
Journal of Inorganic Materials ›› 2013, Vol. 28 ›› Issue (1): 1-11.DOI: 10.3724/SP.J.1077.2012.12082
• Orginal Article • Next Articles
SHI Jian-Lin, CHEN Yu, CHEN Hang-Rong
Received:2012-02-11
Revised:2012-04-10
Published:2013-01-10
Online:2012-12-20
Supported by:CLC Number:
SHI Jian-Lin, CHEN Yu, CHEN Hang-Rong. Progress on the Multifunctional Mesoporous Silica-based Nanotheranostics[J]. Journal of Inorganic Materials, 2013, 28(1): 1-11.
Add to citation manager EndNote|Ris|BibTeX
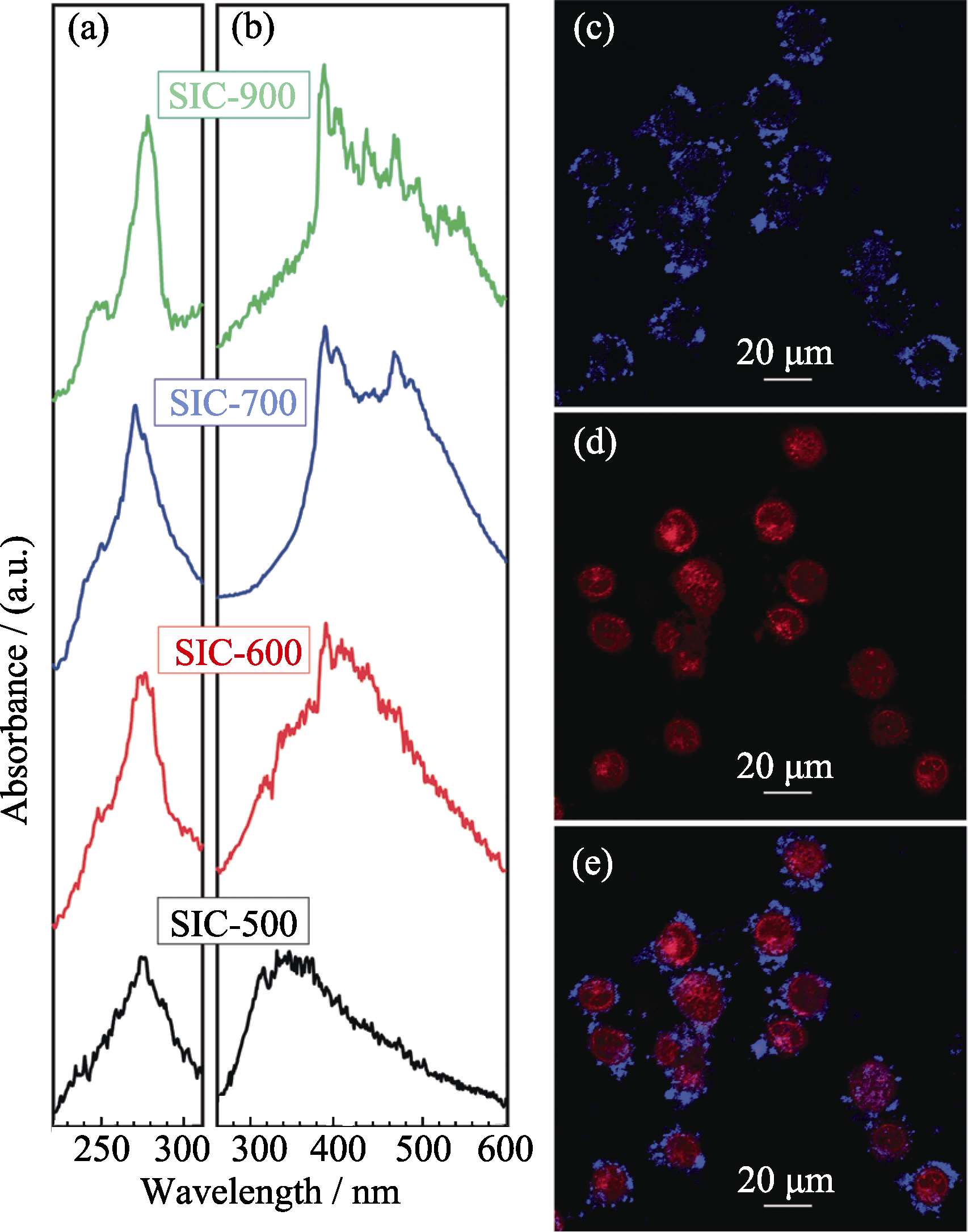
Fig. 3 (a) Luminescent excitation and (b) emission spectra of oxygen-deficient fluorescent MSNs; (c) cell labeling and (b) doxorubicin delivery by obtained fluorescent MSNs (e is the merged figure of c and d)[41]

Fig. 4 (a) Synthetic procedures of MFNEs; (b) Confocal fluorescent microscopic images of breast cancer MCF-7 cells labeled with MFNEs; (c1-c4) In vivo MRI of a tumor-bearing mouse before (c1) and after injection of MFNEs for different time intervals (c2: 0.5 h, c3: 1 h, c4: 1.5 h)[19]
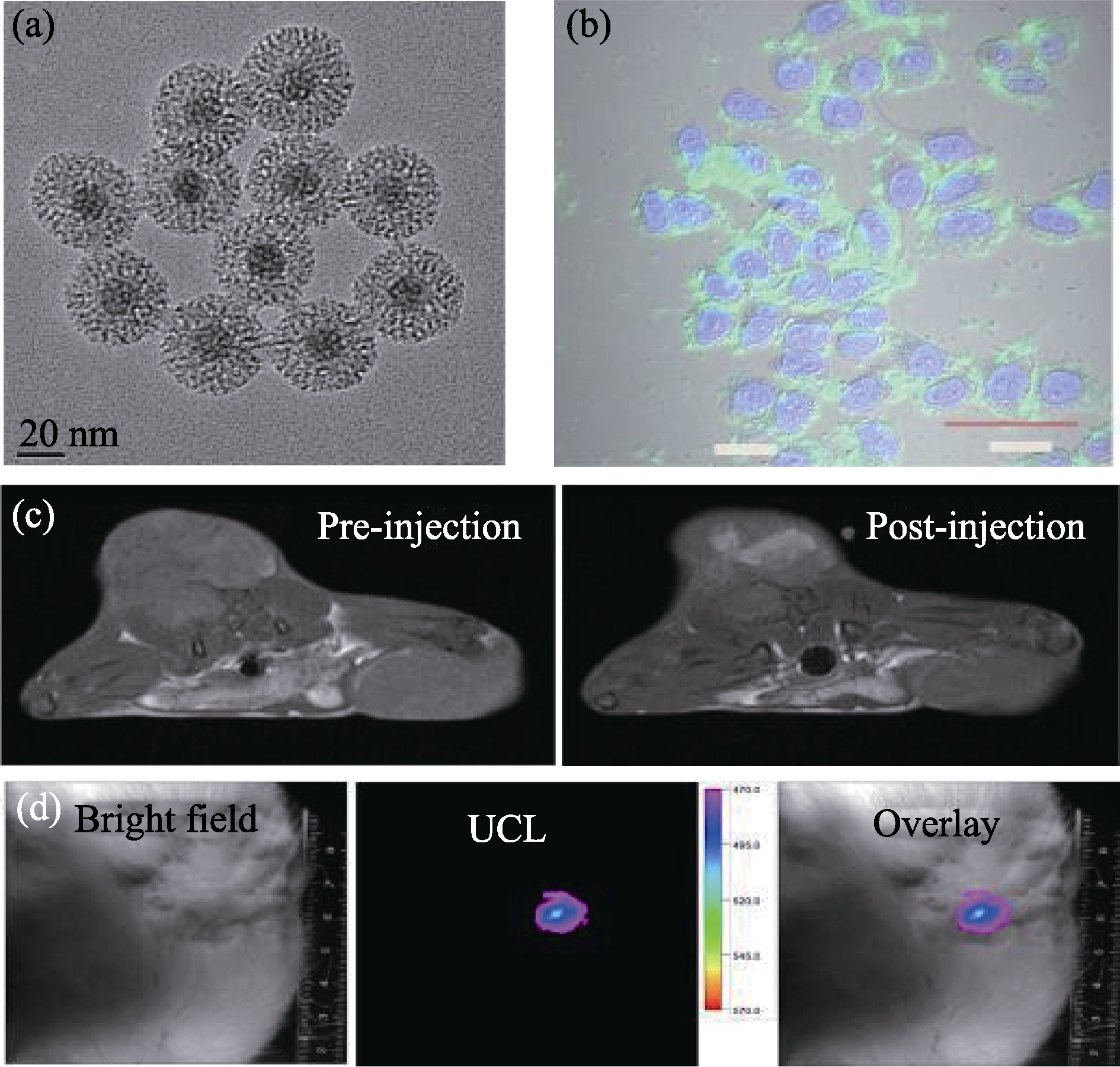
Fig. 5 (a) TEM images of NaYF4:Tm/Yb/Gd@mSiO2 nanocomposites; (b) Confocol images of MCF-7 cells incubated with NaYF4:Tm/Yb/Gd@mSiO2; (c) In vivo MRI-T1 images of tumor (left: pre-injection, right: post-injection); (d) In vivo upconversion luminescence imaging of a tumor-bearing mouse after local injection at the tumor site, from left to right: bright field, upconversion luminescence and overlay images[43]
