无机材料学报 ›› 2025, Vol. 40 ›› Issue (9): 1013-1021.DOI: 10.15541/jim20240492 CSTR: 32189.14.jim20240492
文伸豪1,2( ), 彭德招1,2, 林喆与1,2, 郭霞1,2, 黄培鑫1,2, 章志珍1,2(
), 彭德招1,2, 林喆与1,2, 郭霞1,2, 黄培鑫1,2, 章志珍1,2( )
)
收稿日期:2024-11-22
修回日期:2025-03-04
出版日期:2025-09-20
网络出版日期:2025-03-19
通讯作者:
章志珍, 副教授. E-mail: zhangzhzh28@mail.sysu.edu.cn作者简介:文伸豪(2002-), 博士研究生. E-mail: wenshh9@mail2.sysu.edu.cn
基金资助:
WEN Shenhao1,2( ), PENG Dezhao1,2, LIN Zheyu1,2, GUO Xia1,2, HUANG Peixin1,2, ZHANG Zhizhen1,2(
), PENG Dezhao1,2, LIN Zheyu1,2, GUO Xia1,2, HUANG Peixin1,2, ZHANG Zhizhen1,2( )
)
Received:2024-11-22
Revised:2025-03-04
Published:2025-09-20
Online:2025-03-19
Contact:
ZHANG Zhizhen, associate professor. E-mail: zhangzhzh28@mail.sysu.edu.cnAbout author:WEN Shenhao (2002-), PhD candidate. E-mail: wenshh9@mail2.sysu.edu.cn
Supported by:摘要:
石榴石型固态电解质(LLZTO)具有高离子电导率、宽电化学稳定窗口等优点, 近年来受到了广泛关注。但LLZTO存在与金属锂负极浸润性差、循环过程中锂枝晶生长严重等问题, 限制了其大规模应用。本研究通过熔融金属Li和AlF3, 制备了含氟化物(LiF、AlF3)和Li-Al合金的复合负极(LAF)。元素分布分析表明LAF复合负极与LLZTO电解质接触后, 在界面处形成氟化物。与金属Li相比, 该复合负极能与LLZTO形成较小的界面接触角, 使得界面浸润性显著提升。改性后的LAF3复合负极(Li与AlF3质量比3 : 1)与LLZTO的界面电阻仅为3.9 Ω/cm2, 远小于金属Li负极与LLZTO的界面电阻(138.6 Ω/cm2); 同时, 临界电流密度从0.2 mA/cm2提升至0.8 mA/cm2。组装的LAF3|LLZTO|LAF3对称电池在0.2 mA/cm2电流密度下能稳定沉积/剥离3500 h, 表明LLZTO|LAF3界面处具有稳定的锂离子沉积/剥离过程。LiFePO4|LLZTO|LAF3准固态电池在0.1C(1C=170 mA/g)电流密度下实现了151.1 mAh/g的放电比容量, 且在1C电流密度下循环240圈后, 容量保持率高达96.5%。本研究提出的LAF复合负极有效降低了LLZTO|负极的界面电阻, 并显著提升了锂离子在界面处的沉积/剥离稳定性, 为高性能LLZTO基金属锂电池提供了新的设计思路。
中图分类号:
文伸豪, 彭德招, 林喆与, 郭霞, 黄培鑫, 章志珍. 基于LLZTO电解质的固态锂金属电池负极界面调控[J]. 无机材料学报, 2025, 40(9): 1013-1021.
WEN Shenhao, PENG Dezhao, LIN Zheyu, GUO Xia, HUANG Peixin, ZHANG Zhizhen. Interface Engineering for the Anode in Solid-state Lithium Batteries Based on LLZTO Electrolyte[J]. Journal of Inorganic Materials, 2025, 40(9): 1013-1021.
