无机材料学报 ›› 2023, Vol. 38 ›› Issue (8): 954-962.DOI: 10.15541/jim20220711 CSTR: 32189.14.10.15541/jim20220711
所属专题: 【能源环境】储能电池(202506); 【能源环境】超级电容器(202409)
徐州( ), 刘宇轩, 池俊霖, 张婷婷, 王姝越, 李伟, 马春慧, 罗沙(
), 刘宇轩, 池俊霖, 张婷婷, 王姝越, 李伟, 马春慧, 罗沙( ), 刘守新(
), 刘守新( )
)
收稿日期:2022-11-28
修回日期:2023-02-25
出版日期:2023-03-24
网络出版日期:2023-03-24
通讯作者:
罗 沙, 工程师. E-mail: luo.sha.85@163.com;作者简介:徐 州(1993-), 男, 博士研究生. E-mail: xuzhou0194@126.com
基金资助:
XU Zhou( ), LIU Yuxuan, CHI Junlin, ZHANG Tingting, WANG Shuyue, LI Wei, MA Chunhui, LUO Sha(
), LIU Yuxuan, CHI Junlin, ZHANG Tingting, WANG Shuyue, LI Wei, MA Chunhui, LUO Sha( ), LIU Shouxin(
), LIU Shouxin( )
)
Received:2022-11-28
Revised:2023-02-25
Published:2023-03-24
Online:2023-03-24
Contact:
LUO Sha, engineer. E-mail: luo.sha.85@163.com;About author:XU Zhou (1993-), male, PhD candidate. E-mail: xuzhou0194@126.com
Supported by:摘要:
以木糖为碳源, 利用嵌段共聚物聚环氧乙烷-聚环氧丙烷-聚环氧乙烷 (P123)/十二烷基硫酸钠(SDS)混合乳液构筑微反应器, 水热炭化制备马蹄形中空多孔炭。研究表明木糖在微反应器与溶液界面发生水热反应, 160 ℃水热条件下P123的亲水聚环氧乙烷嵌段(PEO)亲水性下降并向乳液内部增溶, 使乳液逐渐润胀和破裂。P123/SDS质量比会影响微反应器的完整度, 而水热时间可以调控微反应器的开口角度和空腔直径。开放性空腔能储存更多电荷和离子并缩短传输距离, 使多孔炭的比电容和能量密度增大且与空腔直径呈正相关关系。当P123/SDS质量比为1.25 : 1、水热时间为12 h时, 马蹄形中空多孔炭的开口角度(63°)和空腔直径(80 nm)最大、电化学性能最佳, 在6 mol·L-1 KOH三电极体系中电流密度1 A·g-1时比电容达292 F·g-1; 在两电极体系中电流密度0.2 A·g-1时比电容达185 F·g-1, 能量密度达6.44 Wh·kg-1; 电流密度5 A·g-1时5000次充放电循环后电容保持率达94.83%。
中图分类号:
徐州, 刘宇轩, 池俊霖, 张婷婷, 王姝越, 李伟, 马春慧, 罗沙, 刘守新. 双模板-水热炭化制备马蹄形中空多孔炭及其电化学性能[J]. 无机材料学报, 2023, 38(8): 954-962.
XU Zhou, LIU Yuxuan, CHI Junlin, ZHANG Tingting, WANG Shuyue, LI Wei, MA Chunhui, LUO Sha, LIU Shouxin. Horseshoe-shaped Hollow Porous Carbon: Synthesis by Hydrothermal Carbonization with Dual-template and Electrochemical Property[J]. Journal of Inorganic Materials, 2023, 38(8): 954-962.