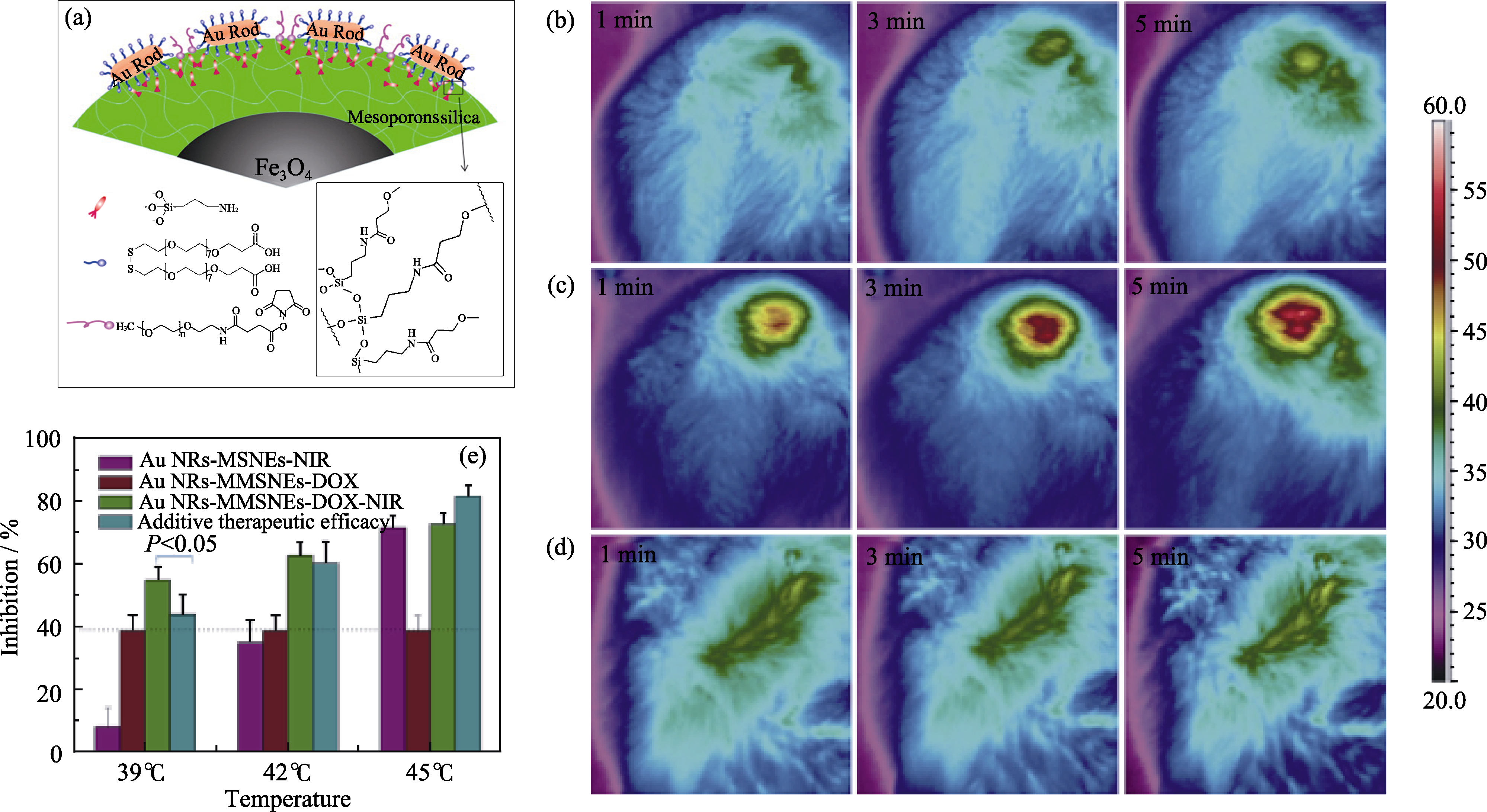
Fig. 6 (a) Microscopic structure of GMMNs; (b-d) Thermographic surveillance of photothermal heating at different time points in GMMNs -injected tumor under 1 W/cm2 (b) and 2 W/cm2 (c) irradiations and PBS solution-injected tumor under 2 W/cm2 irradiation (d); (e) Comparison of inhibition rates for MCF cells treated by GMMNs-NIR (purple), GMMNs-DOX (red) and GMMNs-DOX-NIR (green)[44]
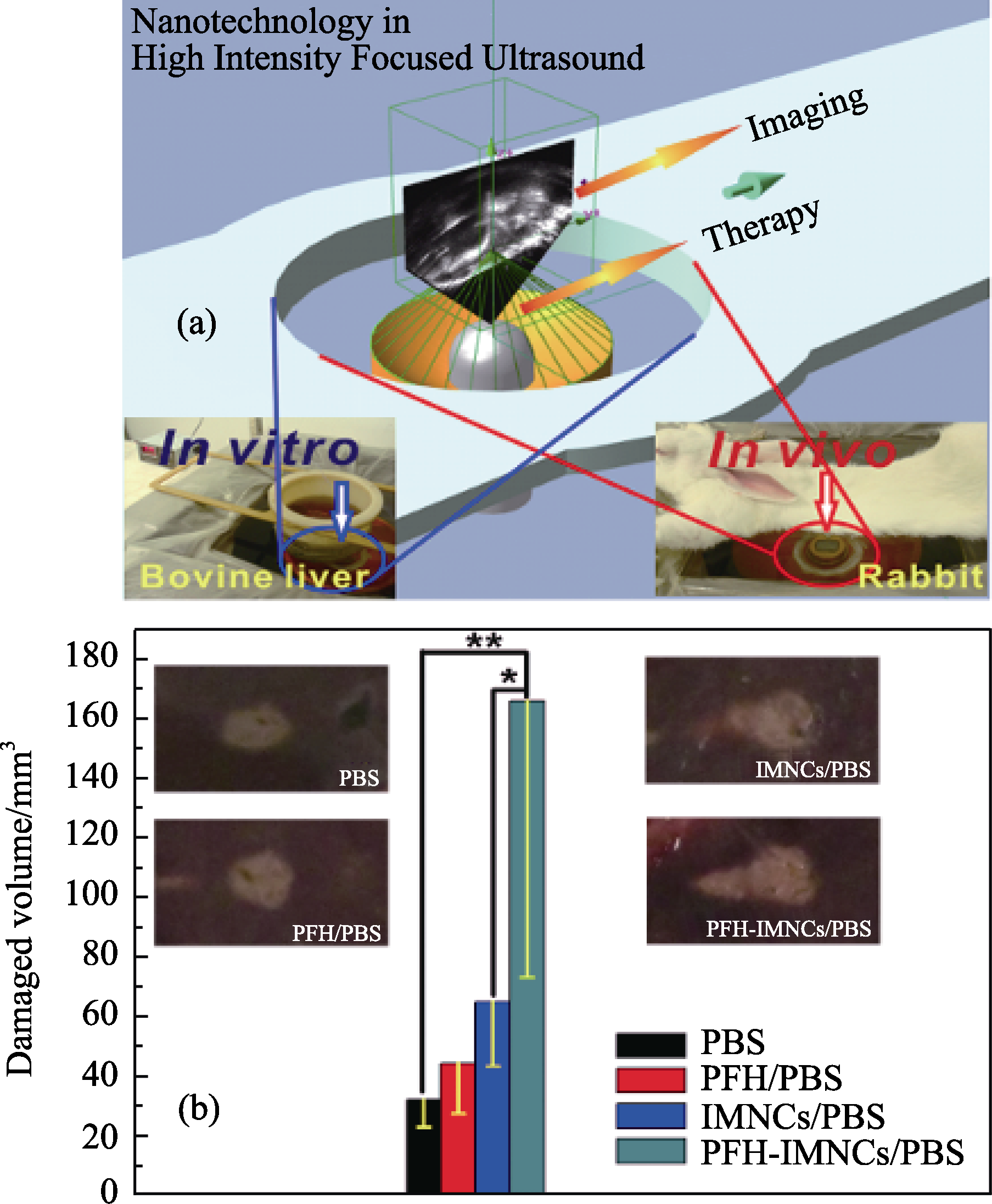
Fig. 7 (a) Schematic illustration of the high intensity focused ultrasound (HIFU) therapeutic principle. The HIFU radiates to the targeted site of the body and the process is monitored by the outside ultrasound imaging. The ex vivo experiment was conducted using bovine liver as a radiation substrate (left digital picture) while the in vivo experiment was carried out using rabbits as a model animal (the right digital picture); (b) Coagulated tissue volume of bovine liver by the intra-tissue injection of different agents such as PBS (200 μL), PFH/PBS (200 μL), IMNCs/PBS (200 μL) and PFH-IMNCs/PBS (200 μL) under the same irradiation power and duration (150 W/cm2, 5 s; *P < 0.1, **P < 0.05). Insets in b are the macroscopic appearances of bovine liver tissues exposed to HIFU with or without using the synergistic agents[45]
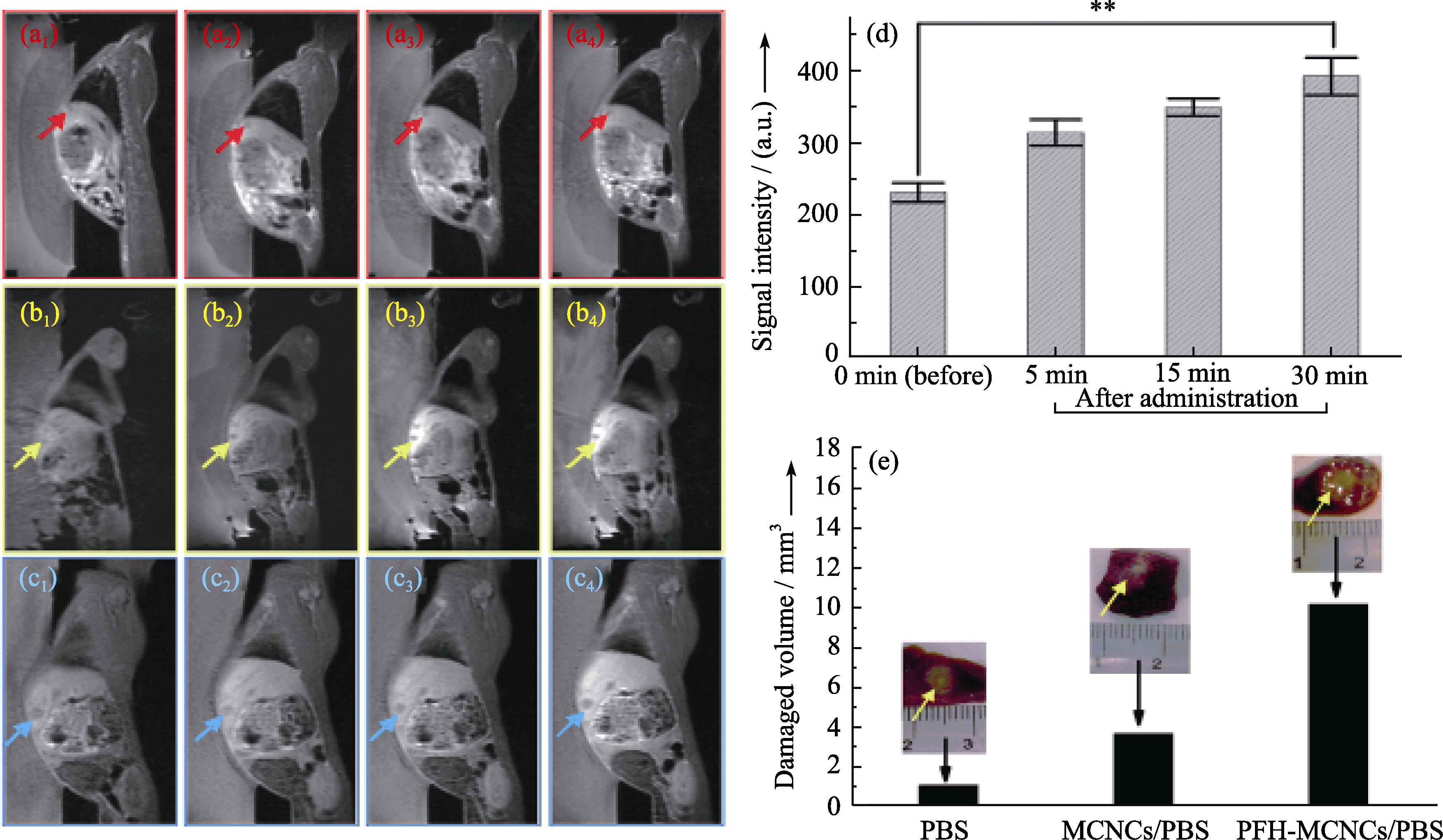