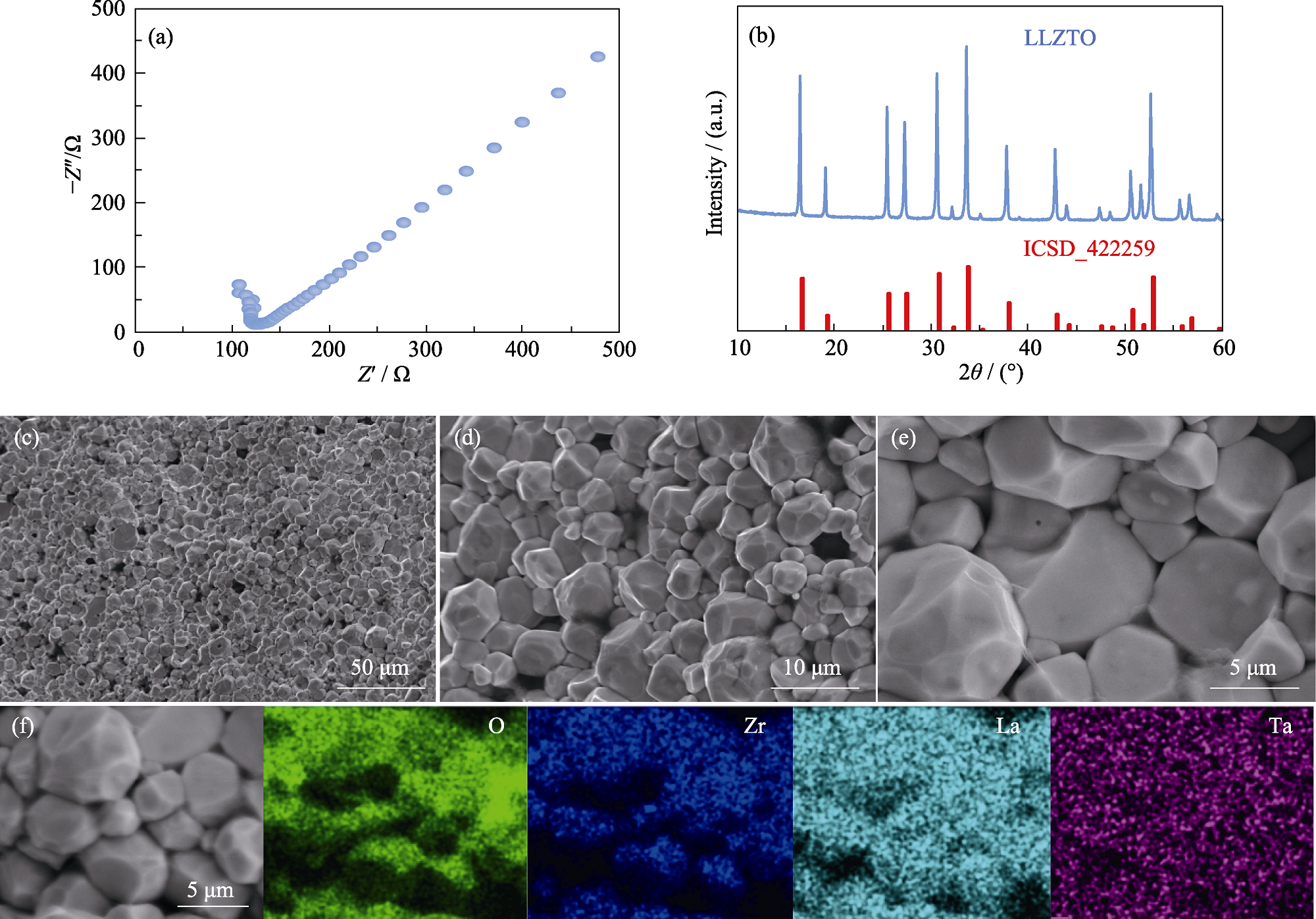
图1 LLZTO电解质片表征
Fig. 1 Characterization of LLZTO electrolyte (a) EIS spectra; (b) XRD pattern; (c-e) Cross-sectional SEM images; (f) Cross-sectional SEM image and EDS mappings
| Element | Mass ratio/% | Molar ratio/% |
|---|---|---|
| La | 46.3 | 14.0 |
| Zr | 13.2 | 6.0 |
| Ta | 10.9 | 2.5 |
| O | 29.6 | 77.4 |
表1 LLZTO电解质片的截面元素比例
Table 1 Proportion of cross-sectional elements in LLZTO electrolyte
| Element | Mass ratio/% | Molar ratio/% |
|---|---|---|
| La | 46.3 | 14.0 |
| Zr | 13.2 | 6.0 |
| Ta | 10.9 | 2.5 |
| O | 29.6 | 77.4 |
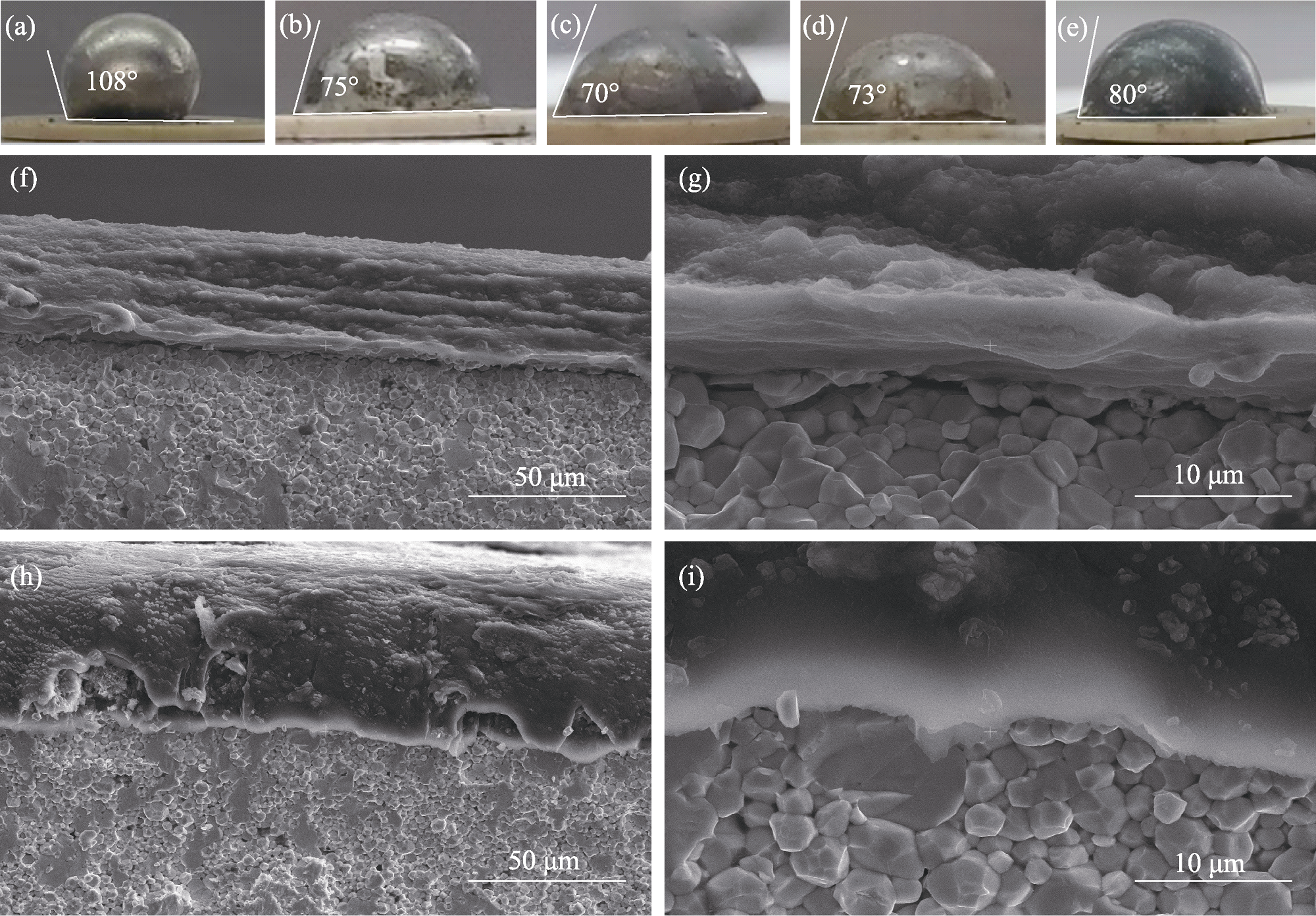
图3 LLZTO复合负极表征
