
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (8): 871-887.DOI: 10.15541/jim20250013
• REVIEW • Previous Articles Next Articles
LUO Xiaomin1( ), QIAO Zhilong1, LIU Ying1, YANG Chen3, CHANG Jiang2,3(
), QIAO Zhilong1, LIU Ying1, YANG Chen3, CHANG Jiang2,3( )
)
Received:2025-01-08
Revised:2025-02-18
Published:2025-08-20
Online:2025-05-22
Contact:
CHANG Jiang, professor. E-mail: jchang@mail.sic.ac.cnAbout author:LUO Xiaomin (1966-), female, professor. E-mail: luoxiaomin@sust.edu.cn
Supported by:CLC Number:
LUO Xiaomin, QIAO Zhilong, LIU Ying, YANG Chen, CHANG Jiang. Inorganic Bioactive Materials Regulating Myocardial Regeneration[J]. Journal of Inorganic Materials, 2025, 40(8): 871-887.
| Composite material | Main composition | Biological mechanism | Ref. |
|---|---|---|---|
| Composite scaffold | Cellulose nanofibrils, sulfonated carbon nanotubes (SCNTs) | Endowing hydrogels with superior mechanical performance and conductivity, thus providing a favorable environment for normal intercellular communication of myocardial tissue; Inducing more expression of connexin 43 (Cx-43) protein around the cell nucleus and promoting proliferation and maturity of myocardial cells | [ |
| Composite scaffold | Au, CO, chitosan (CS) | Increasing Cx-43 content and improving conduction velocity and contractility of the infarct area | [ |
| Composite microsphere | Poly-L-lactic acid, hydroxyapatite (HAP) | Improving cell adhesion to microspheres; Inhibiting inflammation and promoting angiogenesis | [ |
| Composite hydrogel | Mesoporous silica, puerarin, CS | Inhibiting M1-type polarization of macrophages and production of pro-inflammatory factors; Promoting formation of tubular network structure of human umbilical vein endothelial cells (HUVECs) in vitro by its released silicon ions | [ |
| Composite hydrogel | Methacrylated gelatin (GelMA), carbon nanotubes (CNTs) | Inducing orientation of cardiomyocytes and facilitating their synchronous activity by favorable electrical conductivity | [ |
Table 1 Mechanisms of inorganic bioactive composites in regulating myocardial regeneration[27-31]
| Composite material | Main composition | Biological mechanism | Ref. |
|---|---|---|---|
| Composite scaffold | Cellulose nanofibrils, sulfonated carbon nanotubes (SCNTs) | Endowing hydrogels with superior mechanical performance and conductivity, thus providing a favorable environment for normal intercellular communication of myocardial tissue; Inducing more expression of connexin 43 (Cx-43) protein around the cell nucleus and promoting proliferation and maturity of myocardial cells | [ |
| Composite scaffold | Au, CO, chitosan (CS) | Increasing Cx-43 content and improving conduction velocity and contractility of the infarct area | [ |
| Composite microsphere | Poly-L-lactic acid, hydroxyapatite (HAP) | Improving cell adhesion to microspheres; Inhibiting inflammation and promoting angiogenesis | [ |
| Composite hydrogel | Mesoporous silica, puerarin, CS | Inhibiting M1-type polarization of macrophages and production of pro-inflammatory factors; Promoting formation of tubular network structure of human umbilical vein endothelial cells (HUVECs) in vitro by its released silicon ions | [ |
| Composite hydrogel | Methacrylated gelatin (GelMA), carbon nanotubes (CNTs) | Inducing orientation of cardiomyocytes and facilitating their synchronous activity by favorable electrical conductivity | [ |
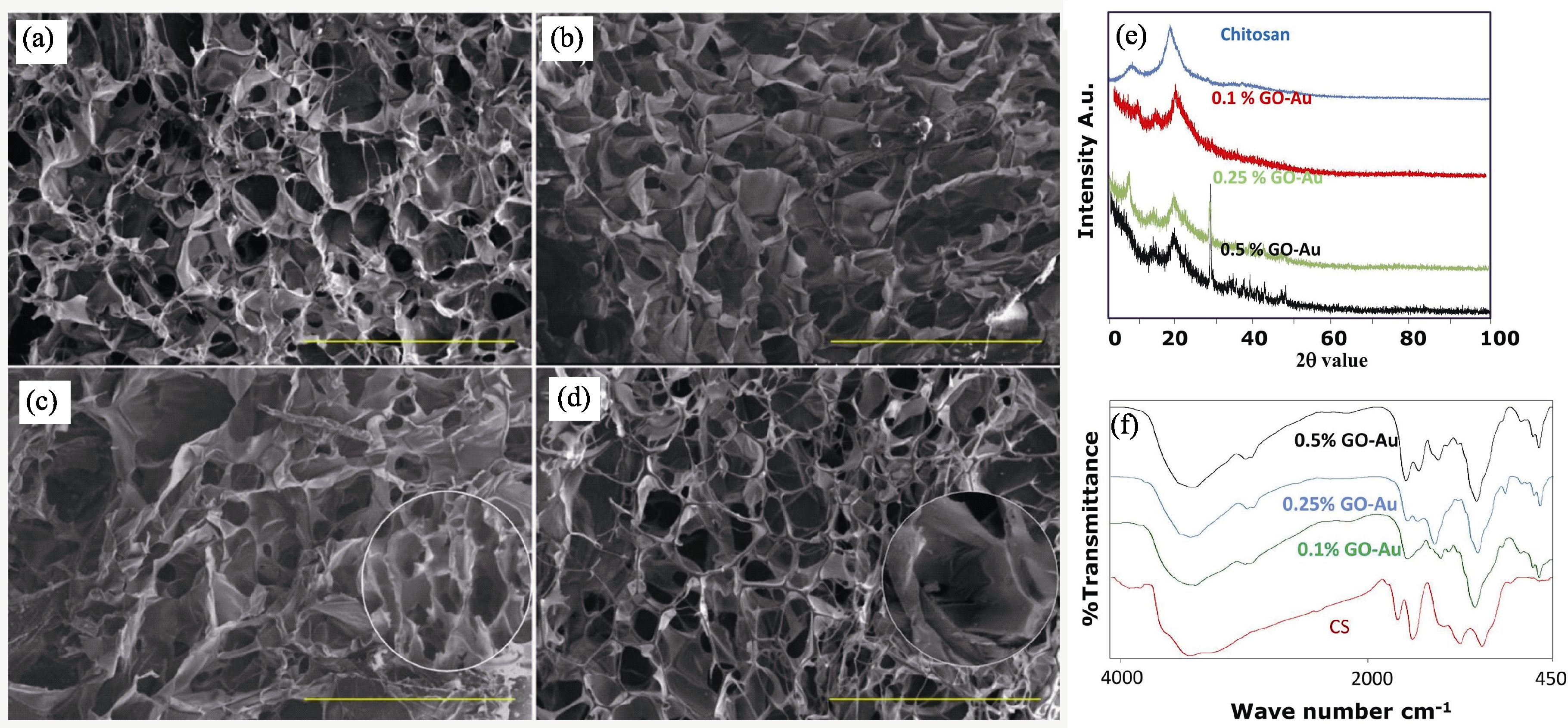
Fig. 1 Physicochemical characterization of GO-Au/CS composite scaffolds[28] (a-d) SEM images of (a) CS, (b) CS/0.1% GO-Au, (c) CS/0.25% GO-Au, and (d) CS/0.5% GO-Au (scale bar is 500 µm); (e) XRD patterns and (f) FT-IR spectra of composite scaffolds at different concentrations

Fig. 2 Schematic diagrams on preparation and application of SiO2-Fe3O4 composite microspheres[33] (a) Schematic of treatment with SiO2-Fe3O4 composite microspheres; (b) Schematic synthesis of composite microspheres

Fig. 3 Overall effects and mechanism of ion therapy on MI treatment[39] (a) Mechanism of ion therapy; (b) Effect of silicon-enriched ion extract on the expression of gap junction associated Cx-43 in NRCMs under glucose/oxygen deprived conditions in vitro; (c) Effect of silicon-enriched ion extract on VEGF-mediated angiogenesis of NRCMs and HUVECs co-cultures under glucose/oxygen deprived conditions in vitro; (d) Effect of ion therapy on cardiac apoptosis in vivo post-MI. NRCMs: neonatal rat cardiomyocytes; VEGF: vascular endothelial growth factor

