无机材料学报 ›› 2025, Vol. 40 ›› Issue (12): 1356-1364.DOI: 10.15541/jim20240535 CSTR: 32189.14.jim20240535
• 专栏:高温燃料电池关键材料(客座编辑:凌意瀚) • 上一篇 下一篇
姜玥宏( ), 宋云峰(
), 宋云峰( ), 张磊磊(
), 张磊磊( ), 马季, 宋昭远, 龙文
), 马季, 宋昭远, 龙文
收稿日期:2024-12-24
修回日期:2025-03-18
出版日期:2025-12-20
网络出版日期:2025-04-09
通讯作者:
宋云峰, 实验师. E-mail: yunfs@lnpu.edu.cn;作者简介:姜玥宏(1994-), 女, 硕士研究生. E-mail: jyh_940315@163.com
基金资助:
JIANG Yuehong( ), SONG Yunfeng(
), SONG Yunfeng( ), ZHANG Leilei(
), ZHANG Leilei( ), MA Ji, SONG Zhaoyuan, LONG Wen
), MA Ji, SONG Zhaoyuan, LONG Wen
Received:2024-12-24
Revised:2025-03-18
Published:2025-12-20
Online:2025-04-09
Contact:
SONG Yunfeng, lecturer. E-mail: yunfs@lnpu.edu.cn;About author:JIANG Yuehong (1994-), female, Master candidate. E-mail: jyh_940315@163.com
Supported by:摘要:
质子传导型固体氧化物燃料电池(H+-SOFC)因温度依赖性弱和能量转换效率高而备受关注。本工作通过氟化诱导提高了电解质BaZr0.1Ce0.7Y0.1Yb0.1O3(BZCYYb)的质子传导性。在450~800 ℃温区, 氟化后的BaZr0.1Ce0.7Y0.1Yb0.1O2.9F0.1 (BZCYYbF)钙钛矿在干氢气中的电导率(σ)为4.59×10-3~2.14×10-2 S/cm, 高于未氟化BZCYYb电解质的电导率(σ=3.99×10-3~1.86×10-2 S/cm)。电解质氟化可以明显降低阳极对氢氧化反应的极化阻抗, 700 ℃条件下从氟化前的2.50 Ω·cm2降低到1.94 Ω·cm2, 300 μm厚电解质支撑单电池(BSCN|电解质|LSFMN)的总阻抗从氟化前的1.54 Ω·cm2降低到氟化后的1.47 Ω·cm2。因此, 电解质氟化的单电池输出功率明显高于电解质未氟化的单电池。700 ℃时, 氟化电解质支撑单电池的最大输出功率密度Pmax为172 mW·cm-2, 明显高于未氟化电解质支撑单电池(Pmax=144 mW·cm-2)。追本溯源, 这是由于电解质氟化不但提高了电解质质子传导能力, 而且强化了阳极侧三相界面对氢燃料的吸附/解离和扩散速率。综上, 氟化能明显改善BZCYYb电解质的质子传导能力, 有助于提升H+-SOFC的电化学性能。
中图分类号:
姜玥宏, 宋云峰, 张磊磊, 马季, 宋昭远, 龙文. 质子传导型固体氧化物燃料电池BaZr0.1Ce0.7Y0.1Yb0.1O3电解质的氟化研究[J]. 无机材料学报, 2025, 40(12): 1356-1364.
JIANG Yuehong, SONG Yunfeng, ZHANG Leilei, MA Ji, SONG Zhaoyuan, LONG Wen. Fluorination of BaZr0.1Ce0.7Y0.1Yb0.1O3 as Electrolyte Material for Proton-conducting Solid Oxide Fuel Cell[J]. Journal of Inorganic Materials, 2025, 40(12): 1356-1364.
