无机材料学报 ›› 2022, Vol. 37 ›› Issue (7): 802-808.DOI: 10.15541/jim20220196 CSTR: 32189.14.10.15541/jim20220196
• 研究快报 • 上一篇
收稿日期:2022-04-07
修回日期:2022-05-15
出版日期:2022-07-20
网络出版日期:2022-05-27
通讯作者:
翟朋博, 副教授. E-mail: woshizpb@qdu.edu.cn; 郭向欣, 教授. E-mail: xxguo@qdu.edu.cn作者简介:苏东良(1995-), 男, 硕士研究生. E-mail: 13994381640@163.com
SU Dongliang( ), CUI Jin, ZHAI Pengbo(
), CUI Jin, ZHAI Pengbo( ), GUO Xiangxin(
), GUO Xiangxin( )
)
Received:2022-04-07
Revised:2022-05-15
Published:2022-07-20
Online:2022-05-27
Contact:
ZHAI Pengbo, associate professor. E-mail: woshizpb@qdu.edu.cn; GUO Xiangxin, professor. E-mail: xxguo@qdu.edu.cnAbout author:SU Dongliang (1995-), male, Master candidate. E-mail: 13994381640@163.com
Supported by:摘要:
硅(Si)负极在充放电过程中巨大的体积变化会导致固态电解质中间相(SEI)破裂和硅颗粒粉化, 进而造成容量快速衰减。本研究报道了一种利用Li6.4La3Zr1.4Ta0.6O12(LLZTO)固体电解质调节Si/C负极表面SEI成分的策略。将LLZTO层均匀地涂覆在商用化聚丙烯(PP)隔膜表面, 不仅提高了电解液对隔膜的润湿性, 均匀化锂离子通量, 并且增大了SEI中无机组分的比例, 从而增强Si/C负极的界面稳定性。得益于上述优势, 使用LLZTO修饰的PP隔膜所组装的锂离子电池表现出更为优异的循环稳定性和倍率性能。Li-Si/C半电池的可逆容量为876 mAh·g-1, 在0.3C (1C=1.5 A·g-1)的倍率下, 200次循环的容量保持率为81%; 而LFP-Si/C全电池的比容量为125 mAh·g-1, 在0.3C (1C=170 mA·g-1)的倍率下循环100次后容量保持率为91.8%。该工作中LLZTO固体电解质调节了Si/C负极表面SEI成分, 为开发高性能硅基锂离子电池提供了新思路。
中图分类号:
苏东良, 崔锦, 翟朋博, 郭向欣. 石榴石型Li6.4La3Zr1.4Ta0.6O12对Si/C负极表面固体电解质中间相的调控机制研究[J]. 无机材料学报, 2022, 37(7): 802-808.
SU Dongliang, CUI Jin, ZHAI Pengbo, GUO Xiangxin. Mechanism Study on Garnet-type Li6.4La3Zr1.4Ta0.6O12 Regulating the Solid Electrolyte Interphases of Si/C Anodes[J]. Journal of Inorganic Materials, 2022, 37(7): 802-808.
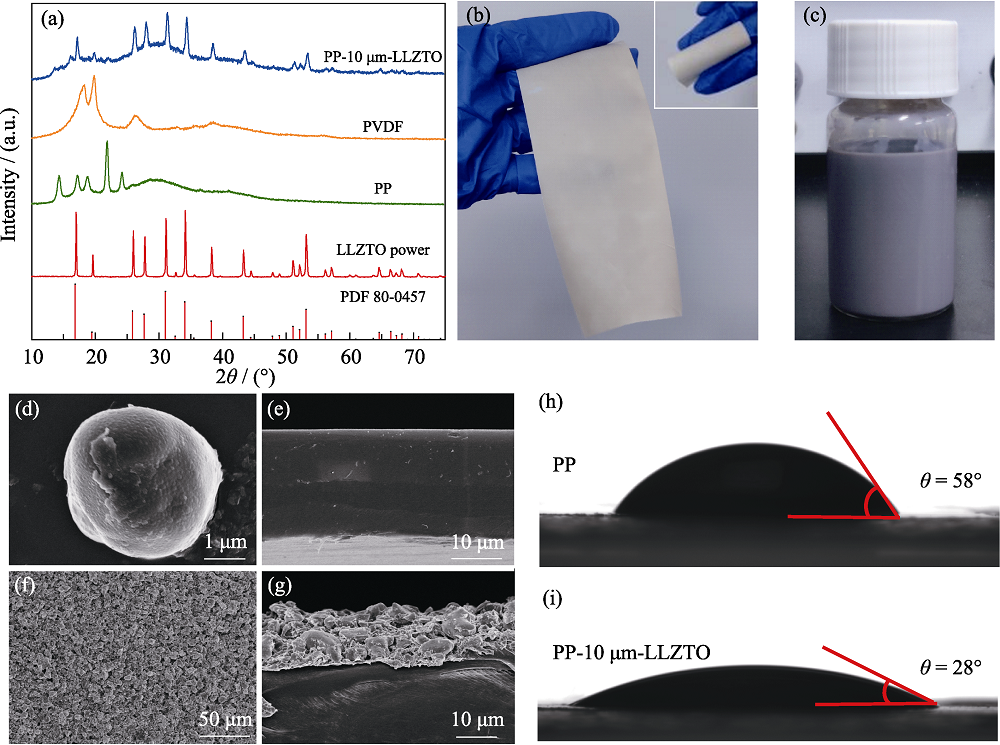
Fig. 2 Structural characterization of PP-10 μm-LLZTO separator (a) XRD patterns of LLZTO powder, bare PP separator, PVDF and PP-10 μm-LLZTO separator; (b) Optical photos of PP-10 μm-LLZTO separator; (c) Photograph of LLZTO-PVDF slurry after stirring; (d) SEM image of LLZTO powder; (e) Cross-sectional SEM image of PP separator; (f) Top-view and (g) cross-sectional SEM images of PP-10 μm-LLZTO separator; Electrolyte contact angles of (h) PP and (i) PP-10 μm-LLZTO separators

Fig. 3 Electrochemical performance of Li-Si/C half cells (a) CV curves of the initial 5 cycles of half-cell using PP-10 μm-LLZTO separator; (b) Rate tests of half-cells using PP and PP-10 μm-LLZTO separators; (c) Charge-discharge curves of Li/Si half-cell using PP-10 μm-LLZTO separator; (d) Stability tests of Li/Si half-cells using PP and PP-10 μm-LLZTO separators with different thicknesses; (e) EIS spectra of half-cells with PP and PP-10 μm-LLZTO separators before cycling. Colorful figures are available on website
| Separator | Bulk resistance/Ω | Interfacial resistance/Ω |
|---|---|---|