Fig. 4 Component of the PSi NP and its therapeutic effect on MI[44] (a) Schematic components of the PSi NP and overview of studies conducted in vitro and in vivo; (b) Single-photon emission computed tomography (SPECT/CT) image quantification of the standardized uptake values in the rat heart at different time after intravenous (i.v.) administration of NPs; (c) SPECT/CT images showing the biodistribution of the nanoparticles at 10 min after i.v. administration; (d) H&E stainings and autoradiograms of apical, basal, and medial rat heart sections; (e) Autoradiographic quantification of radioactivity in the endocardium (Endo) and epicardium (Epi) for Un-P-D-ANP and Un-P-D NPs; (f) Autoradiographic quantification of radioactivity in the Endo and Epi regions of the heart in the apical, medial, and basal sections in isoprenaline-induced MI and normal rat groups; (g) TEM image of Un-P-D-ANP NPs in the NP Endo region of a heart section; (h) Elemental composition of the selected area by EDX analysis showing the presence of the Si element. PSi, NP, Un-P-D-ANP, and Un-P-D denote porous silicon, nanoparticle, undecylenic acid-porous silicon-atrial natriuretic peptide, and undecylenic acid-porous silicon, respectively
| Nanocarrier | Loading | Biological mechanism | Ref. |
|---|---|---|---|
| MSN | miRNA | Inhibiting inflammatory response by inhibiting polarization of M1 macrophage within infarcted myocardium, delivering microRNA-21-5p to endothelial cells, and markedly promoting local neovascularization and rescuing at-risk cardiomyocytes | [ |
| MSN | Quercetin | Effectively inhibiting cell apoptosis and oxidative stress, reducing myocardial infarction size, improving ventricular remodeling and cardiac function-related biochemical indexes, and promoting recovery of cardiac blood flow | [ |
| Porous silicon (PSi) | ANP | Improving colloidal stability, enhancing cellular interactions with cardiomyocytes and nonmyocytes, and attenuating hypertrophic signaling in the endocardium | [ |
| PSi | Wnt3a protein | Prolonging Wnt3a release, and increasing antioxidative stress activity in labeled mesenchymal stem cells, highly beneficial for cell protection in stem cell therapy for MI | [ |
Table 2 Mechanisms of inorganic bioactive nanoparticle materials in regulating myocardial regeneration[26,43 -45]
| Nanocarrier | Loading | Biological mechanism | Ref. |
|---|---|---|---|
| MSN | miRNA | Inhibiting inflammatory response by inhibiting polarization of M1 macrophage within infarcted myocardium, delivering microRNA-21-5p to endothelial cells, and markedly promoting local neovascularization and rescuing at-risk cardiomyocytes | [ |
| MSN | Quercetin | Effectively inhibiting cell apoptosis and oxidative stress, reducing myocardial infarction size, improving ventricular remodeling and cardiac function-related biochemical indexes, and promoting recovery of cardiac blood flow | [ |
| Porous silicon (PSi) | ANP | Improving colloidal stability, enhancing cellular interactions with cardiomyocytes and nonmyocytes, and attenuating hypertrophic signaling in the endocardium | [ |
| PSi | Wnt3a protein | Prolonging Wnt3a release, and increasing antioxidative stress activity in labeled mesenchymal stem cells, highly beneficial for cell protection in stem cell therapy for MI | [ |
| Metal oxide | Composition/modification | Biological mechanism | Ref. |
|---|---|---|---|
| Fe2O3 | Fe2O3@DMSA NPs | Protecting myocardium from ischemia injury in vivo and inhibiting calcium influx | [ |
| SPION | MSCs/coated with polyethylene glycol | Improving heart function and myocardial hypertrophy and reducing fibrosis | [ |
| Fe3O4 | Polycaprolactone, polyvinylidene fluoride | Stimulating maturation, featuring superior sarcomeric structures, improving calcium transients, and upregulating maturation genes | [ |
| CeO2 | Macrophage-derived extracellular vesicles, Pd | Dissipating interstitial edema, triggering prominent angiogenesis, and finally improving cardiac function and ventricular remodeling | [ |
| MgO | MgO NPs | Modulating apoptosis-related markers (caspase-3 and p53), upregulating antiapoptotic (Bcl-2) and antioxidant (SOD) markers | [ |
| ZnO | — | Formation of reactive oxygen species, especially hydrogen peroxid | [ |
Table 3 Mechanisms of several common metal oxides in regulating myocardial regeneration[52-57]
| Metal oxide | Composition/modification | Biological mechanism | Ref. |
|---|---|---|---|
