无机材料学报 ›› 2020, Vol. 35 ›› Issue (7): 827-833.DOI: 10.15541/jim20190488 CSTR: 32189.14.10.15541/jim20190488
所属专题: 能源材料论文精选(一):锂离子电池(2020)
收稿日期:2019-09-25
修回日期:2019-12-16
出版日期:2020-07-20
网络出版日期:2020-01-15
作者简介:湛 菁(1974-), 女, 副教授. E-mail: 80560381@qq.com基金资助:
ZHAN Jing1,2,XU Changfan1,LONG Yiyu1,LI Qihou1( )
)
Received:2019-09-25
Revised:2019-12-16
Published:2020-07-20
Online:2020-01-15
Supported by:摘要:
Bi2Mn4O10具有高的理论比容量, 被认为是一种理想的锂离子电池负极材料。本研究以硝酸铋和乙酸锰为原料, 采用聚丙烯酰胺凝胶法制备Bi2Mn4O10负极材料, 考察了制备条件对Bi2Mn4O10负极材料的物相、形貌及电化学性能的影响。结果表明: 在丙烯酰胺含量与总金属离子摩尔比为8 : 1, 葡萄糖浓度为1.11 mol/L, 热处理温度为873 K的条件下, 可得类球型、分散性良好的纯相Bi2Mn4O10粉末。作为负极材料, Bi2Mn4O10粉末在0.2C (1C=800 mA/g)倍率下循环50圈后可保持496.8 mAh/g的比容量, 容量保持率为76.9%; 3C倍率下放电容量为232 mAh/g。
中图分类号:
湛菁,徐昌藩,龙怡宇,李启厚. 聚丙烯酰胺凝胶法制备Bi2Mn4O10及其电化学性能[J]. 无机材料学报, 2020, 35(7): 827-833.
ZHAN Jing,XU Changfan,LONG Yiyu,LI Qihou. Bi2Mn4O10: Preparation by Polyacrylamide Gel Method and Electrochemical Performance[J]. Journal of Inorganic Materials, 2020, 35(7): 827-833.
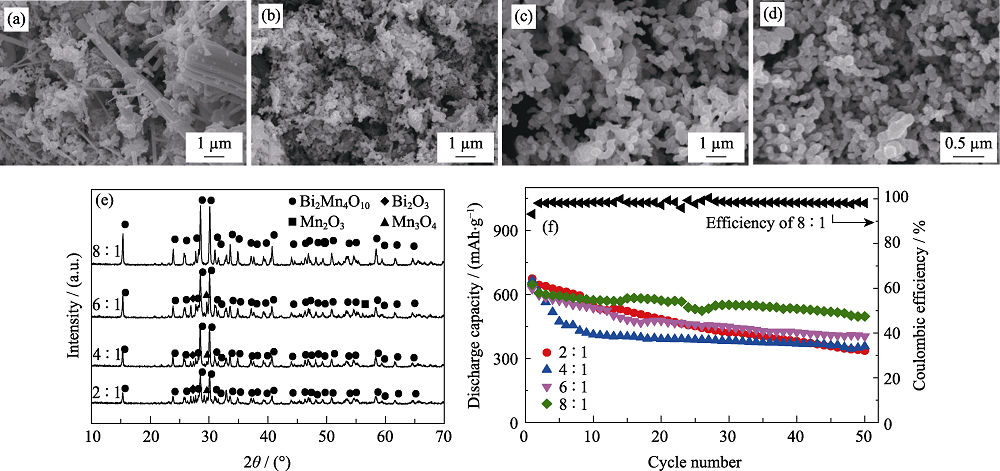
图1 不同丙烯酰胺与总金属离子摩尔比下所得产物的SEM照片((a) 2 : 1, (b) 4 : 1, (c) 6 : 1, (d) 8 : 1), (e) XRD图谱, (f)作为负极材料在0.2C时的比容量循环性能以及摩尔比为8 : 1时产品的库伦效率曲线(0.1C活化3圈, 1C=800 mA/g)
Fig. 1 SEM images ((a) 2 : 1, (b) 4 : 1, (c) 6 : 1, (d)8 : 1), (e) XRD patterns and (f) cycling performance at 0.2C of the products obtained with different molar ratios of acrylamide to total metal ions, and Coulombic efficiency of the product with molar ratio of acrylamide to total metal ions of 8 : 1 (after 3 cycles at 0.1C, 1C=800 mA/g) Glucose concentrations: 1.11 mol/L, heat treatment temperature: 873 K, weight ratio of acrylamide to N,N’-methylene bisacrylamide: 5 : 1