Fig. 8 In vivo T1-weighted MR imaging of rabbits bearing VX2 liver tumor before (a1, b1 and c1) and after (5 min: a2, b2 and c2; 15 min: a3, b3 and c3; 30 min: a4, b4 and c4) administration of different agents (PBS: a1-a4; MCNCs/PBS: b1-b4; PFH-MCNCs/PBS: c1-c4) via ear vein. Arrows indicate the tumor. (d) T1-weighted MRI signal intensities of tumor tissue before and after intravenous administration of PFH-MCNCs/PBS (**P < 0.005); (e) In vivo coagulated necrotic tumor volume by MRI-guided HIFU exposure under the irradiation power of 150 W/cm2 and duration of 5 s in rabbit liver tumors after receiving different agents via ear vein (inset: digital pictures of tumor tissue after HIFU exposure)[18]
| [1] | Lammers T, Aime S, Hennink W E, et al. Theranostic nanomedicine. Accounts Chem. Res., 2011, 44(10): 1029-1038. |
| [2] | Kievit F M, Zhang M Q. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater., 2011, 23(36): H217-H247. |
| [3] | Lammers T, Kiessling F, Hennink W E, et al. Nanotheranostics and image-guided drug delivery: current concepts and future directions. Mol. Pharm., 2010, 7(6): 1899-1912. |
| [4] | Slowing II, Vivero-Escoto J L, Wu C W, et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev., 2008, 60(11): 1278-1288. |
| [5] | Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed., 2007, 46(40): 7548-7558. |
| [6] | Liong M, Angelos S, Choi E, et al. Mesostructured multifunctional nanoparticles for imaging and drug delivery. J. Mater. Chem., 2009, 19(35): 6251-6257. |
| [7] | Wang S B. Ordered mesoporous materials for drug delivery. Micro. Meso. Mater., 2009, 117(1/2): 1-9. |