| Fe2O3 | Fe2O3@DMSA NPs | Protecting myocardium from ischemia injury in vivo and inhibiting calcium influx | [ |
| SPION | MSCs/coated with polyethylene glycol | Improving heart function and myocardial hypertrophy and reducing fibrosis | [ |
| Fe3O4 | Polycaprolactone, polyvinylidene fluoride | Stimulating maturation, featuring superior sarcomeric structures, improving calcium transients, and upregulating maturation genes | [ |
| CeO2 | Macrophage-derived extracellular vesicles, Pd | Dissipating interstitial edema, triggering prominent angiogenesis, and finally improving cardiac function and ventricular remodeling | [ |
| MgO | MgO NPs | Modulating apoptosis-related markers (caspase-3 and p53), upregulating antiapoptotic (Bcl-2) and antioxidant (SOD) markers | [ |
| ZnO | — | Formation of reactive oxygen species, especially hydrogen peroxid | [ |

Fig. 5 PCL/Fe3O4/PVDF composite piezoelectric myocardial patch promoting myocardial regeneration by rebuilding electrical signaling at the damaged myocardium[54] (a) Strategy of engineering cardiac constructs with synergistic mechanical/piezoelectric stimulation; (b) Engineered cardiac constructs with synergistic mechanical/piezoelectric stimulation preserving left ventricular function after acute MI in vivo
| Carbon-based biomaterial | Composition | Biological mechanism | Ref. |
|---|---|---|---|
| CNTs | Single walled CNTs incorporated into collagen substrates | Growth supports for neonatal cardiomyocytes, enhancing cardiomyocyte adhesion and maturation | [ |
| CNTs | GelMA-dielectrophoretically aligned CNT hydrogels | Enhancing the cardiac differentiation of embryoid bodies | [ |
| CNFs | Incorporating CNFs into chitosan | Increasing expression of cardiac-specific genes involved in muscle contraction and electrical coupling | [ |
| GPE | Incorporating GPE into poly(glycerol sebacate) | Graphen, with cytocompatibility and proliferation-promoting property, endowing it conductive for conduction of electrical signals between cardiomyocytes and cardiac tissue | [ |
| GO | Incorporating GO into thiol-modified hyaluronic acid | Promoting transmission of mechanical and electrical signals | [ |
| rGO | Incorporating rGO into alginate | Boosting angiogenic capability of alginate against HUVECs | [ |
Table 4 Mechanisms of several common carbon-based biomaterials in regulating myocardial regeneration[74-79]
| Carbon-based biomaterial | Composition | Biological mechanism | Ref. |
|---|---|---|---|
| CNTs | Single walled CNTs incorporated into collagen substrates | Growth supports for neonatal cardiomyocytes, enhancing cardiomyocyte adhesion and maturation | [ |
| CNTs | GelMA-dielectrophoretically aligned CNT hydrogels | Enhancing the cardiac differentiation of embryoid bodies | [ |
| CNFs | Incorporating CNFs into chitosan | Increasing expression of cardiac-specific genes involved in muscle contraction and electrical coupling | [ |
| GPE | Incorporating GPE into poly(glycerol sebacate) | Graphen, with cytocompatibility and proliferation-promoting property, endowing it conductive for conduction of electrical signals between cardiomyocytes and cardiac tissue | [ |
| GO | Incorporating GO into thiol-modified hyaluronic acid | Promoting transmission of mechanical and electrical signals | [ |
| rGO | Incorporating rGO into alginate | Boosting angiogenic capability of alginate against HUVECs | [ |

Fig. 6 Carbon-based materials promoting myocardial repair by reconstructing mechanical-electrical microenvironment[77-78,103] (a) Schematic diagram of preparation of elastic conductive myocardial patch with GPE and its application[77]; (b-d) MI, MI + PGS and MI + PGS-Gr1 group heart tissue images[77]; (e) Statistical results of infarct area[77]; (f) Ultrasound imaging being performed at 4 weeks post-MI to assess cardiac functionality[77]; (g) Assessment of left ventricular internal dimension in systole (LVIDs), LVID in diastole (LVIDd), fractional shortening (FS) and ejection fraction (EF) at 4 weeks post-MI[77]; (h) Scheme of application of soft and conductive PEG-MEL/HA-SH/GO hydrogel system[78]; (i) Schematics illustrating fabrication of rGO/silkA/R scaffolds and their application in restoring electrical integrity in infarcted myocardium[103]