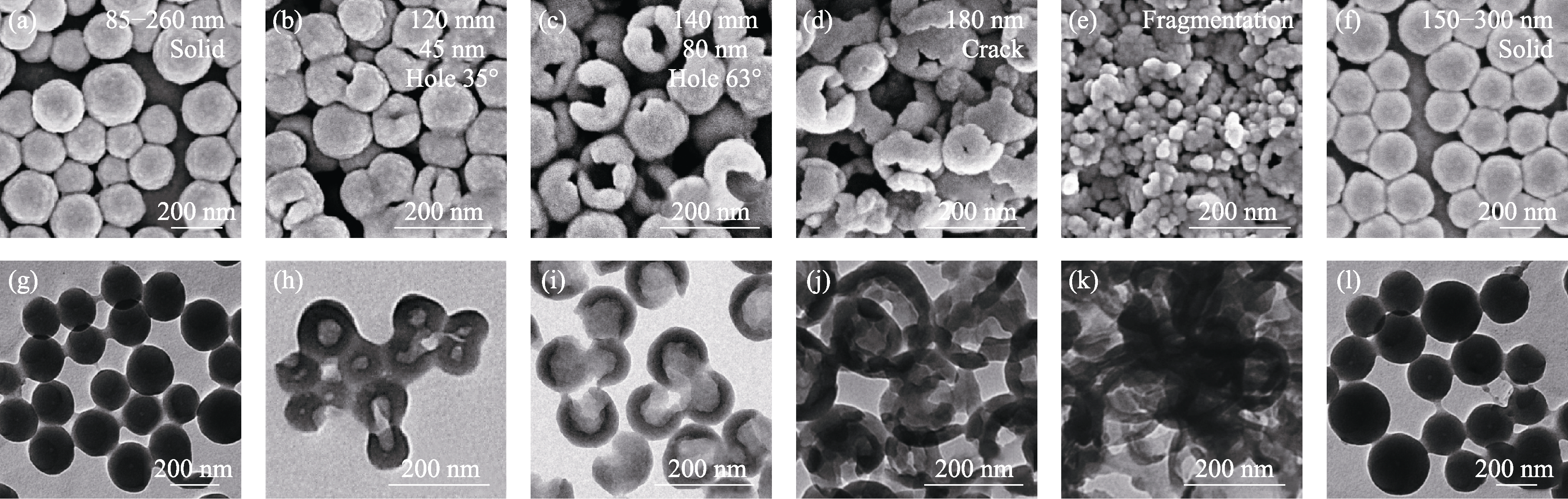
图1 不同P123/SDS质量比炭前驱体的(a~f)SEM和(g~l)TEM照片
Fig. 1 (a-f) SEM and (g-l) TEM images of carbon precursors with different P123/SDS mass ratios (a, g) HNS-S-12; (b, h) HNS-0.625-12; (c, i) HNS-1.25-12; (d, j) HNS-2.5-12; (e, k) HNS-5-12; (f, l) HNS-P-12

图2 不同水热时间炭前驱体的(a~e)SEM和(f~j)TEM照片
Fig. 2 (a-e) SEM and (f-j) TEM images of carbon precursors after hydrothermal treatment for different periods (a, f) HNS-1.25-3; (b, g) HNS-1.25-8; (c, h) HNS-1.25-12; (d, i) HNS-1.25-18; (e, j) HNS-1.25-24
| Sample | Average diameter of particle/nm | Average diameter of inner cavity/nm | Average diameter of carbon wall/nm | Opening angle/(°) | pH dependence |
|---|---|---|---|---|---|
| HNS-1.25-0 | - | - | - | - | 7.27 |
| HNS-1.25-3 | - | - | - | - | 3.53 |
| HNS-1.25-8 | 110 | 60 | 25 | 48 | 3.35 |
| HNS-1.25-12 | 140 | 80 | 30 | 63 | 3.32 |
| HNS-1.25-18 | 230 | 40 | 95 | 39 | 3.25 |
| HNS-1.25-24 | 300 | 0 | 150 | 0 | 3.18 |
表1 不同样品的平均粒径、空腔直径、壁厚、开口角度和pH变化
Table 1 Average diameters of particles, inner cavities, carbon walls, opening angles, and pH dependence of different samples
| Sample | Average diameter of particle/nm | Average diameter of inner cavity/nm | Average diameter of carbon wall/nm | Opening angle/(°) | pH dependence |
|---|---|---|---|---|---|
| HNS-1.25-0 | - | - | - | - | 7.27 |
| HNS-1.25-3 | - | - | - | - | 3.53 |
| HNS-1.25-8 | 110 | 60 | 25 | 48 | 3.35 |
| HNS-1.25-12 | 140 | 80 | 30 | 63 | 3.32 |