Fig. 3 Characterization of LLZTO composite anode (a-e) Optical photographs for contact angles of (a) Li|LLZTO, (b) LAF2|LLZTO, (c) LAF3|LLZTO, (d) LAF4|LLZTO, and (e) Li-Al|LLZTO; (f-i) Cross-sectional SEM images of (f, g) Li|LLZTO and (h, i) LAF3|LLZTO
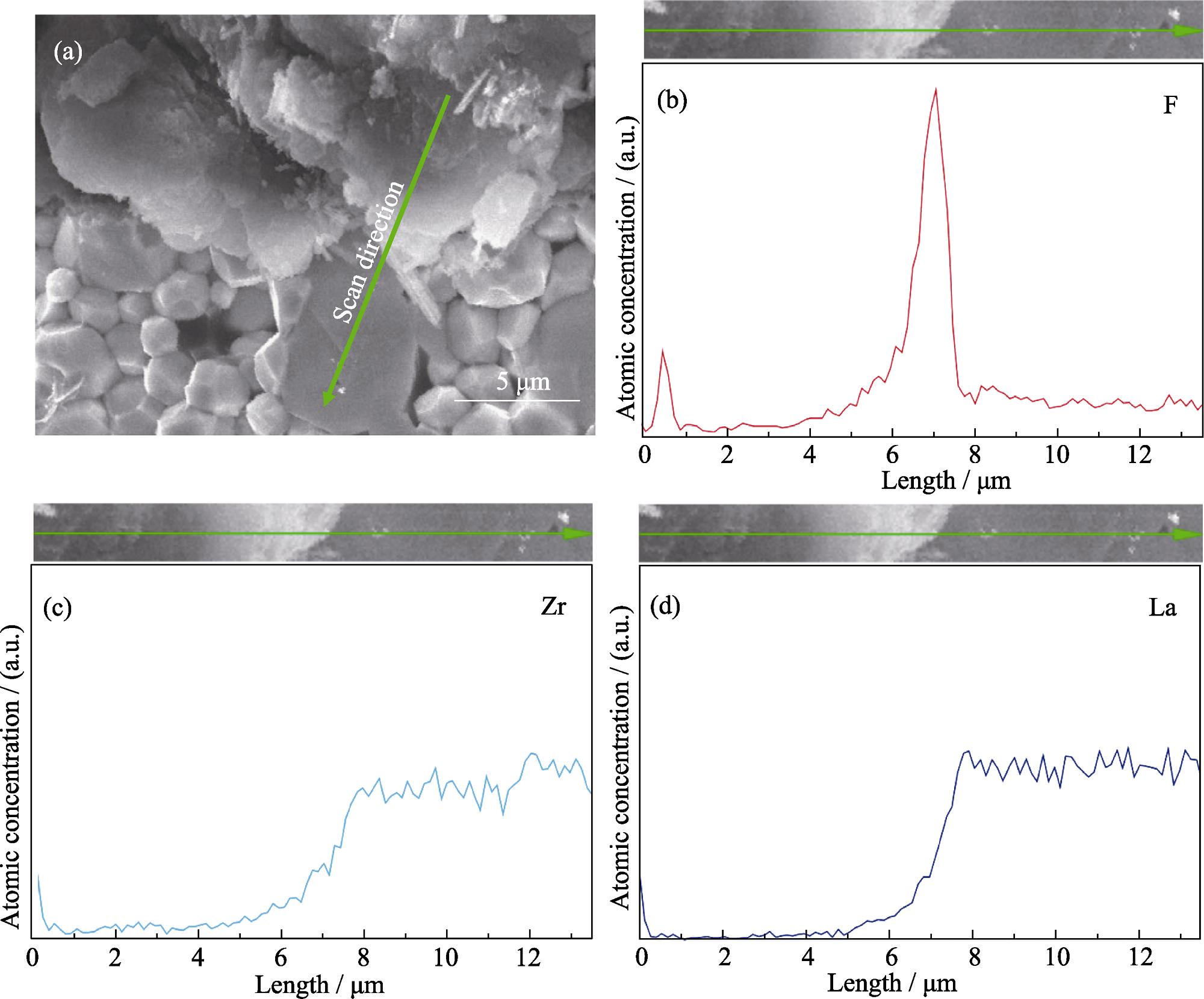
图4 LAF3|LLZTO的界面形貌和元素分布
Fig. 4 Morphology and element distribution of LAF3|LLZTO interface (a) SEM image of the interface; (b-d) EDS spectra across the interface displaying (b) F, (c) Zr, and (d) La element distributions
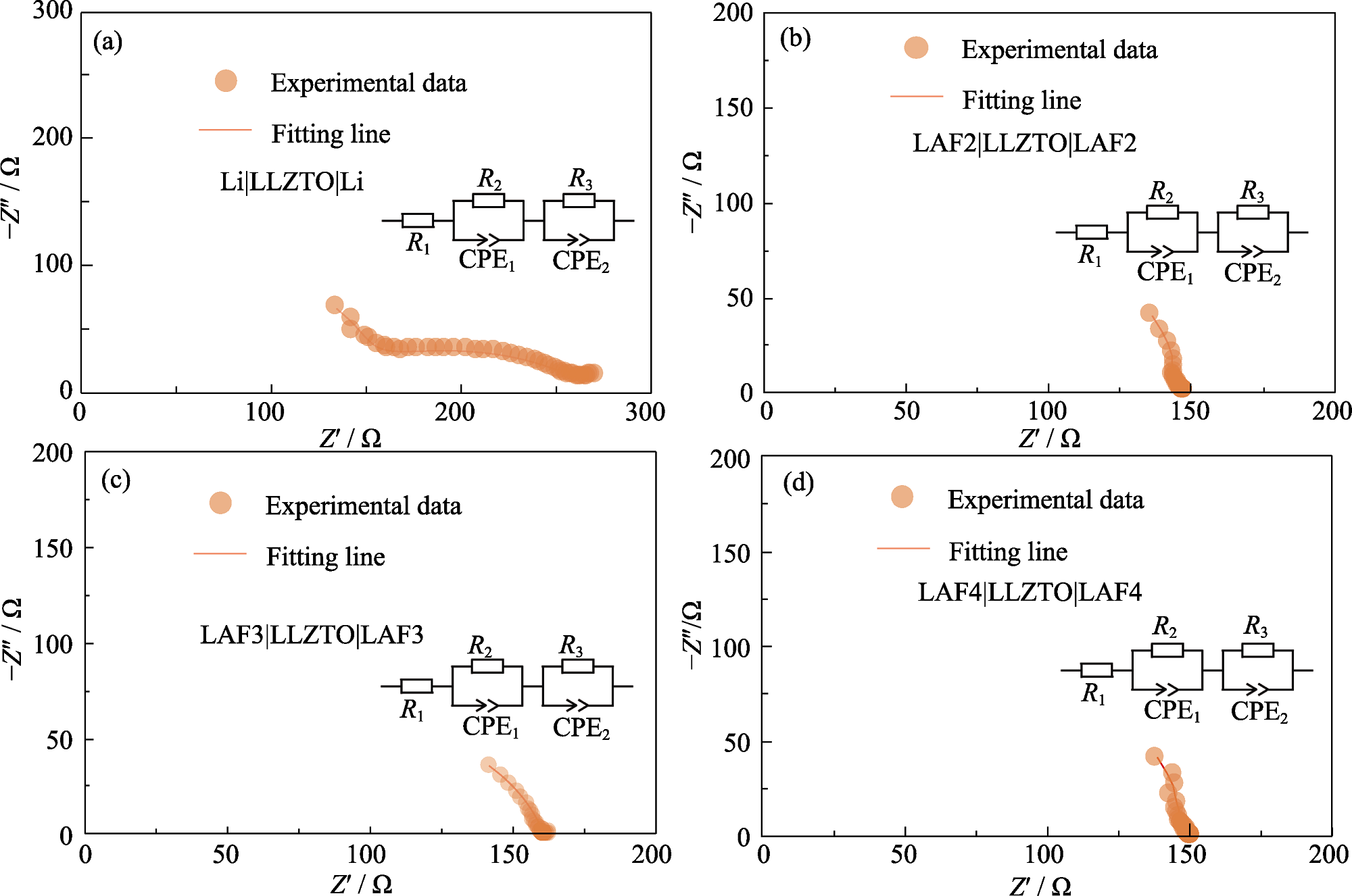
图5 (a) Li|LLZTO|Li、(b) LAF2|LLZTO|LAF2、(c) LAF3|LLZTO|LAF3、(d) LAF4|LLZTO|LAF4对称电池的EIS谱图
Fig. 5 EIS spectra of (a) Li|LLZTO|Li, (b) LAF2|LLZTO|LAF2, (c) LAF3|LLZTO|LAF3, and (d) LAF4|LLZTO|LAF4 symmetrical cells with insets showing the coresponding equivalent circuits