Fig. 7 Zn2SiO4 bioceramic repairing myocardial injury by manipulating cell behaviors[105] (a) Schematic diagram of the Zn2SiO4 for treatment of MI; (b) Zn2SiO4 bioceramic extract preventing H2O2-induced oxidative stress and apoptosis in H9C2 cells; (c) Zn2SiO4 bioceramic extract promoting angiogenesis of MCAECs; (d) Intravenous injection of Zn2SiO4 bioceramic extract reversing ventricular remodeling after MI in mice, including improving the cardiac function and decreasing the infarct size and the fibrosis area
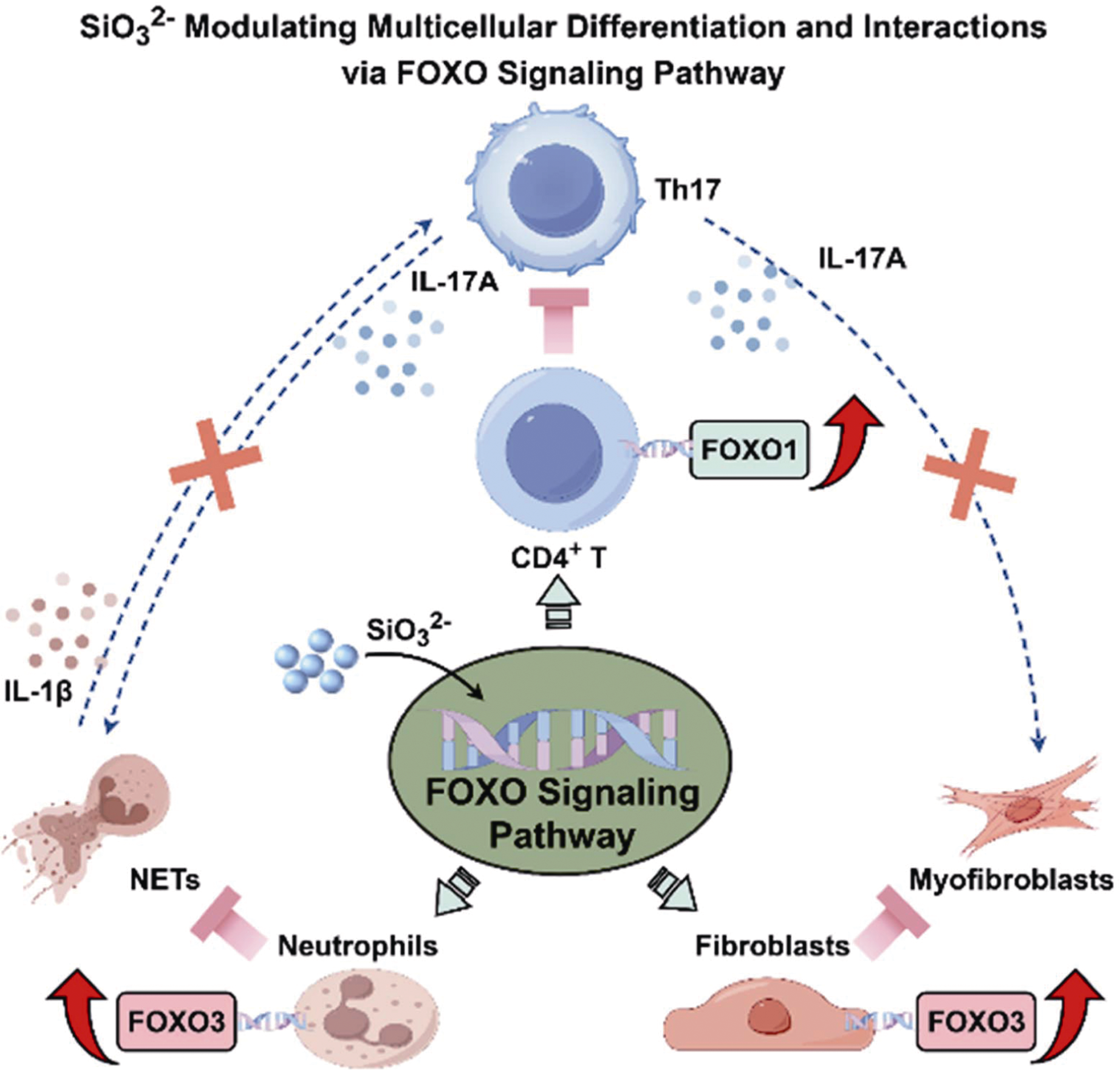
Fig. 8 Biological mechanism of SiO32- treating iDCM[109] SiO32- modulated multicellular differentiation and interactions via FOXO signaling pathway. SiO32- activated the FOXO1 signaling pathway in CD4+ T cells, leading to a significant reduction in their differentiation into Th17 cells. Concurrently, CS promoted FOXO3 expression in neutrophils and fibroblasts, thereby inhibiting their differentiation into NETs and myofibroblasts, respectively. This comprehensive and multi-cellular regulatory mechanism disrupted the harmful interactions between CD4+ T/Th17 cells and neutrophils/ fibroblasts, breaking the vicious cycle of myocardial inflammation and blocking the progression of fibrotic lesions post iDCM
| [1] |
ROTH G A, MENSAH G A, FUSTER V. The global burden of cardiovascular diseases and risks: a compass for global action. Journal of the American College of Cardiology, 2020, 76(25): 2980.
DOI PMID |
| [2] |
DE GASPARI M, TOSCANO G, BAGOZZI L, et al. Endomyocardial fibrosis and myocardial infarction leading to diastolic and systolic dysfunction requiring transplantation. Cardiovascular Pathology, 2019, 38: 21.
DOI PMID |
| [3] | 梁婷婷. 多功能水凝胶包裹Chrysin-7-O-glucuronide促进心肌梗死修复的研究. 重庆: 重庆理工大学硕士学位论文, 2024. |
| [4] | PAULINO E T. Development of the cardioprotective drugs class based on pathophysiology of myocardial infarction: a comprehensive review. Current Problems in Cardiology, 2024, 49(5): 102480. |
| [5] |
SABATINE M S, BERGMARK B A, MURPHY S A, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: an individual patient data meta-analysis. Lancet, 2021, 398(10318): 2247.
DOI PMID |
| [6] | WANG Y B, LI G C, YANG L, et al. Development of innovative biomaterials and devices for the treatment of cardiovascular diseases. Advanced Materials, 2022, 34(46): 2201971. |
| [7] |
XIN M, OLSON E N, BASSEL-DUBY R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nature Reviews Molecular Cell Biology, 2013, 14(8): 529.
DOI PMID |
| [8] |
ITOU J, OISHI I, KAWAKAMI H, et al. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development, 2012, 139(22): 4133.
DOI PMID |
| [9] | BASSAT E, MUTLAK Y E, GENZELINAKH A, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature, 2017, 547(7662): 179. |
| [10] |
LIU T L, HAO Y, ZHANG Z X, et al. Advanced cardiac patches for the treatment of myocardial infarction. Circulation, 2024, 149(25): 2002.
DOI PMID |
| [11] | LIANG J H, LV R H, LI M R, et al. Hydrogels for the treatment of myocardial infarction: design and therapeutic strategies. Macromolecular Bioscience, 2024, 24(2): e2300302. |
| [12] |
YAN W J, XIA Y L, ZHAO H S, et al. Stem cell-based therapy in cardiac repair after myocardial infarction: promise, challenges, and future directions. Journal of Molecular and Cellular Cardiology, 2024, 188: 1.
DOI PMID |
| [13] |
FENG Y J, WANG Y, LI L, et al. Exosomes induce crosstalk between multiple types of cells and cardiac fibroblasts: therapeutic potential for remodeling after myocardial infarction. International Journal of Nanomedicine, 2024, 19: 10605.
DOI PMID |
| [14] | 高龙. 生物玻璃-高分子复合微球/水凝胶的制备及组织再生应用研究. 上海: 中国科学院上海硅酸盐研究所博士学位论文, 2020. |
| [15] | FERRAGE L, BERTRAND G, LENORMAND P, et al. A review of the additive manufacturing (3DP) of bioceramics: alumina, zirconia (PSZ) and hydroxyapatite. Journal of the Australian Ceramic Society, 2017, 53: 11. |
| [16] | STEFANIC M, KOSMAČ T. β-TCP coatings on zirconia bioceramics: the importance of heating temperature on the bond strength and the substrate/coating interface. Journal of the European Ceramic Society, 2018, 38(15): 5264. |
| [17] | EGE D, LU H H, BOCCACCINI A R. Bioactive glass and silica particles for skeletal and cardiac muscle tissue regeneration. Tissue Engineering Part B: Reviews, 2024, 30(4): 448. |
| [18] | XU W F. Biocompatibility and medical application of carbon material. Key Engineering Materials, 2010, 452/453: 477. |
| [19] | ROUALDES O, DUCLOS M E, GUTKNECHT D, et al. In vitro and in vivo evaluation of an alumina-zirconia composite for arthroplasty applications. Biomaterials, 2010, 31(8): 2043. |
| [20] |
ZHAO C, WANG X, GAO L, et al. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomaterialia, 2018, 73: 509.