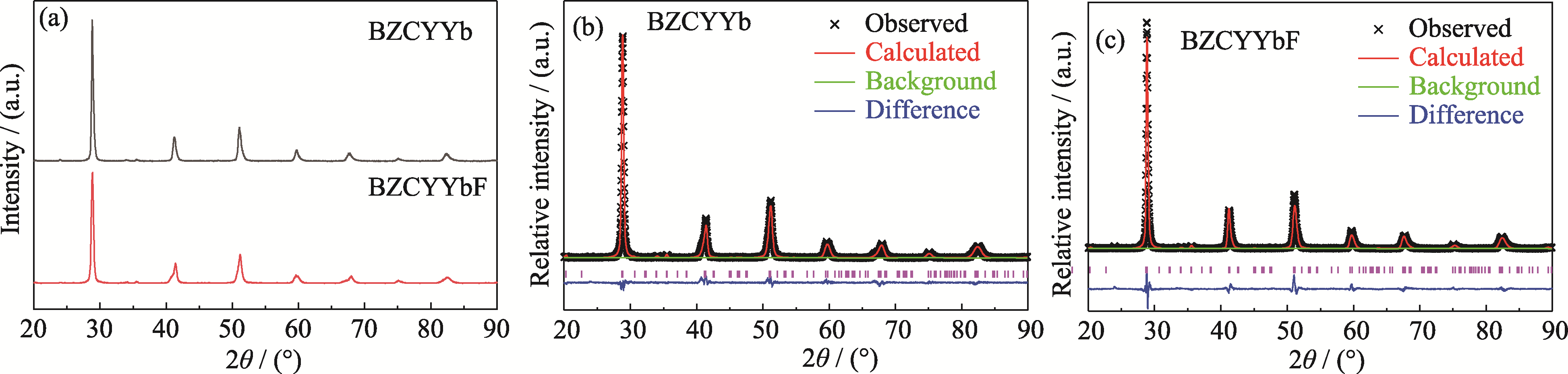
图1 BZCYYb和BZCYYbF样品的XRD图谱及其Rietveld精修图谱
Fig. 1 XRD patterns and Rietveld refinements for BZCYYb and BZCYYbF samples (a) XRD patterns; Rietveld refinements for (b) BZCYYb and (c) BZCYYbF. Colorful figures are available on website

图4 (a)空气中BZCYYb和BZCYYbF的电导率; (b) BZCYYb和(c) BZCYYbF的表面SEM照片; (d)干氢气中BZCYYb和BZCYYbF的电导率
Fig. 4 (a) Electrical conductivities of BZCYYb and BZCYYbF in air; (b, c) Surface SEM images of (b) BZCYYb and (c) BZCYYbF; (d) Electrical conductivities of BZCYYb and BZCYYbF in dry H2

图5 BZCYYb和BZCYYbF对称电池在(a, b, e, f) 600和(c, d, g, h) 700 ℃, (a~d)空气和(e~h)湿氢气下的(a, c, e, g) EIS谱图以及(b, d, f, h) DRT曲线
Fig. 5 (a, c, e, g) EIS spectra and (b, d, f, h) DRT curves for BZCYYb and BZCYYbF symmetric cells in (a-d) air and (e-h) wet H2 at (a, b, e, f) 600 and (c, d, g, h) 700 ℃

图8 (a)阳极支撑单电池在600 ℃的I-V/I-P曲线以及(b)以BZCYYbF作电解质的阳极支撑电池稳定性测试后的断面SEM照片
Fig. 8 (a) I-V/I-P curves for anode-supported single cell at 600 ℃ and (b) cross-sectional SEM image for anode-supported single cell with BZCYYbF electrolyte after cell stability test
| Sample | BZCYYb | BZCYYbF | ||
|---|---|---|---|---|
| Refined | Sigmas | Refined | Sigmas | |
| a/Å | 6.2146 | 0.000851 | 6.210605 | 0.000363 |
| b/Å | 6.2443 | 0.000511 | 6.197102 | 0.000486 |
| c/Å | 8.7578 | 0.00058 | 8.701261 | 0.00077 |
| α/(°) | 90 | - | 90 | - |
| β/(°) | 90 | - | 90 | - |
| γ/(°) | 90 | - | 90 | - |
| V/Å3 | 339.858 | 0.054 | 334.892 | 0.04 |
| χ2 | 1.711 | - | 2.346 | - |
| Rwp/% | 14.83 | - | 14.07 | - |
| Rp/% | 9.99 | - | 11.54 | - |
表S1 基于Pbnm空间群的BZCYYb和BZCYYbF样品的Rietveld精修结果
Table S1 Rietveld refinement results for BZCYYb and BZCYYbF samples based on space group Pbnm
| Sample | BZCYYb | BZCYYbF | ||
|---|---|---|---|---|
| Refined | Sigmas | Refined | Sigmas | |
| a/Å | 6.2146 | 0.000851 | 6.210605 | 0.000363 |
| b/Å | 6.2443 | 0.000511 | 6.197102 | 0.000486 |
| c/Å | 8.7578 | 0.00058 | 8.701261 | 0.00077 |
| α/(°) | 90 | - | 90 | - |
| β/(°) | 90 | - | 90 | - |
| γ/(°) | 90 | - | 90 | - |
| V/Å3 | 339.858 | 0.054 | 334.892 | 0.04 |
| χ2 | 1.711 | - | 2.346 | - |
| Rwp/% | 14.83 | - | 14.07 | - |
| Rp/% | 9.99 | - | 11.54 | - |

图S2 (a, b)不同模式测得的(a) BZCYYb和(b) BZCYYbF在空气下的电导率曲线; (c)三种电导率测试模式的示意图; (d, e)按不同测试模式所测得(d) BZCYYb和(e) BZCYYbF的电导率柱状图; (f) BZCYYb与BZCYYbF的电导率柱状对比图