| HNS-1.25-18 | 230 | 40 | 95 | 39 | 3.25 |
| HNS-1.25-24 | 300 | 0 | 150 | 0 | 3.18 |
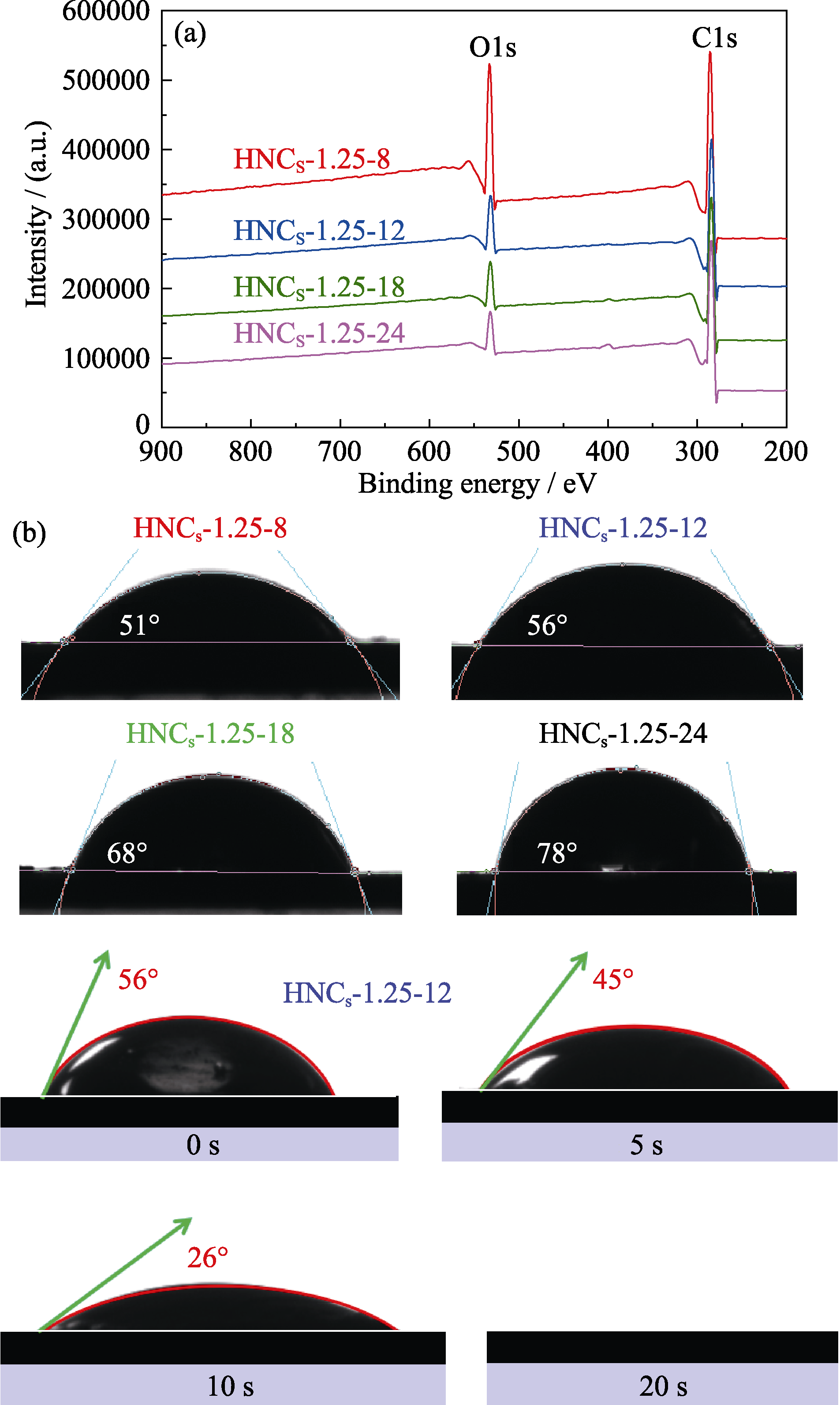
图6 HNCS-1.25-8、HNCS-1.25-12、HNCS-1.25-18和HNCS- 1.25-24的(a) XPS全谱图和(b)润湿性
Fig. 6 (a) XPS total survey and (b) wettability of HNCS-1.25-8, HNCS-1.25-12, HNCS-1.25-18, and HNCS-1.25-24
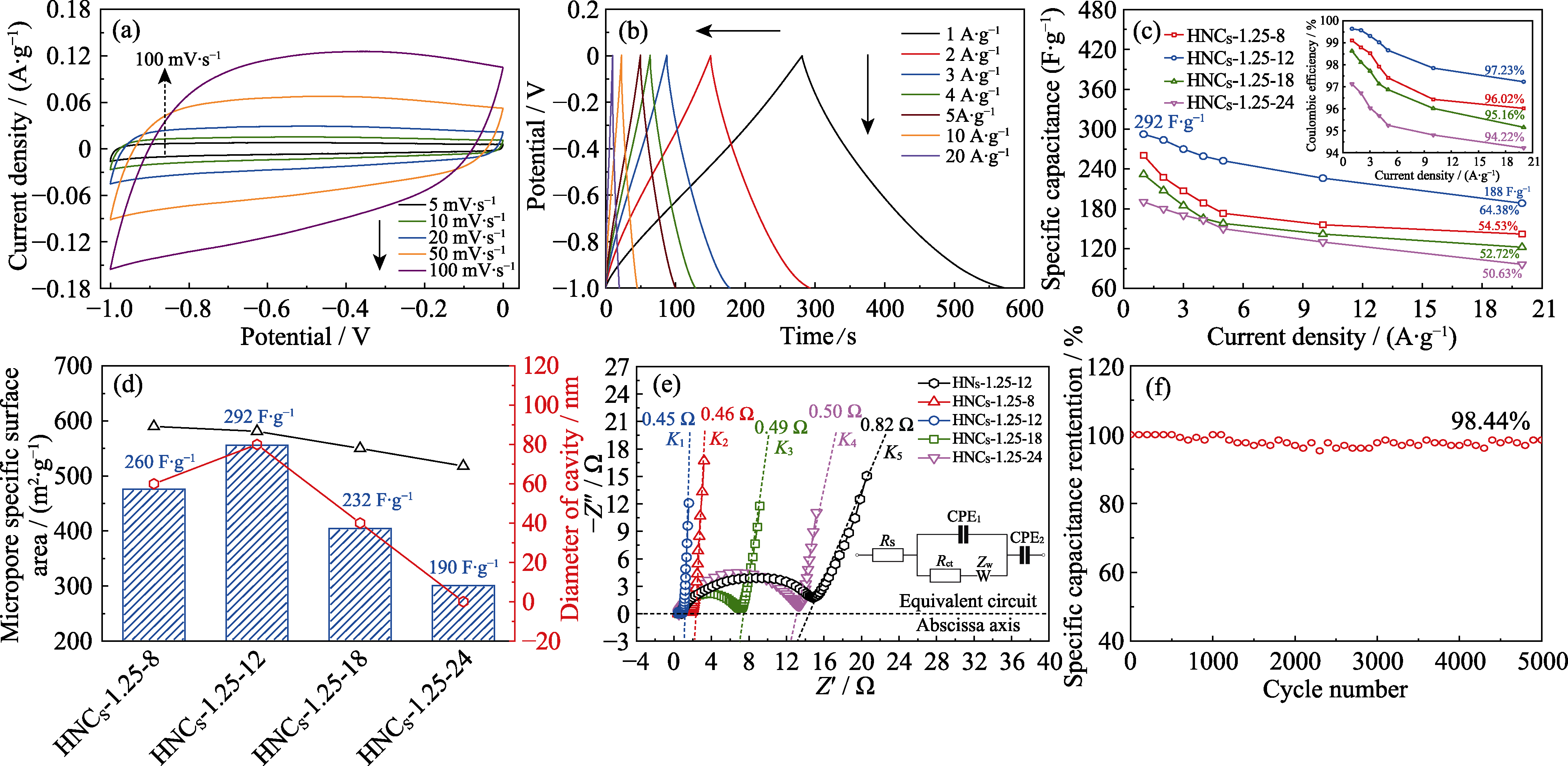
图7 HNCS-1.25-12在(a) 5~100 mV∙s-1的CV曲线和(b) 1~20 A∙g-1的GCD曲线; 不同样品(c)在1~20 A∙g-1的比电容和库仑效率(插图), (d)在1 A∙g-1比电容和空腔直径关系及(e) Nyquist曲线(插图为等效电路图); (f) HNCS-1.25-12的循环稳定性能
Fig. 7 (a) CV curves at 5-100 mV∙s-1 and (b) GCD curves at 1-20 A∙g-1 of HNCS-1.25-12; (c) Specific capacitances and Coulombic efficiencies (inset) at 1-20 A∙g-1, (d) relationship between specific capacitance with diameter of cavity at 1 A∙g-1 and (e) Nyquist plots with equivalent circuit (inset) of different samples; (f) Cycling stability of HNCS-1.25-12

图S2 (a~d)HNS-1.25-12和(e~h)HNCS-1.25-12的(a, e) TEM照片和(b~d)C、(f~h)O元素映射图
Fig. S2 (a, e) TEM images and (b-d) C, (f-h) O element mappings of (a-d) HNS-1.25-12 and (e-h) HNCS-1.25-12
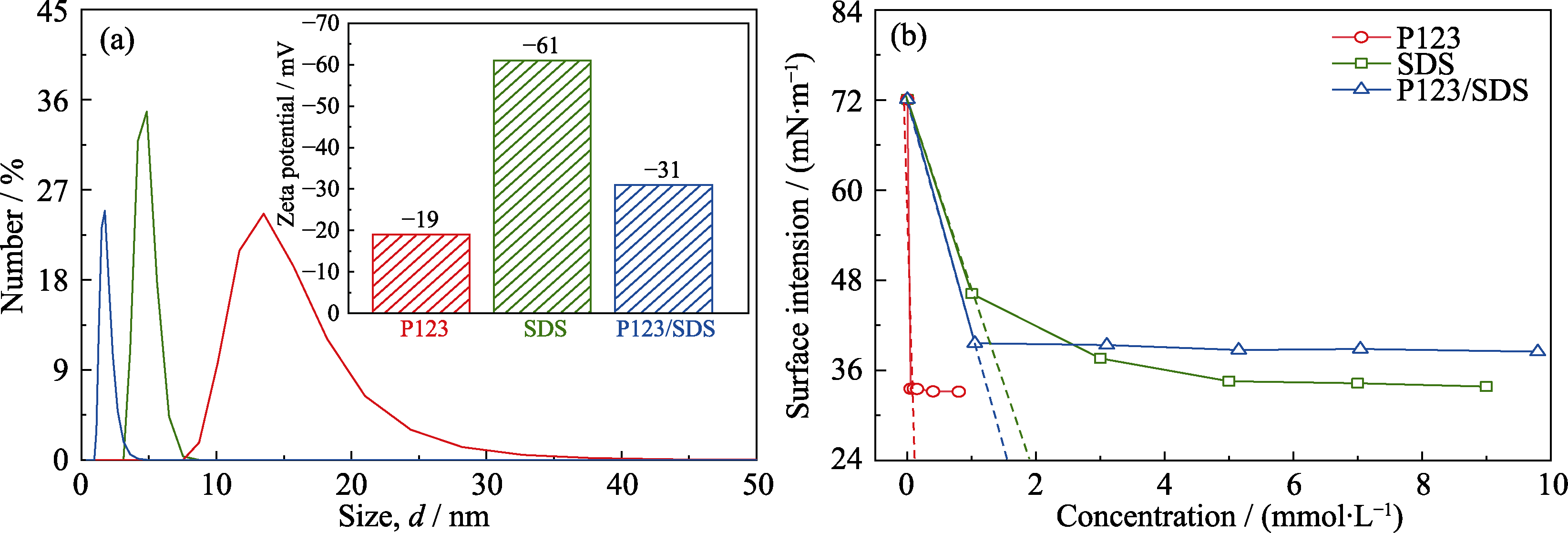
图S3 P123、SDS和混合乳液(P123/SDS的质量比恒定为1.25:1)在溶液中的(a)尺寸分布和Zeta电位、(b)表面张力
Fig. S3 (a) Size distribution and Zeta potential, and (b) surface tension of micelle/emulsion in H2O
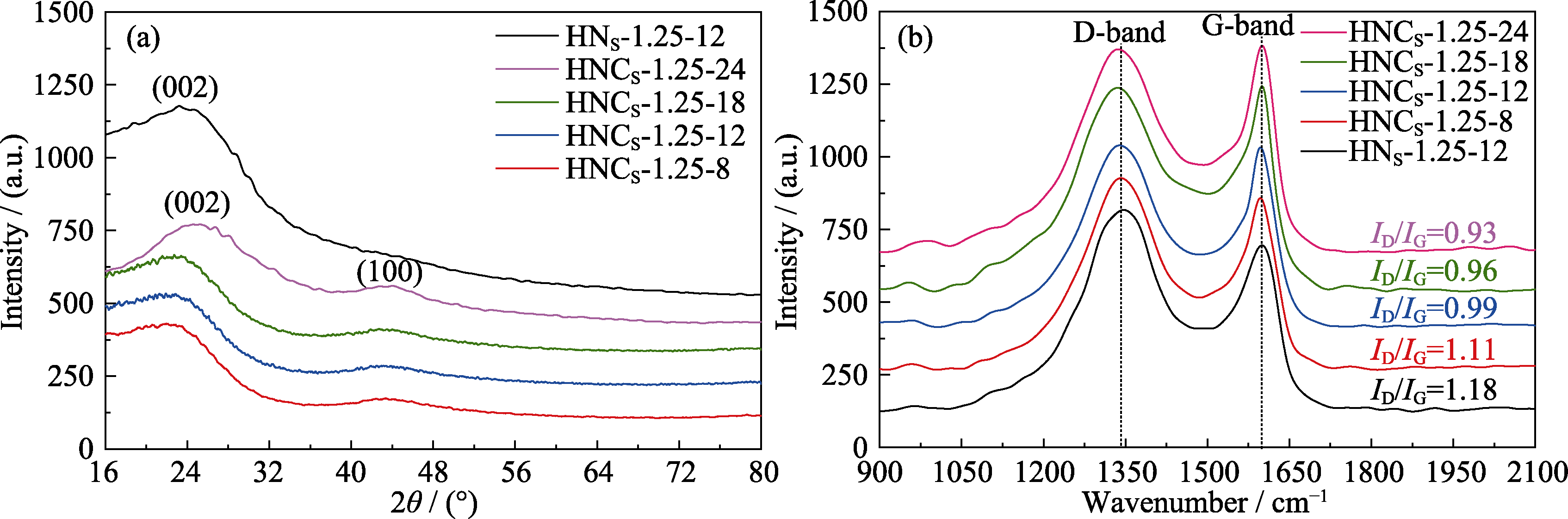
图S5 HNS-1.25-12、HNCS-1.25-8、HNCS-1.25-12、HNCS-1.25-18和HNCS-1.25-24的(a) XRD谱图和(b) Raman谱图
Fig. S5 (a) XRD patterns, (b) Raman spectra of HNS-1.25-12, HNCS-1.25-8, HNCS-1.25-12, HNCS-1.25-18, and HNCS-1.25-24
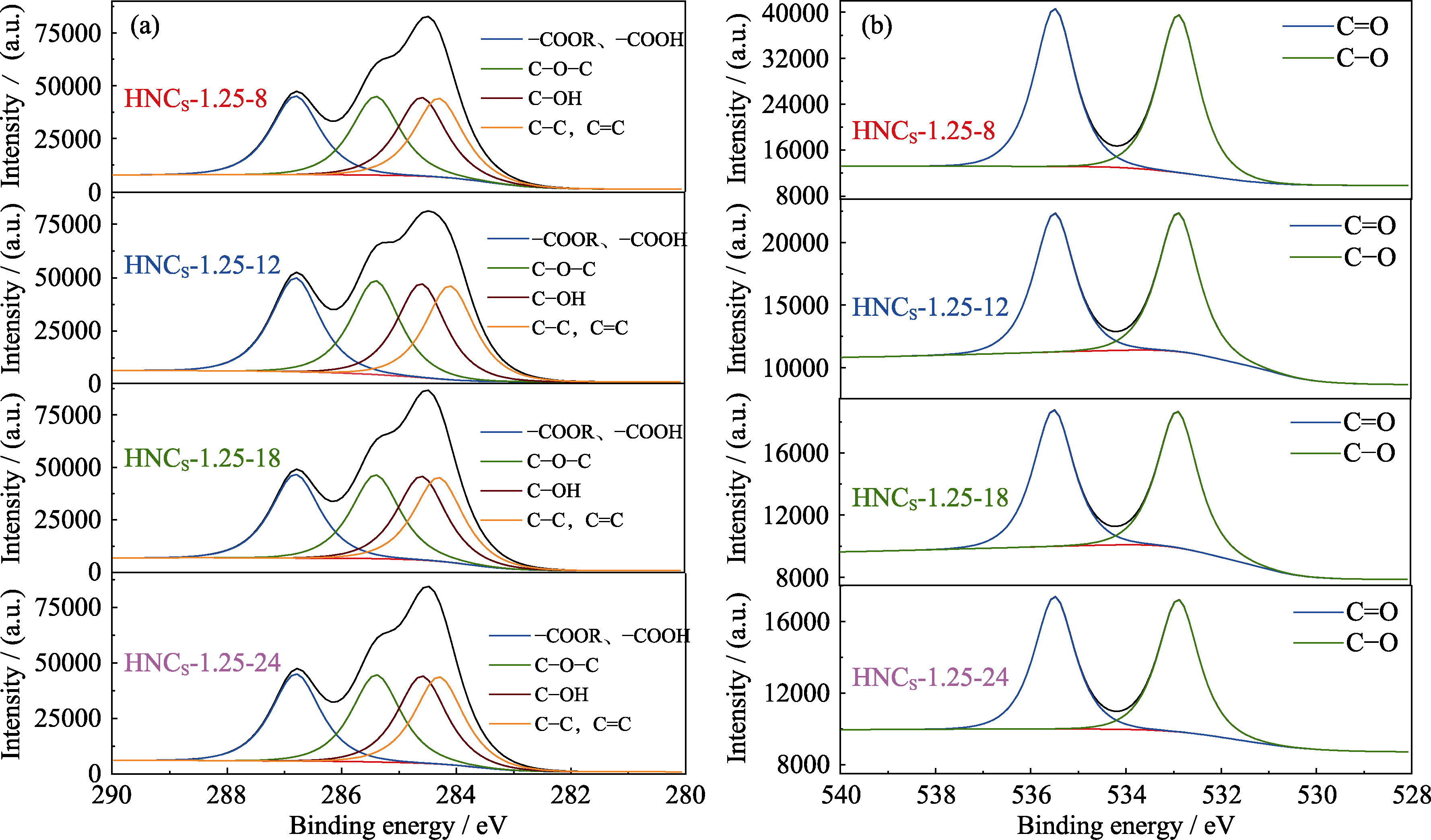