DOI PMID |
| [21] | WANG X T, WANG L Y, WU Q, et al. Chitosan/calcium silicate cardiac patch stimulates cardiomyocyte activity and myocardial performance after infarction by synergistic effect of bioactive ions and aligned nanostructure. ACS Applied Materials & Interfaces, 2019, 11(1): 1449. |
| [22] |
MEIRA R M, RIBEIRO S, IRASTORZA I, et al. Electroactive poly(vinylidene fluoride-trifluoroethylene)/graphene composites for cardiac tissue engineering applications. Journal of Colloid and Interface Science, 2024, 663: 73.
DOI PMID |
| [23] |
OTTERSBACH A, MYKHAYLYK O, HEIDSIECK A, et al. Improved heart repair upon myocardial infarction: combination of magnetic nanoparticles and tailored magnets strongly increases engraftment of myocytes. Biomaterials, 2018, 155: 176.
DOI PMID |
| [24] | ZULKIFLEE I, MASRI S, ZAWANI M, et al. Silicon-based scaffold for wound healing skin regeneration applications: a concise review. Polymers, 2022, 14(19): 4219. |
| [25] |
BAI R, LIU J F, ZHANG J, et al. Conductive single-wall carbon nanotubes/extracellular matrix hybrid hydrogels promote the lineage-specific development of seeding cells for tissue repair through reconstructing an integrin-dependent niche. Journal of Nanobiotechnology, 2021, 19: 252.
DOI PMID |
| [26] | LI Y, CHEN X, JIN R, et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Science Advances, 2021, 7(9): eabd6740. |
| [27] |
SUN C Y, XIE Y Y, ZHU H F, et al. Highly electroactive tissue engineering scaffolds based on nanocellulose/sulfonated carbon nanotube composite hydrogels for myocardial tissue repair. Biomacromolecules, 2023, 24(12): 5989.
DOI PMID |
| [28] |
SARAVANAN S, SAREEN N, ABU-EL-RUB E, et al. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Scientific Reports, 2018, 8: 15069.
DOI PMID |
| [29] |
MALDA J, FRONDOZA C G. Microcarriers in the engineering of cartilage and bone. Trends in Biotechnology, 2006, 24(7): 299.
DOI PMID |
| [30] | FENG J, XING M, QIAN W, et al. An injectable hydrogel combining medicine and matrix with anti-inflammatory and pro-angiogenic properties for potential treatment of myocardial infarction. Regenerative Biomaterials, 2023, 10: rbad036. |
| [31] | SUN L, ZHU X, ZHANG X, et al. Induced cardiomyocytes- integrated conductive microneedle patch for treating myocardial infarction. Chemical Engineering Journal, 2021, 414: 128723. |
| [32] | MENG J, XIAO B, WU F, et al. Co-axial fibrous scaffolds integrating with carbon fiber promote cardiac tissue regeneration post myocardial infarction. Materials Today Bio, 2022, 16: 100415. |
| [33] | CHEN F, ZHAO E R, HABLEEL G, et al. Increasing the efficacy of stem cell therapy via triple-function inorganic nanoparticles. ACS Nano, 2019, 13(6): 6605. |
| [34] | LI H, ZHU J, XU Y, et al. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biology, 2022, 54: 102384. |
| [35] | HASAN A, KHATTAB A, ISLAM M A, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Advanced Science, 2015, 2(11): 1500122. |
| [36] |
LIN K, XIA L, LI H, et al. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials, 2013, 34(38): 10028.
DOI PMID |
| [37] |
MAO L, XIA L, CHANG J, et al. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomaterialia, 2017, 61: 217.
DOI PMID |
| [38] | XING M, JIANG Y, BI W, et al. Strontium ions protect hearts against myocardial ischemia/reperfusion injury. Science Advances, 2021, 7(3): eabe0726. |
| [39] | YI M, LI H K, WANG X Y, et al. Ion therapy: a novel strategy for acute myocardial infarction. Advanced Science, 2019, 6(1): 1801260. |
| [40] | HONG X Q, TIAN G E, DAI B Y, et al. Copper-loaded milk- protein derived microgel preserves cardiac metabolic homeostasis after myocardial infarction. Advanced Science, 2024, 11(35): 2401527. |
| [41] | LV Q B, MA B X, LI W J, et al. Nanomaterials-mediated therapeutics and diagnosis strategies for myocardial infarction. Frontiers in Chemistry, 2022, 10: 943009. |
| [42] |
SONG L, JIA K W, YANG F Q, et al. Advanced nanomedicine approaches for myocardial infarction treatment. International Journal of Nanomedicine, 2024, 19: 6399.
DOI PMID |
| [43] | LIU C J, YAO L, HU Y M, et al. Effect of quercetin-loaded mesoporous silica nanoparticles on myocardial ischemia-reperfusion injury in rats and its mechanism. International Journal of Nanomedicine, 2021, 16: 741. |
| [44] | FERREIRA M P A, RANJAN S, KINNUNEN S, et al. Drug- loaded multifunctional nanoparticles targeted to the endocardial layer of the injured heart modulate hypertrophic signaling. Small, 2017, 13(33): 1701276. |
| [45] | QI S C, ZHANG P F, MA M, et al. Cellular internalization-induced aggregation of porous silicon nanoparticles for ultrasound imaging and protein-mediated protection of stem cells. Small, 2019, 15(1): 1804332. |
| [46] | GALAGUDZA M, KOROLEV D, POSTNOV V, et al. Passive targeting of ischemic-reperfused myocardium with adenosine- loaded silica nanoparticles. International Journal of Nanomedicine, 2012, 7: 1671. |
| [47] | LIU S Y, CHEN X, BAO L L, et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nature Biomedical Engineering, 2020, 4(11): 1063. |
| [48] | KIM M, SAHU A, HWANG Y, et al. Targeted delivery of anti-inflammatory cytokine by nanocarrier reduces atherosclerosis in Apo E-/- mice. Biomaterials, 2020, 226: 119550. |
| [49] | HONG X Q, TIAN G E, ZHU Y, et al. Exogeneous metal ions as therapeutic agents in cardiovascular disease and their delivery strategies. Regenerative Biomaterials, 2023, 11: rbad103. |
| [50] | NORDIN A H, AHMAD Z, HUSNA S M N, et al. The state of the art of natural polymer functionalized Fe3O4 magnetic nanoparticle composites for drug delivery applications: a review. Gels, 2023, 9(2): 121. |
| [51] | WANG F, HAN D, QIAO Z, et al. Neutrophil-targeted Mn3O4 nanozyme treats myocardial ischemia reperfusion injury by scavenging reactive oxygen species. Research Square, DOI: 10.21203/rs.3.rs-2288620/v1. |
| [52] |
XIONG F, WANG H, FENG Y D, et al. Cardioprotective activity of iron oxide nanoparticles. Scientific Reports, 2015, 5: 8579.