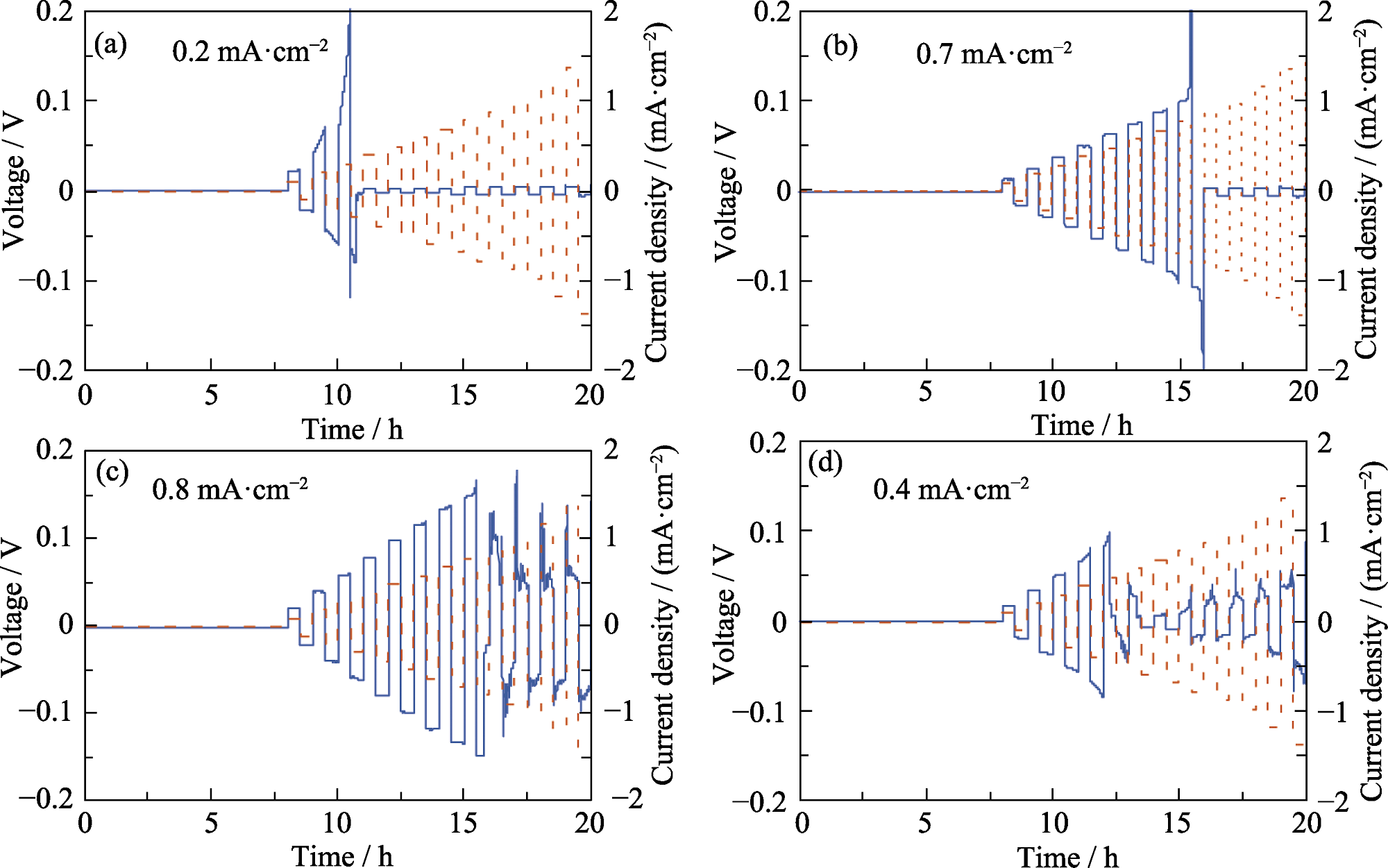
图6 (a) Li|LLZTO|Li、(b) LAF2|LLZTO|LAF2、(c) LAF3|LLZTO|LAF3、(d) LAF4|LLZTO|LAF4对称电池的临界电流密度
Fig. 6 Critical current densities of (a) Li|LLZTO|Li, (b) LAF2|LLZTO|LAF2, (c) LAF3|LLZTO|LAF3, and (d) LAF4|LLZTO|LAF4 symmetrical cells
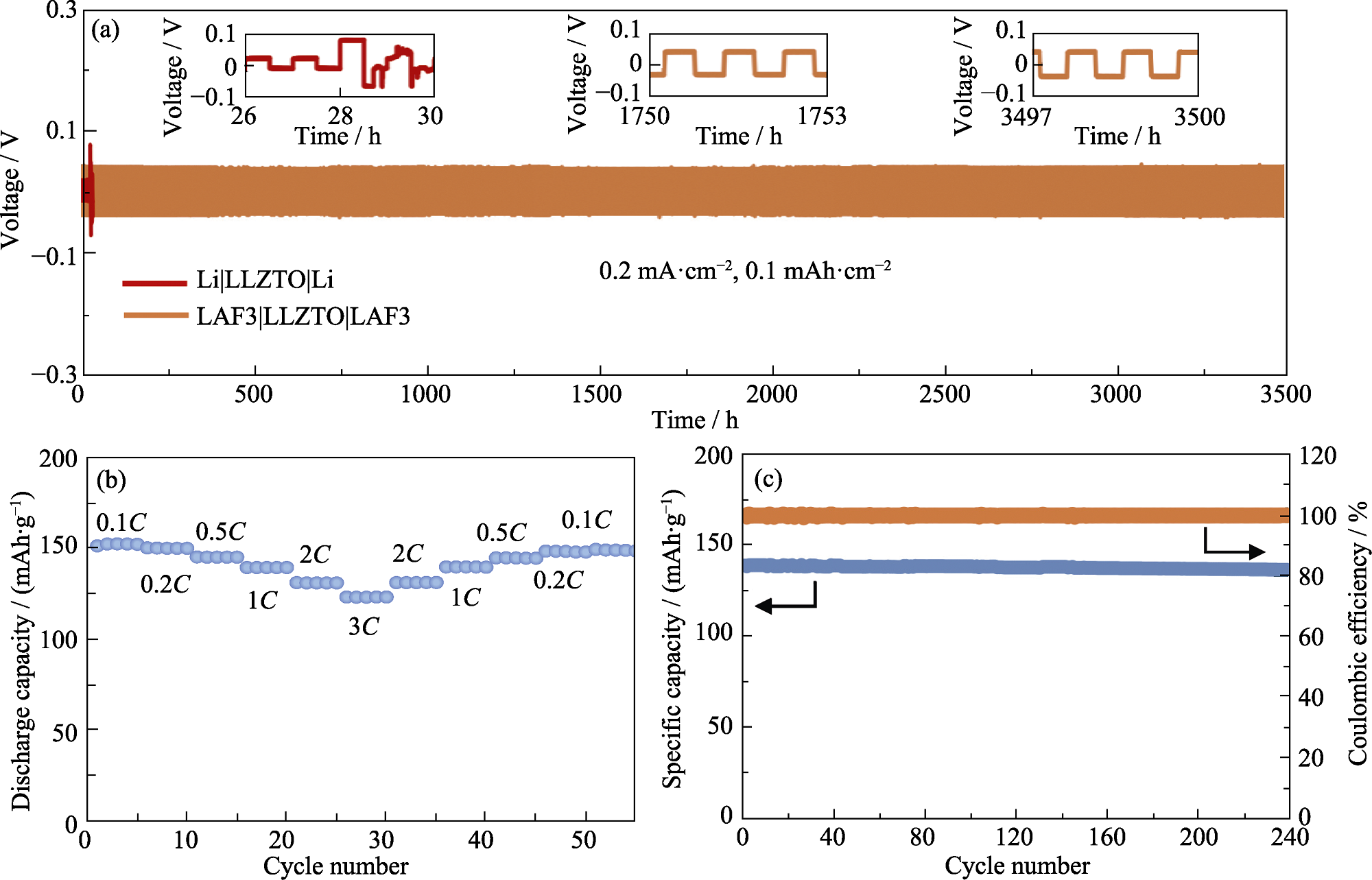
图7 (a) LAF3|LLZTO|LAF3和Li|LLZTO|Li对称电池在0.2 mA/cm2电流密度下的循环测试; (b) LFP|LLZTO|LAF3电池的倍率性能以及(c)在1C倍率下的循环稳定性
Fig. 7 (a) Cycling performance of LAF3|LLZTO|LAF3 and Li|LLZTO|Li symmetrical cells at a current density of 0.2 mA/cm2; (b) Rate performance and (c) cycling performance of LFP|LLZTO|LAF3 cell at 1C rate