图S6 HNCS-1.25-8、HNCS-1.25-12、HNCS-1.25-18和HNCS-1.25-24的(a) C1s和(b) O1s XPS高分辨率谱图
Fig. S6 (a) C1s and (b) O1s high resolution XPS spectra of HNCS-1.25-8, HNCS-1.25-12, HNCS-1.25-18 and HNCS-1.25-24
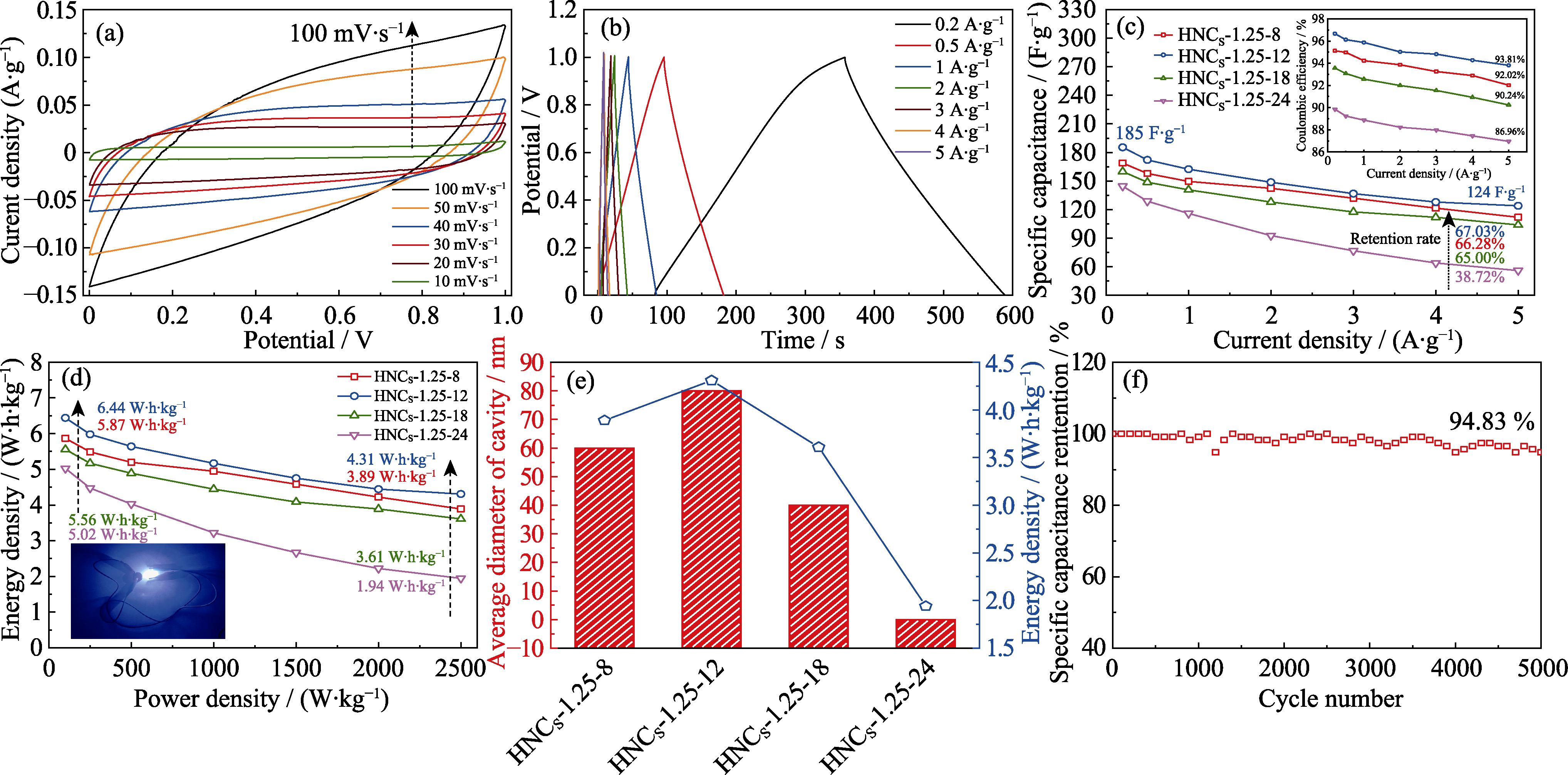
图S8 不同材料在二电极体系下的电化学性能
Fig. S8 Electrochemical performance of different materials in two-electrode system (a) CV curves at 10-100 mV∙s-1 and (b) GCD curves at 0.2-5 A∙g-1 of HNCS-1.25-12; (c) Specific capacitances and Coulombic efficiencies (inset) at 0.2-5 A∙g-1;, (d) Ragone plot with inset showing picture of lit-up LED and (e) relationship between energy density and diameter of cavity at power density of 2500 W∙kg-1 for different samples; (g) Cycling stability of HNCS-1.25-12
| Sample | Specific surface area, SBET/(m2·g-1) | Micropore specific surface area, Smicro /(m2·g-1) | Ratio of micropore, Smicro/SBET | Total pore volume /(cm3·g-1) | Pore volume of micropore/(cm3·g-1) | Average pore size/nm |
|---|---|---|---|---|---|---|
| HNS-1.25-12 | 9 | - | - | 0.03 | - | 11.90 |
| HNCS-1.25-8 | 619 | 590 | 95.32% | 0.23 | 0.22 | 1.49 |
| HNCS-1.25-12 | 611 | 581 | 95.09% | 0.23 | 0.22 | 1.52 |
| HNCS-1.25-18 | 617 | 550 | 89.14% | 0.32 | 0.21 | 1.84 |
| HNCS-1.25-24 | 588 | 518 | 88.10% | 0.32 | 0.20 | 1.87 |
表S1 不同样品的孔结构参数
Table S1 Textural parameters of different samples
| Sample | Specific surface area, SBET/(m2·g-1) | Micropore specific surface area, Smicro /(m2·g-1) | Ratio of micropore, Smicro/SBET | Total pore volume /(cm3·g-1) | Pore volume of micropore/(cm3·g-1) | Average pore size/nm |
|---|---|---|---|---|---|---|
| HNS-1.25-12 | 9 | - | - | 0.03 | - | 11.90 |
