2023, 38(6): 656-662. |
| [9] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [10] | 李涛, 曹鹏飞, 胡力涛, 夏勇, 陈一, 刘跃军, 孙翱魁. NH4+扩层MoS2的制备及其储锌性能研究[J]. 无机材料学报, 2023, 38(1): 79-86. |
| [11] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [12] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [13] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [14] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [15] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||