| [1] | 徐学笛. 化学电源的发展及展望. 化学工程与装备, 2008(2): 95. |
| [2] | XIE J, LU Y C. A retrospective on lithium-ion batteries. Nature Communications, 2020, 11: 2499. |
| [3] |
赵光金, 李晶晶, 胡玉霞, 等. 锂离子电池储能电站安全风险及应对策略. 电源技术, 2024, 48(12): 2343.
DOI |
| [4] | 徐琛. 固态锂电池复合固态电解质研究进展. 中外能源, 2023, 28(9): 18. |
| [5] | KIM A, WOO S, KANG M, et al. Research progresses of garnet-type solid electrolytes for developing all-solid-state Li batteries. Frontiers in Chemistry, 2020, 8: 468. |
| [6] | WU J, CHEN L, SONG T, et al. A review on structural characteristics, lithium ion diffusion behavior and temperature dependence of conductivity in perovskite-type solid electrolyte Li3-xLa2/3-xTiO3. Functional Materials Letters, 2017, 10(3): 1730002. |
| [7] | LI C, LI R, LIU K, et al. NaSICON: a promising solid electrolyte for solid-state sodium batteries. Interdisciplinary Materials, 2022, 1(3): 396. |
| [8] | ZHAO Y, DAEMEN L L. Superionic conductivity in lithium- rich anti-perovskites. Journal of the American Chemical Society, 2012, 134(36): 15042. |
| [9] | AIMI A, ONODERA H, SHIMONISHI Y, et al. High Li-ion conductivity in pyrochlore-type solid electrolyte Li2-xLa(1+x)/3M2O6F (M=Nb, Ta). Chemistry of Materials, 2024, 36(8): 3717. |
| [10] |
WANG C, FU K, KAMMAMPATA S P, et al. Garnet-type solid-state electrolytes: materials, interfaces, and batteries. Chemical reviews, 2020, 120(10): 4257.
DOI PMID |
| [11] | MURUGAN R, THANGADURAI V, WEPPNER W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angewandte Chemie International Edition, 2007, 46(41): 7778. |
| [12] | CHEN R, NOLAN A M, LU J, et al. The thermal stability of lithium solid electrolytes with metallic lithium. Joule, 2020, 4(4): 812. |
| [13] | CHENG E J, DUAN H, WANG M J, et al. Li-stuffed garnet solid electrolytes: current status, challenges, and perspectives for practical. Energy Storage Materials, 2024, 75: 103970. |
| [14] | 张念, 任国玺, 章辉, 等. 石榴石型固态电解质表界面问题及优化的研究进展. 物理学报, 2020, 69(22): 224. |
| [15] |
HAN X, GONG Y, FU K, et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nature Materials, 2017, 16(5): 572.