Fig. S2 (a, b) Electrical conductivity curves for (a) BZCYYb and (b) BZCYYbF in air measured by different models; (c) Schematic diagram for three different conductivity testing models; (d, e) Histograms of conductivities for (d) BZCYYb and (e) BZCYYbF measured by different models; (f) Comparison of conductivities for BZCYYb and BZCYYbF

图S3 (a) BZCYYb和(b) BZCYYbF在H2中的电导率曲线; (c)两种电导率测试模式的示意图; (d)按不同测试模式所测得BZCYYbF的电导率柱状图; (e) BZCYYb与BZCYYbF的电导率柱状对比图
Fig. S3 (a, b) Electrical conductivity curves for (a) BZCYYb and (b) BZCYYbF in H2 measured by different models; (c) Schematic diagram for two different conductivity testing models; (d) Histogram for BZCYYbF measured by different models; (e) Comparison of conductivities of BZCYYb and BZCYYbF
| [1] |
TSVETKOV N, KIM D, JEONG I, et al. Advances in materials and interface understanding in protonic ceramic fuel cells. Advanced Materials Technologies, 2023, 8(20): 2201075.
DOI URL |
| [2] | GAO Y, LIU K C, LI Q, et al. The approaches to conducting in-situ heterostructure electrodes for SOCs: a mini review. Sustainable Materials and Technologies, 2024, 41: e01107. |
| [3] |
LUO Y, ZHANG D, LIU T, et al. In situ exsolution of quaternary alloy nanoparticles for CO2-CO mutual conversion using reversible solid oxide cells. Advanced Functional Materials, 2024, 34(40): 2403922.
DOI URL |
| [4] |
ZHOU M Y, LIU Z J, CHEN M L, et al. Electrochemical performance and chemical stability of proton-conducting BaZr0.8-xCexY0.2O3-δ electrolytes. Journal of the American Ceramic Society, 2022, 105(9): 5711.
DOI URL |
| [5] |
YANG L, WANG S, BLINN K, et al. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2-xYbxO3-δ. Science, 2009, 326(5949): 126.
DOI URL |
| [6] |
GUO Y, LIN Y, RAN R, et al. Zirconium doping effect on the performance of proton-conducting BaZryCe0.8-yY0.2O3-δ (0.0≤y≤0.8) for fuel cell applications. Journal of Power Sources, 2009, 193(2): 400.
DOI URL |
| [7] |
ZHONG Z. Stability and conductivity study of the BaCe0.9-xZrxY0.1O2.95 systems. Solid State Ionics, 2007, 178(3/4): 213.
DOI URL |
| [8] |
CHOI S, KUCHARCZYK C J, LIANG Y, et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nature Energy, 2018, 3(3): 202.
DOI |
| [9] |
CHOI S Y, TIMOTH C, SOSSINA M H. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: exceptional reversibility, stability, and demonstrated Faradaic efficiency. Energy and Environmental Science, 2019, 12(1): 206.