| HNCS-1.25-8 | 619 | 590 | 95.32% | 0.23 | 0.22 | 1.49 |
| HNCS-1.25-12 | 611 | 581 | 95.09% | 0.23 | 0.22 | 1.52 |
| HNCS-1.25-18 | 617 | 550 | 89.14% | 0.32 | 0.21 | 1.84 |
| HNCS-1.25-24 | 588 | 518 | 88.10% | 0.32 | 0.20 | 1.87 |
| Samples | Capacitance/ (F·g-1) | Current density/ (A·g-1) | Electrolyte | Ref. |
|---|---|---|---|---|
| NMHCSS | 240 | 0.2 | 6 mol∙L-1 KOH | [ |
| HFC | 238 | 0.5 | 6 mol∙L-1 KOH | [ |
| Fe2O3@Gr-CNT/NF | 114 | 1 | 2 mol∙L-1 KOH | [ |
| BHPC | 187 | 0.5 | 6 mol∙L-1 KOH | [ |
| ACS | 218 | 0.2 | 6 mol∙L-1 NaOH | [ |
| N-MWCNTs | 184 | 0.5 | 5 mol∙L-1 KOH | [ |
| SC-ZN | 263 | 0.5 | 6 mol∙L-1 KOH | [ |
| PN-ECB | 265 | 0.5 | 6 mol∙L-1 NaOH | [ |
| NHPC | 225 | 0.25 | 3 mol∙L-1 NaOH | [ |
| BPCS | 217 | 1 | 6 mol∙L-1 KOH | [ |
| rGONS | 200 | 0.5 | 6 mol∙L-1 KOH | [ |
| HNCS-1.25-12 | 292 | 1 | 6 mol∙L-1 KOH | This work |
表S2 文献报道的多孔炭基材料的电容性能
Table S2 Capacitive properties of doped-carbon materials reported in literature
| Samples | Capacitance/ (F·g-1) | Current density/ (A·g-1) | Electrolyte | Ref. |
|---|---|---|---|---|
| NMHCSS | 240 | 0.2 | 6 mol∙L-1 KOH | [ |
| HFC | 238 | 0.5 | 6 mol∙L-1 KOH | [ |
| Fe2O3@Gr-CNT/NF | 114 | 1 | 2 mol∙L-1 KOH | [ |
| BHPC | 187 | 0.5 | 6 mol∙L-1 KOH | [ |
| ACS | 218 | 0.2 | 6 mol∙L-1 NaOH | [ |
| N-MWCNTs | 184 | 0.5 | 5 mol∙L-1 KOH | [ |
| SC-ZN | 263 | 0.5 | 6 mol∙L-1 KOH | [ |
| PN-ECB | 265 | 0.5 | 6 mol∙L-1 NaOH | [ |
| NHPC | 225 | 0.25 | 3 mol∙L-1 NaOH | [ |
| BPCS | 217 | 1 | 6 mol∙L-1 KOH | [ |
| rGONS | 200 | 0.5 | 6 mol∙L-1 KOH | [ |
| HNCS-1.25-12 | 292 | 1 | 6 mol∙L-1 KOH | This work |