| [8] | Kresge C T, Leonowicz M E, Roth W J, et al. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature, 1992, 359(6397): 710-712. |
| [9] | Piao Y, Burns A, Kim J, et al. Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv. Funct. Mater., 2008, 18(23): 3745-3758. |
| [10] | He Q J, Shi J L. Mesoporous silica nanoparticle based nano drug delivery systems: synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem., 2011, 21(16): 5845-5855. |
| [11] | Vallet-Regi M, Ramila A, del Real R P, et al. A new property of MCM-41: drug delivery system. Chem. Mater., 2001, 13(2): 308-311. |
| [12] | Coti K K, Belowich M E, Liong M, et al. Mechanised nanoparticles for drug delivery. Nanoscale, 2009, 1(1): 16-39. |
| [13] | Lu J, Liong M, Li Z X, et al. Biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small, 2010, 6(16): 1794-1805. |
| [14] | Li L L, Tang F Q, Liu H Y, et al. In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy. ACS Nano, 2010, 4(11): 6874-6882. |
| [15] | He Q J, Zhang J M, Shi J L, et al. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials, 2010, 31(6): 1085-1092. |
| [16] | Huang X L, Li L L, Liu T L, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano, 2011, 5(7): 5390-5399. |
| [17] | Chen Y, Chen H R, Zeng D P, et al. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano, 2010, 4(10): 6001-6013. |
| [18] | Chen Y, Chen H R, Sun Y, et al. Multifunctional mesoporous composite nanocapsules for highly efficient MRI-guided high-intensity focused ultrasound cancer surgery. Angew. Chem. Int. Ed., 2011, 50(52): 12505-12509. |