| Bare PP | 3.3 | 525.5 |
| PP-10 μm-LLZTO | 7.0 | 149.1 |
Table S1 Bulk resistance and ionic conductivity of PP and PP-10 μm-LLZTO separators
| Separator | Bulk resistance/Ω | Interfacial resistance/Ω |
|---|---|---|
| Bare PP | 3.3 | 525.5 |
| PP-10 μm-LLZTO | 7.0 | 149.1 |

Fig. S11 (a) Charge-discharge curves during cycling of LFP|PP-10 μm-LLZTO|Si/C at 0.3C; (b) Cycling performances of LFP|PP|Si/C and LFP|PP-10 μm-LLZTO|Si/C at 0.3C
| [1] |
LI K, HU X, ZHANG Z, et al. Three-dimensional porous biogenic Si/C composite for high performance lithium-ion battery anode derived from equisetum fluviatile. Journal of Inorganic Materials, 2021, 36(9): 929-935.
DOI URL |
| [2] | CERVERA R B, SUZUKI N, OHNISHI T, et al. High performance silicon-based anodes in solid-state lithium batteries. Energy & Environmental Science, 2014, 7(2): 662-666. |
| [3] |
FENG M Y, TIAN J H, LIU Y Y, et al. Effect of silicon anode additives on lithium ion batteries. Journal of Inorganic Materials, 2015, 30(6): 647-652.
DOI URL |
| [4] |
OZANAM F, ROSSO M. Silicon as anode material for Li-ion batteries. Materials Science and Engineering: B, 2016, 213: 2-11.
DOI URL |
| [5] |
SUNG J, KIM N, MA J, et al. Subnano-sized silicon anode via crystal growth inhibition mechanism and its application in a prototype battery pack. Nature Energy, 2021, 6(12): 1164-1175.
DOI URL |
| [6] |
HUANG X, SUI X, YANG H, et al. HF-free synthesis of Si/C yolk/shell anodes for lithium-ion batteries. Journal of Materials Chemistry A, 2018, 6(6): 2593-2599.
DOI URL |
| [7] |
LIU Y, BAI H, ZHAO Q, et al. Storage aging mechanism of LiNi0.8Co0.15Al0.05O2/graphite Li-ion batteries at high state of charge. Journal of Inorganic Materials, 2021, 36(2): 175-180.
DOI URL |
| [8] |
MENG X L, HUO H Y, GUO X X, et al. Influence of film thickness on the electrochemical performance of α-SiOx thin-film anodes. Journal of Inorganic Materials, 2018, 33(10): 1141-1146.
DOI URL |
| [9] |
OHTA N, KIMURA S, SAKABE J. et al. Anode properties of Si nanoparticles in all-solid-state Li batteries. ACS Applied Energy Materials, 2019, 2(10): 7005-7008.
DOI URL |
| [10] |
HA J, PAIK U. Hydrogen treated, cap-opened Si nanotubes array anode for high power lithium ion battery. Journal of Power Sources, 2013, 244: 463-468.
DOI URL |
| [11] |
KIM H, SEO M, PARK M H, et al. A critical size of silicon nano-anodes for lithium rechargeable batteries. Angewandte Chemie International Edition, 2010, 49(12): 2146-2149.
DOI URL |
| [12] |
CHAN C K, PENG H, LIU G, et al. High-performance lithium battery anodes using silicon nanowires. Nature Nanotechnology, 2008, 3(1): 31-35.