| [1] | LIU F, CHENG Y, TAN J C, et al. Carbon nanomaterials with hollow structures: a mini-review. Frontiers in Chemistry, 2021, 9: 668336. |
| [2] |
SUNDER A, KRÄMER M, HANSELMANN R, et al. Molecular nanocapsules based on amphiphilic hyperbranched polyglycerols. Angewandte Chemie International Edition, 1999, 38(23): 3552.
DOI URL |
| [3] | LIANG J, HU H, PARK H, et al. Construction of hybrid bowl-like structures by anchoring NiO nanosheets on flat carbon hollow particles with enhanced lithium storage properties. Energy & Environmental Science, 2015, 8(6): 1707. |
| [4] |
LIANG J, YU X Y, ZHOU H, et al. Bowl-like SnO2@carbon hollow particles as an advanced anode material for lithium-ion batteries. Angewandte Chemie International Edition, 2014, 53(47): 12803.
DOI URL |
| [5] |
ZHANG W, CHENG R R, BI H H, et al. A review of porous carbons produced by template methods for supercapacitor applications. New Carbon Materials, 2021, 36(1): 69.
DOI URL |
| [6] |
ZHAO R G, WANG H, ZHANG X Y, et al. Hierarchically porous three-dimensional (3D) carbon nanorod networks with a high content of FeNx sites for efficient oxygen reduction reaction. Langmuir, 2022, 38(37): 11372.
DOI URL |
| [7] | YU L, YU X Y, LOU X W. The design and synthesis of hollow micro-/nanostructures: present and future trends. Advanced Materials, 2018, 30(38): 1800939. |
| [8] |
KAKANI V, RAMESH S, YADAV H M, et al. Hydrothermal synthesis of CuO@MnO2 on nitrogen-doped multiwalled carbon nanotube composite electrodes for supercapacitor applications. Scientific Reports, 2022, 12(1): 12951.