DOI URL |
| [10] |
REN R, YU X, WANG Z, et al. Fluorination inductive effect enables rapid bulk proton diffusion in BaCo0.4Fe0.4Zr0.1Y0.1O3-δ perovskite oxide for high-activity protonic ceramic fuel cell cathode. Applied Catalysis B: Environmental, 2022, 317(1): 121759.
DOI URL |
| [11] |
LI W, LI Y, F L, et al. Enhancing performance of proton ceramic fuel cells through fluorine-doped perovskite oxides. Rare Metals, 2025, 44: 2405.
DOI URL |
| [12] |
JIANG T, LIU Y, WANG Z, et al. An improved direct current sintering technique for proton conductor - BaZr0.1Ce0.7Y0.1Yb0.1O3-δ: the effect of direct current on sintering process. Journal of Power Sources, 2014, 248(8): 70.
DOI URL |
| [13] | ZHANG Y, XIE D, CHI B, et al. Basic properties of proton conductor BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb) material. Asia-Pacific Journal of Chemical Engineering, 2019, 14(4): 58. |
| [14] | CHEN T, JING Y H, ANDERSON L O, et al. Toward durable protonic ceramic cells: hydration-induced chemical expansion correlates with symmetry in the Y-doped BaZrO3-BaCeO3 solid solution. The Journal of Physical Chemistry, 2021, 125(47): 26216. |
| [15] |
LOKEN A, RICOTE S, WACHOWSKI S, et al. Thermal and chemical expansion in proton ceramic electrolytes and compatible electrodes. Crystals, 2018, 8(9): 365.
DOI URL |
| [16] |
STOKES S J, SAIFUL I. Defect chemistry and proton-dopant association in BaZrO3 and BaPrO3. Journal of Materials Chemistry, 2010, 20(6): 6258.
DOI URL |
| [17] |
ZHONG Z Y, SONG T, ZHAO S K, et al. High-performance BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb) protonic ceramic fuel cell electrolytes by the Ba evaporation inhibition strategy. Ceramics International, 2024, 50(2): 3633.
DOI URL |
| [18] |
ZHU H, RICOTE S, COORS W G, et al. Interpreting equilibrium- conductivity and conductivity-relaxation measurements to establish thermodynamic and transport properties for multiple charged defeat conducting ceramics. Faraday Discussions, 2015, 182(3): 49.
DOI URL |
| [19] | ZHU H Y, SANDRINE R, DUAN C C, et al. Defect chemistry and transport within dense BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb) proton- conducting membranes. Journal of the Electrochemical Society, 2018, 10(2): 3633. |
| [20] |
SOMEKAWA T, TACHIKAWA Y, TACHIKAWA Y, et al. Physicochemical properties of proton conductive Ba(Zr0.1Ce0.7Y0.1Yb0.1)O3-δ solid electrolyte in terms of electrochemical performance of solid oxide fuel cells. International Journal of Hydrogen Energy, 2016, 41(39): 17539.
DOI URL |
| [21] |
WANG J, ZHANG D, LIU T, et al. Self-assembled FeRu bimetallic nano catalysts for efficient and durable mutual CO-CO2 conversion in a reversible solid oxide electrochemical cell. Science China Materials, 2024, 67(5): 1471.
DOI |
| [22] |
CHEN H N, ZHANF H C, ZHOU Y J, et al. Structure-conduction correlations in a chlorine-rich superionic lithium-argyrodite solid electrolyte: a DRT analysis. Journal of Power Sources, 2023, 583(1): 233579.
DOI URL |
| [23] |
YANG Q, TIAN D, LIU R, et al. Exploiting rare-earth-abundant layered perovskite cathodes of LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln=La and Nd) for SOFCs. International Journal of Hydrogen Energy, 2021, 46(7): 5630.
DOI URL |
| [24] | XIE D, LING A, YAN D, et al. A comparative study on the composite cathodes with proton conductor and oxygen ion conductor for proton-conducting solid oxide fuel cell. Electrchimica Acta, 2020, 344: 136143. |
| [25] |
SUMI H, SHIMADA H, WAANABE K, et al. External current dependence of polarization resistances for reversible solid oxide and protonic ceramic cells with current leakage. ACS Applied Energy Materials, 2023, 6(3): 1853.