DOI URL |
| [13] |
WEN Z, LU G, MAO S, et al. Silicon nanotube anode for lithium-ion batteries. Electrochemistry Communications, 2013, 29: 67-70.
DOI URL |
| [14] |
ZHANG H, ZONG P, CHEN M, et al. In situ synthesis of multilayer carbon matrix decorated with copper particles: enhancing the performance of Si as anode for Li-ion batteries. ACS Nano, 2019, 13(3): 3054-3062.
DOI URL |
| [15] |
WANG G X, AHN J H, YAO J, et al. Nanostructured Si-C composite anodes for lithium-ion batteries. Electrochemistry Communications, 2004, 6(7): 689-692.
DOI URL |
| [16] |
SHEN T, XIA X H, XIE D, et al. Encapsulating silicon nanoparticles into mesoporous carbon forming pomegranate- structured microspheres as a high-performance anode for lithium ion batteries. Journal of Materials Chemistry A, 2017, 5(22): 11197-11203.
DOI URL |
| [17] |
DU F M, ZHAO N, LI Y Q, et al. All solid state lithium batteries based on lamellar garnet-type ceramic electrolytes. Journal of Power Sources, 2015, 300: 24-28.
DOI URL |
| [18] | ZHAO C Z, CHEN P Y, ZHANG R, et al. An ion redistributor for dendrite-free lithium metal anodes. Science Advances, 2018, 4(11): eaat3446. |
| [19] |
HUO H, LI X, CHEN Y, et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Materials, 2020, 29: 361-366.
DOI URL |
| [20] |
LIANG T CAO, J H, LIANG W H, et al. Asymmetrically coated LAGP/PP/PVDF-HFP composite separator film and its effect on the improvement of NCM battery performance. RSC Advances, 2019, 9(70): 41151-41160.
DOI URL |
| [21] |
ZHOU X, YIN Y X, WAN L J, et al. Self-assembled nanocomposite of silicon nanoparticles encapsulated in graphene through electrostatic attraction for lithium-ion batteries. Advanced Energy Materials, 2012, 2(9): 1086-1090.
DOI URL |
| [22] |
YAO Y, MCDOWELL M T, RYU I, et al. Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Letters, 2011, 11(7): 2949-2954.
DOI URL |
| [23] |
DHANABALAN A, SONG B F, BISWAL S L, et al. Extreme rate capability cycling of porous silicon composite anodes for lithium- ion batteries. ChemElectroChem, 2021, 8(17): 3318-3325.
DOI URL |
| [24] | ZHANG X, WENG S, YANG G, et al. Interplay between solid-electrolyte interphase and (in) active LixSi in silicon anode. Cell Reports Physical Science, 2021, 2(12): 100668. |
| [25] |
LIU J Y, GAO X W, HARTLEY G O, et al. The interface between Li6.5La3Zr1.5Ta0.5O12 and liquid electrolyte. Joule, 2020, 4(1): 101-108.
DOI URL |
| [26] |
JAUMANN T, BALACH J, KLOSE M, et al. SEI-component formation on sub 5 nm sized silicon nanoparticles in Li-ion batteries: the role of electrode preparation, FEC addition and binders. Physical Chemistry Chemical Physics, 2015, 17(38): 24956-24967.
DOI URL |
| [27] | LI Y JIN, B, WANG K, et al. Coordinatively-intertwined dual anionic polysaccharides as binder with 3D network conducive for stable SEI formation in advanced silicon-based anodes. Chemical Engineering Journal, 2022, 429: 132235. |
| [28] | LÜ L, WANG Y, HUANG W, et al. The effect of cathode type on the electrochemical performance of Si-based full cells. Journal of Power Sources, 2022, 520: 230855. |
| [1] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [2] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [3] | 程节, 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直. 蛋黄壳结构FeF3·0.33H2O@N掺杂碳纳米笼正极材料的构筑及其电化学性能[J]. 无机材料学报, 2024, 39(3): 299-305. |
| [4] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| [5] | 苏楠, 邱介山, 王治宇. 高容量氟掺杂碳包覆纳米硅负极材料: 气相氟化法制备及其储锂性能[J]. 无机材料学报, 2023, 38(8): 947-953. |
| [6] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [7] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [8] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [9] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [10] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [11] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [12] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| [13] | 肖美霞, 李苗苗, 宋二红, 宋海洋, 李钊, 毕佳颖. 表面端基卤化Ti3C2 MXene应用于锂离子电池高容量电极材料的研究[J]. 无机材料学报, 2022, 37(6): 660-668. |
| [14] | 王禹桐, 张非凡, 许乃才, 王春霞, 崔立山, 黄国勇. 水系锂离子电池负极材料LiTi2(PO4)3的研究进展[J]. 无机材料学报, 2022, 37(5): 481-492. |
| [15] | 李昆儒, 胡省辉, 张正富, 郭玉忠, 黄瑞安. 源于溪木贼的高性能锂离子电池三维多孔生物质硅/碳复合负极材料[J]. 无机材料学报, 2021, 36(9): 929-935. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||