DOI PMID |
| [53] |
NASEROLESLAMI M, ABOUTALEB N, PARIVAR K. The effects of superparamagnetic iron oxide nanoparticles-labeled mesenchymal stem cells in the presence of a magnetic field on attenuation of injury after heart failure. Drug Delivery and Translational Research, 2018, 8(5): 1214.
DOI PMID |
| [54] | HAN K, MAO M, FU L Y, et al. Multimaterial printing of serpentine microarchitectures with synergistic mechanical/piezoelectric stimulation for enhanced cardiac-specific functional regeneration. Small, 2024, 20(42): 2401561. |
| [55] | LI B, ZHANG Q, DU W X, et al. Reshaping cardiac microenvironments by macrophage-derived extracellular vesicles-coated Pd@CeO2 heterostructures for myocardial ischemia/reperfusion injury therapy. Materials Today, 2023, 65: 47. |
| [56] | NISA F Y, RAHMAN M A, SAHA S, et al. Unraveling Tamarindus indica pulp-derived green magnesium oxide nanoparticles for cardioprotective potential against doxorubicin-induced cardiomyopathy: a comprehensive biochemical and gene expression study. ACS Omega, 2023, 8(48): 45626. |
| [57] |
BARUI A K, VEERIAH V, MUKHERJEE S, et al. Zinc oxide nanoflowers make new blood vessels. Nanoscale, 2012, 4(24): 7861.
DOI PMID |
| [58] | KUMAR A, JENA P, BEHERA S, et al. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine: Nanotechnology, Biology and Medicine, 2010, 6(1): 64. |
| [59] | CHAUDEURGE A, WILHELM C, CHEN-TOURNOUX A, et al. Can magnetic targeting of magnetically labeled circulating cells optimize intramyocardial cell retention. Cell Transplantation, 2012, 21(4): 679. |
| [60] | JANSMAN M M T, HOSTA-RIGAU L. Cerium- and iron-oxide-based nanozymes in tissue engineering and regenerative medicine. Catalysts, 2019, 9(8): 691. |
| [61] |
KORNFELD O S, HWANG S, DISATNIK M H, et al. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circulation Research, 2015, 116(11): 1783.
DOI PMID |
| [62] |
FAN Q, TAO R, ZHANG H, et al. Dectin-1 contributes to myocardial ischemia/reperfusion injury by regulating macrophage polarization and neutrophil infiltration. Circulation, 2019, 139(5): 663.
DOI PMID |
| [63] |
CELARDO I, PEDERSEN J Z, TRAVERSA E, et al. Pharmacological potential of cerium oxide nanoparticles. Nanoscale, 2011, 3(4): 1411.
DOI PMID |
| [64] |
NIU J L, AZFER A, ROGERS L M, et al. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovascular Research, 2007, 73(3): 549.
DOI PMID |
| [65] |
ZHAO Y D, YANG Y H, WEN Y S, et al. Effect of cerium oxide nanoparticles on myocardial cell apoptosis induced by myocardial ischemia-reperfusion injury. Cellular and Molecular Biology, 2022, 68(3): 43.
DOI PMID |
| [66] |
WU X, REBOLL M R, KORF-KLINGEBIEL M, et al. Angiogenesis after acute myocardial infarction. Cardiovascular Research, 2021, 117(5): 1257.
DOI PMID |
| [67] |
JACOBS A, RENAUDIN G, FORESTIER C, et al. Biological properties of copper-doped biomaterials for orthopedic applications: a review of antibacterial, angiogenic and osteogenic aspects. Acta Biomaterialia, 2020, 117: 21.
DOI PMID |
| [68] | GAO P, FAN B, YU X, et al. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioactive Materials, 2020, 5(3): 680. |
| [69] |
BEJARANO J, DETSCH R, BOCCACCINI A R, et al. PDLLA scaffolds with Cu- and Zn-doped bioactive glasses having multifunctional properties for bone regeneration. Journal of Biomedical Materials Research Part A, 2017, 105(3): 746.
DOI PMID |
| [70] | SHABANKHAH M, MOGHADDASZADEH A, NAJMODDIN N. 3D printed conductive PCL/GO scaffold immobilized with gelatin/CuO accelerates H9C2 cells attachment and proliferation. Progress in Organic Coatings, 2024, 186: 108013. |
| [71] | FARANI M R, FARSADROOH M, ZARE I, et al. Green synthesis of magnesium oxide nanoparticles and nanocomposites for photocatalytic antimicrobial, antibiofilm and antifungal applications. Catalysts, 2023, 13(4): 642. |
| [72] | MOHAMED A T A E, RAGHEB M A, SHEHATA M R, et al. In vivo cardioprotective effect of zinc oxide nanoparticles against doxorubicin-induced myocardial infarction by enhancing the antioxidant system and nitric oxide production. Journal of Trace Elements in Medicine and Biology, 2024, 86: 127516. |
| [73] |
NGUYEN T P, QU Z L, WEISS J N. Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. Journal of Molecular and Cellular Cardiology, 2014, 70: 83.
DOI PMID |
| [74] | SUN H, LÜ S, JIANG X, et al. Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the β1-integrin-mediated signaling pathway. Biomaterials, 2015, 55: 84. |
| [75] |
AHADIAN S, YAMADA S, RAMÓN-AZCÓN J, et al. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomaterialia, 2016, 31: 134.
DOI PMID |
| [76] |
MARTINS A M, ENG G, CARIDADE S G, et al. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules, 2014, 15(2): 635.
DOI PMID |
| [77] | SHI M, BAI L, XU M, et al. Micropatterned conductive elastomer patch based on poly(glycerol sebacate)-graphene for cardiac tissue repair. Biofabrication, 2022, 14: 035001. |
| [78] |
BAO R, TAN B, LIANG S, et al. A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials, 2017, 122: 63.