| [19] | Chen Y, Chen H R, Zhang S J, et al. Multifunctional mesoporous nanoellipsoids for biological bimodal imaging and magnetically targeted delivery of anticancer drugs. Adv. Funct. Mater., 2011, 21(2): 270-278. |
| [20] | Lee C H, Cheng S H, Wang Y J, et al. Near-infrared mesoporous silica nanoparticles for optical imaging: characterization and in vivo biodistribution. Adv. Funct. Mater., 2009, 19(2): 215-222. |
| [21] | Zhao W R, Gu J L, Zhang L X, et al. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J. Am. Chem. Soc., 2005, 127(25): 8916-8917. |
| [22] | Deng Y, Qi D, Deng C, et al. Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J. Am. Chem. Soc., 2008, 130(1): 28-29. |
| [23] | Kim J, Lee J E, Lee J, et al. Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J. Am. Chem. Soc., 2006, 128(3): 688-689. |
| [24] | Kim J, Kim H S, Lee N, et al. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew. Chem. Int. Ed., 2008, 47(44): 8438-8441. |
| [25] | Lee J E, Lee N, Kim H, et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc., 2010, 132(2): 552-557. |
| [26] | Gan Q, Lu X Y, Yuan Y A, et al. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles- capped mesoporous silica. Biomaterials, 2011, 32(7): 1932-1942. |
| [27] | Viswanathan S, Kovacs Z, Green K N, et al. Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem. Rev., 2010, 110(5): 2960-3018. |