DOI PMID |
| [16] | PORZ L, SWAMY T, SHELDON B W, et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Advanced Energy Materials, 2017, 7(20): 1701003. |
| [17] | SHARAFI A, KAZYAK E, DAVIS A L, et al. Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12. Chemistry of Materials, 2017, 29(18): 7961. |
| [18] | DUAN H, CHEN W P, FAN M, et al. Building an air stable and lithium deposition regulable garnet interface from moderate- temperature conversion chemistry. Angewandte Chemie International Edition, 2020, 132(29): 12167. |
| [19] | BI Z, SUN Q, JIA M, et al. Molten salt driven conversion reaction enabling lithiophilic and air-stable garnet surface for solid-state lithium batteries. Advanced Functional Materials, 2022, 32(52): 2208751. |
| [20] | RUAN Y, LU Y, LI Y, et al. A 3D cross-linking lithiophilic and electronically insulating interfacial engineering for garnet-type solid-state lithium batteries. Advanced Functional Materials, 2021, 31(5): 2007815. |
| [21] | LEE S, LEE K S, KIM S, et al. Design of a lithiophilic and electron-blocking interlayer for dendrite-free lithium-metal solid-state batteries. Science Advances, 2022, 8(30): eabq0153. |
| [22] | BI Z, HUANG W, MU S, et al. Dual-interface reinforced flexible solid garnet batteries enabled by in-situ solidified gel polymer electrolytes. Nano Energy, 2021, 90: 106498. |
| [23] | BI Z, SHI R, LIU X, et al. In situ conversion reaction triggered alloy@antiperovskite hybrid layers for lithiophilic and robust lithium/garnet interfaces. Advanced Functional Materials, 2023, 33(43): 2307701. |
| [24] | ALEXANDER G V, SHI C, O’NEILL J, et al. Extreme lithium- metal cycling enabled by a mixed ion-and electron-conducting garnet three-dimensional architecture. Nature Materials, 2023, 22(9): 1136. |
| [25] | FENG W, DONG X, LI P, et al. Interfacial modification of Li/garnet electrolyte by a lithiophilic and breathing interlayer. Journal of Power Sources, 2019, 419: 91. |
| [26] | LI Z, ZHENG W, LU G, et al. Superionic conductor enabled composite lithium with high ionic conductivity and interfacial wettability for solid-state lithium batteries. Advanced Functional Materials, 2024, 34(12): 2309751. |
| [27] | HU X, YU J, WANG Y, et al. A lithium intrusion-blocking interfacial shield for wide-pressure-range solid-state lithium metal batteries. Advanced Materials, 2024, 36(7): 2308275. |
| [28] | CHEN B, ZHANG J, ZHANG T, et al. Directly using Li2CO3 as a lithiophobic interlayer to inhibit Li dendrites for high- performance solid-state batteries. ACS Energy Letters, 2023, 8(5): 2221. |
| [29] | SHI K, WAN Z, YANG L, et al. In situ construction of an ultra-stable conductive composite interface for high-voltage all-solid-state lithium metal batteries. Angewandte Chemie International Edition, 2020, 59(29): 11784. |
| [30] | LU Y, HUANG X, RUAN Y, et al. An in situ element permeation constructed high endurance Li-LLZO interface at high current densities. Journal of Materials Chemistry A, 2018, 6(39): 18853. |
| [31] | MA C, JIANG W, DUAN Q, et al. Superdense lithium deposition via mixed ionic/electronic conductive interfaces implanted in vivo/vitro for stable lithium metal batteries. Advanced Energy Materials, 2024, 14(25): 2400202. |
| [32] | LI Y, LI J, XIAO H, et al. A novel 3D Li/Li9Al4/Li-Mg alloy anode for superior lithium metal batteries. Advanced Functional Materials, 2023, 33(14): 2213905. |
| [33] | PENG Z, REN F, YANG S, et al. A highly stable host for lithium metal anode enabled by Li9Al4-Li3N-AlN structure. Nano Energy, 2019, 59: 110. |
| [34] | SHI X, PANG Y, WANG B, et al. In situ forming LiF nanodecorated electrolyte/electrode interfaces for stable all- solid-state batteries. Materials Today Nano, 2020, 10: 100079. |
| [35] | CHEN Y, OUYANG C, SONG L, et al. Electrical and lithium ion dynamics in three main components of solid electrolyte interphase from density functional theory study. The Journal of Physical Chemistry C, 2011, 115(14): 7044. |
| [1] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [2] | 易国刚, 吴耀应, 俎喜红. 无溶剂法低温制备双碳包覆多孔硅碳负极材料及储锂性能研究[J]. 无机材料学报, 2025, 40(12): 1379-1386. |
| [3] | 李勇锋, 顾玉萍, 师广照, 胡九林, 雷萌, 彭晖, 曾宇平, 李驰麟. NASICON型陶瓷固态电池的电化学电位界面调控[J]. 无机材料学报, 2025, 40(11): 1201-1211. |
| [4] | 张宇婷, 李晓斌, 刘尊义, 李宁, 赵鹬. 复合蛋黄壳型NiCo2V2O8@TiO2@NC材料用作锂离子电池负极研究[J]. 无机材料学报, 2025, 40(11): 1221-1228. |
| [5] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [6] | 程节, 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直. 蛋黄壳结构FeF3·0.33H2O@N掺杂碳纳米笼正极材料的构筑及其电化学性能[J]. 无机材料学报, 2024, 39(3): 299-305. |
| [7] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| [8] | 苏楠, 邱介山, 王治宇. 高容量氟掺杂碳包覆纳米硅负极材料: 气相氟化法制备及其储锂性能[J]. 无机材料学报, 2023, 38(8): 947-953. |
| [9] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [10] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [11] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [12] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [13] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [14] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [15] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||