DOI PMID |
| [79] | GARIBALDI S, BRUNELLI C, BAVASTRELLO V, et al. Carbon nanotube biocompatibility with cardiac muscle cells. Nanotechnology, 2006, 17(2): 391. |
| [80] | MCCAULEY M D, VITALE F, YAN J S, et al. In vivo restoration of myocardial conduction with carbon nanotube fibers. Circulation: Arrhythmia and Electrophysiology, 2019, 12(8): e007256. |
| [81] | REN J, XU Q, CHEN X, et al. Superaligned carbon nanotubes guide oriented cell growth and promote electrophysiological homogeneity for synthetic cardiac tissues. Advanced Materials, 2017, 29(44): 1702713. |
| [82] |
WANG L, WU Y, HU T, et al. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomaterialia, 2017, 59: 68.
DOI PMID |
| [83] |
STOUT D A, BASU B, WEBSTER T J. Poly(lactic-co-glycolic acid): carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomaterialia, 2011, 7(8): 3101.
DOI PMID |
| [84] |
ASIRI A M, MARWANI H M, KHAN S B, et al. Greater cardiomyocyte density on aligned compared with random carbon nanofibers in polymer composites. International Journal of Nanomedicine, 2014, 9: 5533.
DOI PMID |
| [85] |
MEHRABI A, BAHEIRAEI N, ADABI M, et al. Development of a novel electroactive cardiac patch based on carbon nanofibers and gelatin encouraging vascularization. Applied Biochemistry and Biotechnology, 2020, 190: 931.
DOI PMID |
| [86] | TASHAKORI-MIYANROUDI M, RAKHSHAN K, RAMEZ M, et al. Conductive carbon nanofibers incorporated into collagen bio-scaffold assists myocardial injury repair. International Journal of Biological Macromolecules, 2020, 163: 1136. |
| [87] |
CHEN X, ZOU M, LIU S, et al. Applications of graphene family nanomaterials in regenerative medicine: recent advances, challenges, and future perspectives. International Journal of Nanomedicine, 2024, 19: 5459.
DOI PMID |
| [88] |
KIM T, KAHNG Y H, LEE T, et al. Graphene films show stable cell attachment and biocompatibility with electrogenic primary cardiac cells. Molecules and Cells, 2013, 36: 577.
DOI PMID |
| [89] |
PARK J, PARK S, RYU S, et al. Graphene-regulated cardiomyogenic differentiation process of mesenchymal stem cells by enhancing the expression of extracellular matrix proteins and cell signaling molecules. Advanced Healthcare Materials, 2014, 3(2): 176.
DOI PMID |
| [90] |
HITSCHERICH P, APHALE A, GORDAN R, et al. Electroactive graphene composite scaffolds for cardiac tissue engineering. Journal of Biomedical Materials Research Part A, 2018, 106(11): 2923.
DOI PMID |
| [91] |
PARK S, AN J, JUNG I, et al. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Letters, 2009, 9(4): 1593.
DOI PMID |
| [92] |
ZHANG K, ZHENG H, LIANG S, et al. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomaterialia, 2016, 37: 131.
DOI PMID |
| [93] | SHIN S R, AGHAEI-GHAREH-BOLAGH B, DANG T T, et al. Cell-laden microengineered and mechanically tunable hybrid hydrogels of gelatin and graphene oxide. Advanced Materials, 2013, 25(44): 6385. |
| [94] |
SHIN S R, AGHAEI-GHAREH-BOLAGH B, GAO X G, et al. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Advanced Functional Materials, 2014, 24(39): 6136.
PMID |
| [95] |
ZHOU J, YANG X N, LIU W, et al. Injectable OPF/graphene oxide hydrogels provide mechanical support and enhance cell electrical signaling after implantation into myocardial infarct. Theranostics, 2018, 8(12): 3317.
DOI PMID |
| [96] |
ALAGARSAMY K N, MATHAN S, YAN W A, et al. Carbon nanomaterials for cardiovascular theranostics: promises and challenges. Bioactive Materials, 2021, 6(8): 2261.
DOI PMID |
| [97] | ZARGAR S M, MEHDIKHANI M, RAFIENIA M. Reduced graphene oxide-reinforced gellan gum thermoresponsive hydrogels as a myocardial tissue engineering scaffold. Journal of Bioactive and Compatible Polymers, 2019, 34(4/5): 331. |
| [98] | ZHAO G, QING H, HUANG G, et al. Reduced graphene oxide functionalized nanofibrous silk fibroin matrices for engineering excitable tissues. NPG Asia Materials, 2018, 10: 982. |
| [99] | PARK J, KIM Y S, RYU S, et al. Graphene potentiates the myocardial repair efficacy of mesenchymal stem cells by stimulating the expression of angiogenic growth factors and gap junction protein. Advanced Functional Materials, 2015, 25(17): 2590. |
| [100] |
SHIN S R, ZIHLMANN C, AKBARI M, et al. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small, 2016, 12(27): 3677.
DOI PMID |
| [101] |
BAHEIRAEI N, RAZAVI M, GHAHREMANZADEH R. Reduced graphene oxide coated alginate scaffolds: potential for cardiac patch application. Biomaterials Research, 2023, 27(1): 109.
DOI PMID |
| [102] | FENG Y, ZHAO G, XU M, et al. rGO/silk fibroin-modified nanofibrous patches prevent ventricular remodeling via Yap/taz-TGFβ1/smads signaling after myocardial infarction in rats. Frontiers in Cardiovascular Medicine, 2021, 8: 718055. |
| [103] | ZHAO G, FENG Y, XUE L, et al. Anisotropic conductive reduced graphene oxide/silk matrices promote post-infarction myocardial function by restoring electrical integrity. Acta Biomaterialia, 2022, 139: 190. |
| [104] | ZOU S, IRELAND D, BROOKS R A, et al. The effects of silicate ions on human osteoblast adhesion, proliferation, and differentiation. Journal of Biomedical Materials Research Part B, 2009, 90B(1): 123. |
| [105] | ZHANG Y, LI X, ZHANG Z, et al. Zn2SiO4 bioceramic attenuates cardiac remodeling after myocardial infarction. Advanced Healthcare Materials, 2023, 12(21): 2203365. |
| [106] |
SHIMIZU I, MINAMINO T.Physiological and pathological cardiac hypertrophy. Journal of Molecular and Cellular Cardiology, 2016, 97: 245.
DOI PMID |
| [107] |
LI X, ZHANG Y, JIN Q, et al. Silicate ions derived from calcium silicate extract decelerate ang II-induced cardiac remodeling. Tissue Engineering and Regenerative Medicine, 2023, 20(5): 671.