DOI |
| [9] | LACHOS-PEREZ D, TORRES-MAYANGA P C, ABAIDE E R, et al. Hydrothermal carbonization and liquefaction: differences, progress, challenges, and opportunities. Bioresource Technology, 2022, 343: 126084. |
| [10] | KAN Y N, CHEN B W, ZHAI S C, et al. Chemical compositions of carbon sources affected on surface morphology and spectral properties of the synthetic carbon microspheres. Spectroscopy and Spectral Analysis, 2020, 40(10): 3153. |
| [11] |
KANG S M, LI X L, FAN J, et al. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Industrial & Engineering Chemistry Research, 2012, 51(26): 9023.
DOI URL |
| [12] |
WANG S P, HAN C L, WANG J, et al. Controlled synthesis of ordered mesoporous carbohydrate-derived carbons with flower-like structure and N-doping by self-transformation. Chemistry of Materials, 2014, 26(23): 6872
DOI URL |
| [13] |
XIONG S Q, FAN J C, WANG Y, et al. A facile template approach to nitrogen-doped hierarchical porous carbon nanospheres from polydopamine for high-performance supercapacitors. Journal of Materials Chemistry A, 2017, 5(34): 18242.
DOI URL |
| [14] |
YANG Z C, TANG C H, GONG H, et al. Hollow spheres of nanocarbon and their manganese dioxide hybrids derived from soft template for supercapacitor application. Journal of Power Sources, 2013, 240: 713.
DOI URL |
| [15] |
WU M B, AI P P, TAN M H, et al. Synthesis of starch-derived mesoporous carbon for electric double layer capacitor. Chemical Engineering Journal, 2014, 245: 166.
DOI URL |
| [16] |
WU Q, LI W, TAN J, et al. Flexible cage-like carbon spheres with ordered mesoporous structures prepared via a soft-template/ hydrothermal process from carboxymethylcellulose. RSC Advances, 2014, 4(16): 61518.
DOI URL |
| [17] | XU Z, LIU Y X, XU C. F, et al. B-doped hierarchical porous carbon spheres prepared by xylose-soft template hydrothermal strategy for enhancing electrochemical property. Chinese Journal of Inorganic Chemistry, 2022, 38(10): 2006. |
| [18] |
LIU H T, XU Y J, WANG J J, et al. A triple template of P123/SDS/CS with simultaneously enhanced utilization efficiency of P123 and crystal seeds. Journal of Alloys and Compounds, 2018, 765: 907.
DOI URL |
| [19] |
WU Q, LI W, TAN J, et al. Hydrothermal carbonization of carboxymethylcellulose: one-pot preparation of conductive carbon microspheres and water-soluble fluorescent carbon nanodots. Chemical Engineering Journal, 2015, 266: 112.
DOI URL |
| [20] |
WU Q, LI W, TAN J, et al. Hydrothermal synthesis of magnetic mesoporous carbon microspheres from carboxymethylcellulose and nickel acetate. Applied Surface Science, 2015, 332: 354.
DOI URL |
| [21] |
TITIRICI M M, ANTONIETTI M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chemical Society Reviews, 2010, 39(1): 103.
DOI URL |
| [22] |
WU L M, TONG D S, LI C S, et al. Insight into formation of montmorillonite-hydrochar nanocomposite under hydrothermal conditions. Applied Clay Science, 2016, 119: 116.
DOI URL |
| [23] |
WANG G H, HILGERT J, RICHTER F H, et al. Platinum-cobalt bimetallic nanoparticles in hollow carbon nanospheres for hydrogenolysis of 5-hydroxymethylfurfural. Nature Materials, 2014, 13(3): 293.
DOI |
| [24] | XING Z B, GUO Z J, ZHANG Y W, et al. Regulation of SDS on the surface charge density of SB3-12 micelles and its effect on drug dissolution. Acta Physico-Chimica Sinica, 2020, 36(6): 1906006. |
| [25] |
MONDAL R, GHOSH N, PAUL B K, et al. Triblock-copolymer- assisted mixed-micelle formation results in the refolding of unfolded protein. Langmuir, 2018, 34(3): 896.
DOI URL |
| [26] |
HOSSAIN M K, HINATA S, LOPEZ-QUINTELA A, et al. Phase behavior of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) block copolymer in water and water-C12EO5 systems. Journal of Dispersion Science and Technology, 2003, 24(3/4): 411.
DOI URL |
| [27] |
KANCHARLA S, BEDROV D, TSIANOU M, et al. Structure and composition of mixed micelles formed by nonionic block copolymers and ionic surfactants in water determined by small- angle neutron scattering with contrast variation. Journal of Colloid and Interface Science, 2022, 609: 456.
DOI URL |
| [28] | YIN J, ZHANG W L, ALHEBSHI N A, et al. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods, 2020, 4(3): 1900853. |
| [29] |
LONG S J, SI C D. Integrated gas expansion and activation strategy to prepare shaddock peel-derived nitrogen doped honeycomb carbon for high performance supercapacitor. Journal of Porous Materials, 2022, 29(5): 1639.
DOI |
| [30] |
ZHANG F M, XIAO X S, GANDLA D, et al. Bio-derived carbon with tailored hierarchical pore structures and ultra-high specific surface area for superior and advanced supercapacitors. Nanomaterials, 2022, 12(1): 27.
DOI URL |
| [31] | LIU X, SONG P P, HOU J H, et al. Revealing the dynamic formation process and mechanism of hollow carbon spheres: from bowl to sphere. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 2797. |
| [32] | LIN H H, TAN Z X, YANG J W, et al. Highly porous carbon material from polycyclodextrin for high-performance supercapacitor electrode. Journal of Energy Storage, 2022, 53: 105036. |
| [33] |
YEGANEH F, CHIEWCHAN N, CHONKAEW W. Hydrothermal pretreatment of biomass-waste-garlic skins in the cellulose nanofiber production process. Cellulose, 2022, 29(4): 2333.
DOI |
| [34] | FU J Q, BAI L, CHI M S, et al. Study on the evolution pattern of the chemical structure of Fenton pretreated lignin during hydrothermal carbonization. Journal of Environmental Chemical Engineering, 2022, 10(2): 107184. |
| [35] | JIA X, LI L C, TENG J W, et al. Glycation of rice protein and D-xylose pretreated through hydrothermal cooking-assisted high hydrostatic pressure: focus on the structural and functional properties. LWT, 2022, 160: 113194. |
| [36] |
QIU W L, LEISEN J E, LIU Z Y, et al. Key features of polyimide- derived carbon molecular sieves. Angewandte Chemie International Edition, 2021, 60(41): 22322.