| [28] | Terreno E, Castelli D D, Viale A, et al. Challenges for molecular magnetic resonance imaging. Chem. Rev., 2010, 110(5): 3019-3042. |
| [29] | Taylor K M L, Kim J S, Rieter W J, et al. Mesoporous silica nanospheres as highly efficient MRI contrast agents. J. Am. Chem. Soc., 2008, 130(7): 2154-2155. |
| [30] | Hsiao J K, Tsai C P, Chung T H, et al., Mesoporous silica nanoparticles as a delivery system of gadolinium for effective human stem cell tracking. Small, 2008, 4(9): 1445-1452. |
| [31] | Penfield J G, Reilly R F. What nephrologists need to know about gadolinium. Nat. Clin. Pract. Nephrol., 2007, 3(12): 654-668. |
| [32] | Tromsdorf U I, Bruns O T, Salmen S C, et al. A highly effective, nontoxic T-1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett., 2009, 9(12): 4434-4440. |
| [33] | Perez-Rodriguez J, Lai S, Ehst B D, et al. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment-report of 33 cases. Radiology, 2009, 250(2): 371-377. |
| [34] | Na H B, Lee J H, An K J, et al. Development of a T-1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed., 2007, 46(28): 5397-5401. |
| [35] | Peng Y K, Lai C W, Liu C L, et al. A new and facile method to prepare uniform hollow MnO/functionalized mSiO2 core/shell nanocomposites. ACS Nano, 2011, 5(5): 4177-4187. |
| [36] | Schladt T D, Shukoor M I, Schneider K, et al. Au@MnO nanoflowers: hybrid nanocomposites for selective dual functionalization and imaging. Angew. Chem. Int. Ed., 2010, 49(23): 3976-3980. |