DOI PMID |
| [108] |
WANG C, WANG Q, GAO W, et al. Highly efficient local delivery of endothelial progenitor cells significantly potentiates angiogenesis and full-thickness wound healing. Acta Biomaterialia, 2018, 69: 156.
DOI PMID |
| [109] | SUN P, ZHANG Z, GAO F, et al. Silicate-based therapy for inflammatory dilated cardiomyopathy by inhibiting the vicious cycle of immune inflammation via FOXO signaling. Science Advances, 2025, 11(15): eadr7208. |
| [110] | BARABADI Z, AZAMI M, SHARIFI E, et al. Fabrication of hydrogel based nanocomposite scaffold containing bioactive glass nanoparticles for myocardial tissue engineering. Materials Science and Engineering: C, 2016, 69: 1137. |
| [111] | QI Q, ZHU Y, LIU G, et al. Local intramyocardial delivery of bioglass with alginate hydrogels for post-infarct myocardial regeneration. Biomedicine & Pharmacotherapy, 2020, 129: 110382. |
| [112] | SHI M, ZHAO F, SUN L, et al. Bioactive glass activates VEGF paracrine signaling of cardiomyocytes to promote cardiac angiogenesis. Materials Science and Engineering: C, 2021, 124: 112077. |
| [113] | SHI M, CAO X, ZHUANG J, et al. The cardioprotective effect and mechanism of bioactive glass on myocardial reperfusion injury. Biomedical Materials, 2021, 16(4): 045044. |
| [114] | ZHOU Y, GAO L, PENG J, et al. Bioglass activated albumin hydrogels for wound healing. Advanced Healthcare Materials, 2018, 7(16): 1800144. |
| [115] | GAO L, YI M, XING M, et al. In situ activated mesenchymal stem cells (MSCs) by bioactive hydrogels for myocardial infarction treatment. Journal of Materials Chemistry B, 2020, 8(34): 7713. |
| [116] | POPARA J, ACCOMASSO L, VITALE E, et al. Silica nanoparticles actively engage with mesenchymal stem cells in improving acute functional cardiac integration. Nanomedicine, 2018, 13(10): 1121. |
| [117] | CROISSANT J G, FATIEIEV Y, KHASHAB N M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Advanced Materials, 2017, 29(9): 1604634. |
| [118] |
POUSSARD S, DECOSSAS M, LE BIHAN O, et al. Internalization and fate of silica nanoparticles in C2C12 skeletal muscle cells: evidence of a beneficial effect on myoblast fusion. International Journal of Nanomedicine, 2015, 10: 1479.
DOI PMID |
| [119] | HE Q, SHI J, ZHU M, et al. The three-stage in vitro degradation behavior of mesoporous silica in simulated body fluid. Microporous and Mesoporous Materials, 2010, 131(1/2/3): 314. |
| [120] | VALLET-REGÍ M, BALAS F, ARCOS D. Mesoporous materials for drug delivery. Angewandte Chemie International Edition, 2007, 46(40): 7548. |
| [121] |
CHEN Y, LIU S, LIANG Y, et al. Single dose of intravenous miR199a-5p delivery targeting ischemic heart for long-term repair of myocardial infarction. Nature Communications, 2024, 15: 5565.
DOI PMID |
| [122] | TAN H, SONG Y, CHEN J, et al. Platelet-like fusogenic liposome-mediated targeting delivery of miR-21 improves myocardial remodeling by reprogramming macrophages post myocardial ischemia-reperfusion injury. Advanced Science, 2021, 8(15): 2100787. |
| [123] | WANG Q, SONG Y, CHEN J, et al. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials, 2021, 276: 121028. |
| [124] | OUYANG M Z, OUYANG X N, PENG Z F, et al. Heart-targeted amelioration of sepsis-induced myocardial dysfunction by microenvironment responsive nitric oxide nanogenerators in situ. Journal of Nanobiotechnology, 2022, 20(1): 263. |
| [1] | YU Shengyang, SU Haijun, JIANG Hao, YU Minghui, YAO Jiatong, YANG Peixin. A Review of Pore Defects in Ultra-high Temperature Oxide Ceramics by Laser Additive Manufacturing: Formation and Suppression [J]. Journal of Inorganic Materials, 2025, 40(9): 944-956. |
| [2] | LIU Jiangping, GUAN Xin, TANG Zhenjie, ZHU Wenjie, LUO Yongming. Research Progress on Catalytic Oxidation of Nitrogen-containing Volatile Organic Compounds [J]. Journal of Inorganic Materials, 2025, 40(9): 933-943. |
| [3] | XIAO Xiaolin, WANG Yuxiang, GU Peiyang, ZHU Zhenrong, SUN Yong. Advances in Regulation of Damaged Skin Regeneration by Two-dimensional Inorganic Materials [J]. Journal of Inorganic Materials, 2025, 40(8): 860-870. |
| [4] | MA Jingge, WU Chengtie. Application of Inorganic Bioceramics in Promoting Hair Follicle Regeneration and Hair Growth [J]. Journal of Inorganic Materials, 2025, 40(8): 901-910. |
| [5] | ZHANG Hongjian, ZHAO Ziyi, WU Chengtie. Inorganic Biomaterials on Regulating Neural Cell Function and Innervated Tissue Regeneration: A Review [J]. Journal of Inorganic Materials, 2025, 40(8): 849-859. |
| [6] | AI Minhui, LEI Bo. Micro-nanoscale Bioactive Glass: Functionalized Design and Angiogenic Skin Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 921-932. |
| [7] | WANG Yutong, CHANG Jiang, XU He, WU Chengtie. Advances in Silicate Bioceramic/Bioglass for Wound Healing: Effects, Mechanisms and Application Ways [J]. Journal of Inorganic Materials, 2025, 40(8): 911-920. |
| [8] | MA Wenping, HAN Yahui, WU Chengtie, LÜ Hongxu. Application of Inorganic Bioactive Materials in Organoid Research [J]. Journal of Inorganic Materials, 2025, 40(8): 888-900. |
| [9] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [10] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [11] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [12] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [13] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [14] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [15] | YANG Mingkai, HUANG Zeai, ZHOU Yunxiao, LIU Tong, ZHANG Kuikui, TAN Hao, LIU Mengying, ZHAN Junjie, CHEN Guoxing, ZHOU Ying. Co-production of Few-layer Graphene and Hydrogen from Methane Pyrolysis Based on Cu and Metal Oxide-KCl Molten Medium [J]. Journal of Inorganic Materials, 2025, 40(5): 473-480. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||