DOI URL |
| [26] |
SHI H G, HU Y, FENG Z X, et al. Solid-state synthesis of BaCe0.16Y0.04Fe0.8O3-δ cathode for protonic ceramic fuel cells. Asia-Pacific Journal of Chemical Engineering, 2022, 17(4): e2789.
DOI URL |
| [1] | 闫共芹, 王晨, 蓝春波, 洪雨昕, 叶维超, 付向辉. Al掺杂P2型Na0.8Ni0.33Mn0.67-xAlxO2钠离子电池正极材料的制备与电化学性能[J]. 无机材料学报, 2025, 40(9): 1005-1012. |
| [2] | 王亮君, 欧阳玉昭, 赵俊亮, 杨长. Cu-Mn-I固溶体薄膜制备及其p型透明导电性质调控[J]. 无机材料学报, 2025, 40(9): 1022-1028. |
| [3] | 万俊池, 杜路路, 张永上, 李琳, 刘建德, 张林森. Na4FexP4O12+x/C钠离子电池正极材料的结构演变及其电化学性能[J]. 无机材料学报, 2025, 40(5): 497-503. |
| [4] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [5] | 杨恒强, 张馨月, 马义初, 周青军. 铁基钙钛矿La0.25M0.75FeO3-δ (M=Ba, Sr, Ca)的制备及其作为固体氧化物燃料电池阴极材料的性能研究[J]. 无机材料学报, 2025, 40(12): 1365-1372. |
| [6] | 凌意瀚, 郭胜, 曹志强, 田云峰, 刘方升, 金芳军, 高源. 固体氧化物电池直孔电极结构的制备技术与性能研究进展[J]. 无机材料学报, 2025, 40(12): 1311-1323. |
| [7] | 张宇婷, 李晓斌, 刘尊义, 李宁, 赵鹬. 复合蛋黄壳型NiCo2V2O8@TiO2@NC材料用作锂离子电池负极研究[J]. 无机材料学报, 2025, 40(11): 1221-1228. |
| [8] | 唐阳, 刘立敏, 周晓亮, 张搏, 蒋星洲, 贾浩义, 罗延麟庆. 质子陶瓷膜反应器的制备及低温氨分解性能研究[J]. 无机材料学报, 2025, 40(11): 1277-1284. |
| [9] | 程节, 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直. 蛋黄壳结构FeF3·0.33H2O@N掺杂碳纳米笼正极材料的构筑及其电化学性能[J]. 无机材料学报, 2024, 39(3): 299-305. |
| [10] | 陈正鹏, 金芳军, 李明飞, 董江波, 许仁辞, 徐韩昭, 熊凯, 饶睦敏, 陈创庭, 李晓伟, 凌意瀚. 双钙钛矿Sr2CoFeO5+δ阴极材料的制备及其中温固体氧化物燃料电池性能研究[J]. 无机材料学报, 2024, 39(3): 337-344. |
| [11] | TAM YU Puy Mang, 徐愉, 高泉浩, 周海琼, 张振, 尹浩, 李真, 吕启涛, 陈振强, 马凤凯, 苏良碧. 掺铒CaF2、SrF2、PbF2晶体的光谱性能与团簇结构研究[J]. 无机材料学报, 2024, 39(3): 330-336. |
| [12] | 苏楠, 邱介山, 王治宇. 高容量氟掺杂碳包覆纳米硅负极材料: 气相氟化法制备及其储锂性能[J]. 无机材料学报, 2023, 38(8): 947-953. |
| [13] | 王华进, 寇华敏, 王墉哲, 姜大朋, 张博, 钱小波, 王静雅, 朱琳玲, 曾爱军, 杨秋红, 苏良碧. 193 nm激光下不同含量Y杂质CaF2晶体辐照损伤研究[J]. 无机材料学报, 2023, 38(2): 219-224. |
| [14] | 丁健翔, 张凯歌, 柳东明, 郑伟, 张培根, 孙正明. Ti3AlC2陶瓷及其衍生物Ti3C2Tx增强的Ag基电接触材料[J]. 无机材料学报, 2022, 37(5): 567-573. |
| [15] | 刘芳芳, 传秀云, 杨扬, 李爱军. 氮/硫共掺杂对纤水镁石模板碳纳米管电化学性能的影响[J]. 无机材料学报, 2021, 36(7): 711-717. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||