| [37] | Kim T, Momin E, Choi J, et al. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T(1) contrast agents for labeling and MRI tracking of adipose-derived mesenchyrnal stem cells. J. Am. Chem. Soc., 2011, 133(9): 2955-2961. |
| [38] | Chen Y, Chen H, Zhang S, et al. Structure-property relationships in manganese oxide - mesoporous silica nanoparticles used for T1-weighted MRI and simultaneous anti-cancer drug delivery. Biomaterials, 2012, 33(7): 2388-2398. |
| [39] | Pan J, Wan D, Gong J L. PEGylated liposome coated QDs/mesoporous silica core-shell nanoparticles for molecular imaging. Chem. Commun., 2011, 47(12): 3442-3444. |
| [40] | Qian H S, Guo H C, Ho P C L, et al. Mesoporous-silica-coated up-conversion fluorescent nanoparticles for photodynamic therapy. Small, 2009, 5(20): 2285-2290. |
| [41] | He Q J, Shi J L, Cui X Z, et al. Synthesis of oxygen-deficient luminescent mesoporous silica nanoparticles for synchronous drug delivery and imaging. Chem. Commun., 2011, 47(28): 7947-7949. |
| [42] | Feng J, Song S Y, Deng R P, et al. Novel multifunctional nanocomposites: magnetic mesoporous silica nanospheres covalently bonded with near-infrared luminescent lanthanide complexes. Langmuir, 2010, 26(5): 3596-3600. |
| [43] | Liu J, Bu W, Zhang S, et al. Controlled synthesis of uniform and monodisperse upconversion core/mesoporous silica shell nanocomposites for bimodal imaging. Chem. Eur. J., 2012, 18(8): 2335-2341. |
| [44] | Ma M, Chen H, Chen Y, et al. Au capped magnetic core/mesoporous silica shell nanoparticles for combined photothermo-/chemo-therapy and multimodal imaging. Biomaterials, 2012, 33(3): 989-998. |