DOI URL |
| [37] | MA L N, BI Z J, ZHANG W, et al. Synthesis of a Three-dimensional interconnected oxygen-, boron-, nitrogen-, and phosphorus tetratomic-doped porous carbon network as electrode material for the construction of a superior flexible supercapacitor. ACS Applied Materials & Interfaces, 2020, 12(41): 46170. |
| [38] |
DU J, ZHANG Y, LÜ H J, et al. N/B-co-doped ordered mesoporous carbon spheres by ionothermal strategy for enhancing supercapacitor performance. Journal of Colloid and Interface Science, 2021, 587: 780.
DOI URL |
| [39] | PHOLAUYPHON W, BULAKHE R N, MANYAM J, et al. High- performance supercapacitors using carbon dots/titanium dioxide composite electrodes and carbon dot-added sulfuric acid electrolyte. Journal of Electroanalytical Chemistry, 2022, 910: 116177. |
| [40] |
ZHANG J P, NING X A, LI D P, et al. Nitrogen-enriched micro-mesoporous carbon derived from polymers organic frameworks for high-performance capacitive deionization. Journal of Environmental Sciences, 2022, 111: 282.
DOI URL |
| [41] | ZHANG Y, LIU K Y, ZHANG W, et al. Electrochemical performance of electrodes in MnO2 supercapacitor. Acta Chimica Sinica, 2008, 66(8): 909. |
| [42] |
HU Y R, DONG X L, HOU L, et al. Electrochemical oxidation of 2D B, N-codoped carbon nanosheets to improve their pseudo- capacitance. New Carbon Materials, 2021, 36(6): 1109.
DOI URL |
| [43] |
ZHOU Y, XIAO N, QIU J S, et al. Preparation of carbon microfibers from coal liquefaction residue. Fuel, 2008, 87: 3474.
DOI URL |
| [44] |
LIU C L, DONG W S, CAO G P, et al. Influence of KOH followed by oxidation pretreatment on the electrochemical performance of phenolic based activated carbon fibers. Journal of Electroanalytical Chemistry, 2007, 611: 225.
DOI URL |
| [45] | CHEN Y, MA Y N, HUANG J D, et al. Fabricating dual redox electrolyte to achieve ultrahigh specific capacitance and reasonable Coulombic efficiency for biomass activated carbon. Electrochimica Acta, 2022, 414: 140215. |
| [46] |
WANG M, YANG J, JIA K L, et al. Boosting supercapacitor performance of graphene by coupling with nitrogen-doped hollow carbon frameworks. Chemistry-A European Journal, 2020, 26: 2897.
DOI URL |
| [47] |
LU W J, HAO L N, WANG Y W. Highly active N, S co-doped ultramicroporous carbon for high-performance supercapacitor electrodes. Micromachines, 2022, 13(6): 905.
DOI URL |
| [48] |
JIAO J C, ZHU Y X, PENG X W, et al. Preparation of high capacitive performance porous carbon assisted by sodium dodecyl sulfate. Acta Chimica Sinica, 2021, 79: 778.
DOI URL |
| [49] |
HU X, LIU H B, XIA X H, et al. Polyaniline-carbon pillared graphene composite: preparation and electrochemical performance. Journal of Inorganic Materials, 2019, 34(2): 145.
DOI URL |
| [1] | 魏志帆, 陈国清, 祖宇飞, 刘渊, 李明浩, 付雪松, 周文龙. ZrB2-HfSi2复相陶瓷显微组织及其核-周结构形成机制[J]. 无机材料学报, 2025, 40(7): 817-825. |
| [2] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [3] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [4] | 薛轶凡, 李玮洁, 张中伟, 庞旭, 刘愚. 碳纤维布表面PyC界面相微观结构及均匀性的工艺调控[J]. 无机材料学报, 2024, 39(4): 399-408. |
| [5] | 管皞阳, 张立, 荆开开, 师维刚, 王晶, 李玫, 刘永胜, 张程煜. 国产三代2.5D SiCf/SiC复合材料的界面力学性能[J]. 无机材料学报, 2024, 39(3): 259-266. |
| [6] | 郝永鑫, 秦娟, 孙军, 杨金凤, 李清连, 黄贵军, 许京军. 坩埚底角形状对提拉法生长同成分铌酸锂晶体的影响[J]. 无机材料学报, 2024, 39(10): 1167-1174. |
| [7] | 丁统顺, 丰平, 孙学文, 单沪生, 李琪, 宋健. Fmoc-FF-OH钝化钙钛矿薄膜及其太阳能电池性能研究[J]. 无机材料学报, 2023, 38(9): 1076-1082. |
| [8] | 胡忠良, 傅赟天, 蒋蒙, 王连军, 江莞. Nb/Mg3SbBi界面层热稳定性研究[J]. 无机材料学报, 2023, 38(8): 931-937. |
| [9] | 沈轩逸, 马沁, 薛玉冬, 廖春景, 朱敏, 张翔宇, 杨金山, 董绍明. 复合界面层对SiCf/SiC复合材料力学损伤行为的影响[J]. 无机材料学报, 2023, 38(8): 917-922. |
| [10] | 林俊良, 王占杰. 铁电超晶格的研究进展[J]. 无机材料学报, 2023, 38(6): 606-618. |
| [11] | 穆宏赫, 王鹏飞, 施宇峰, 张中晗, 武安华, 苏良碧. 热交换坩埚下降法制备大尺寸氟化铈晶体的热场设计与优化[J]. 无机材料学报, 2023, 38(3): 288-295. |
| [12] | 孙晗, 李文俊, 贾子璇, 张岩, 殷利迎, 介万奇, 徐亚东. ACRT技术对大尺寸ZnTe晶体溶液法制备及其性能影响[J]. 无机材料学报, 2023, 38(3): 310-315. |
| [13] | 华思恒, 杨东旺, 唐昊, 袁雄, 展若雨, 徐卓明, 吕嘉南, 肖娅妮, 鄢永高, 唐新峰. n型Bi2Te3基材料表面处理对热电单元性能的影响[J]. 无机材料学报, 2023, 38(2): 163-169. |
| [14] | 谭淑雨, 刘晓宁, 毕志杰, 万勇, 郭向欣. 正极包覆与界面修饰: 双策略改善聚氧化乙烯固态电解质对高电压正极稳定性[J]. 无机材料学报, 2023, 38(12): 1466-1474. |
| [15] | 孙铭, 邵溥真, 孙凯, 黄建华, 张强, 修子扬, 肖海英, 武高辉. RGO/Al复合材料界面性质第一性原理研究[J]. 无机材料学报, 2022, 37(6): 651-659. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||