| [45] | Chen Y, Gao Y, Chen H, et al. Engineering inorganic nanoemulsions/nanoliposomes by fluoride-silica chemistry for efficient delivery/Co-delivery of hydrophobic agents. Adv. Funct. Mater., 2012, 22(8): 1586-1597. |
| [46] | Wang X, Chen H, Chen Y, et al. Perfluorohexane-encapsulated mesoporous silica nanocapsules as enhancement agents for highly efficient high intensity focused ultrasound (HIFU). Adv. Mater., 2012, 24(6): 785-791. |
| [47] | Takegami K, Kaneko Y, Watanabe T, et al. Heating and coagulation volume obtained with high-intensity focused ultrasound therapy: comparison of perflutren protein-type A microspheres and MRX-133 in rabbits. Radiology, 2005, 237(1): 132-136. |
| [48] | Kennedy J E, Haar G R, Cranston D. High intensity focused ultrasound: surgery of the future?Br. J. Radiol., 2003, 76(909): 590-599. |
| [49] | Bailey M R, Khokhlova V A, Sapozhnikov O A, et al. Physical mechanisms of the therapeutic effect of ultrasound - a review. Acoust. Phys., 2003, 49(4): 369-388. |
| [50] | Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics, 2010, 50(2): 221-229. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | WANG Yueyue, HUANG Jiahui, KONG Hongxing, LI Huaizhu, YAO Xiaohong. Silver Loaded Radial Mesoporous Silica: Preparation and Application in Dental Resins [J]. Journal of Inorganic Materials, 2025, 40(1): 77-83. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||