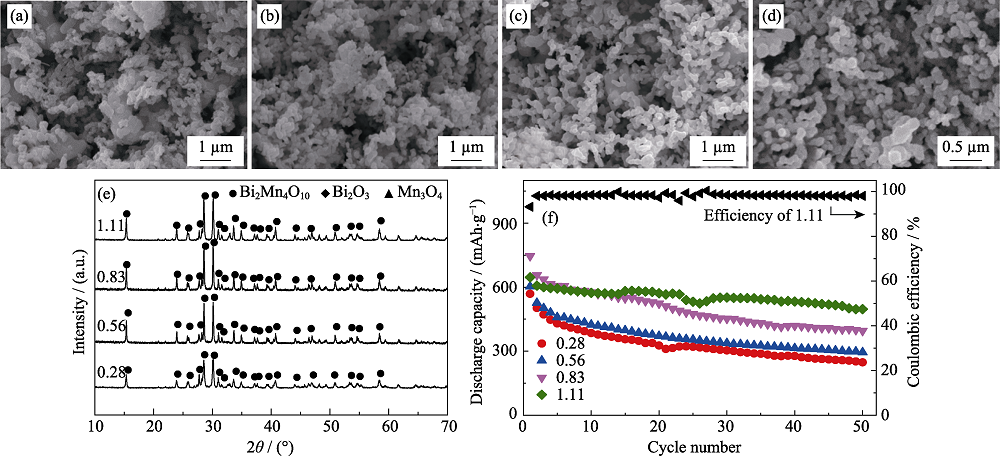
图2 不同葡萄糖浓度下所得产物的SEM照片((a) 0.28 mol/L, (b) 0.56 mol/L, (c)0.83 mol/L, (d) 1.11 mol/L), (e) XRD图谱, (f) 作为负极材料在0.2C时的比容量循环性能以及葡萄糖浓度为1.11 mol/L时产品的库伦效率曲线(0.1C活化3圈, 1C=800 mA/g)
Fig. 2 SEM images ((a) 0.28 mol/L, (b) 0.56 mol/L, (c) 0.83 mol/L, (d) 1.11 mol/L), (e) XRD patterns and (f) cycling performance at 0.2C of the products obtained with different glucose concentrations, and Coulombic efficiency of the product with 1.11 mol/L glucose (after three cycles at 0.1C, 1C=800 mA/g) Molar ratio of acrylamide to total metal ions: 8 : 1; heat treatment temperature: 873 K; weight ratio of acrylamide to N,N’-methylene bisacrylamide of 5 : 1
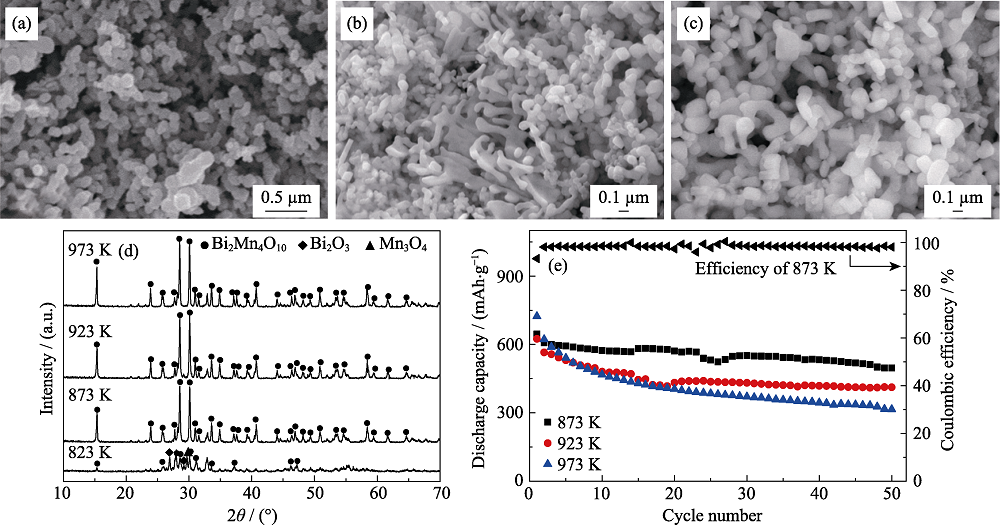
图3 不同热处理温度下所得产物SEM照片((a) 873 K, (b) 923 K, (c) 973 K), (d) XRD图谱, (e)作为负极材料在0.2C时的比容量循环性能以及873 K时产品的库伦效率曲线(0.1C活化3圈, 1C=800 mA/g)
Fig. 3 SEM images ((a) 873 K, (b) 923 K, (c) 973 K), (d) XRD patterns and (e) cycling performance at 0.2C of the products obtained with different heat treatment temperatures, and Coulombic efficiency of the product with heat-treatment temperature of 873 K (after three cycles at 0.1C, 1C=800 mA/g) Molar ratio of acrylamide to total metal ions: 8 : 1, glucose concentrations of 1.11 mol/L, weight ratio of acrylamide to N,N’-methylene bisacrylamide: 5 : 1
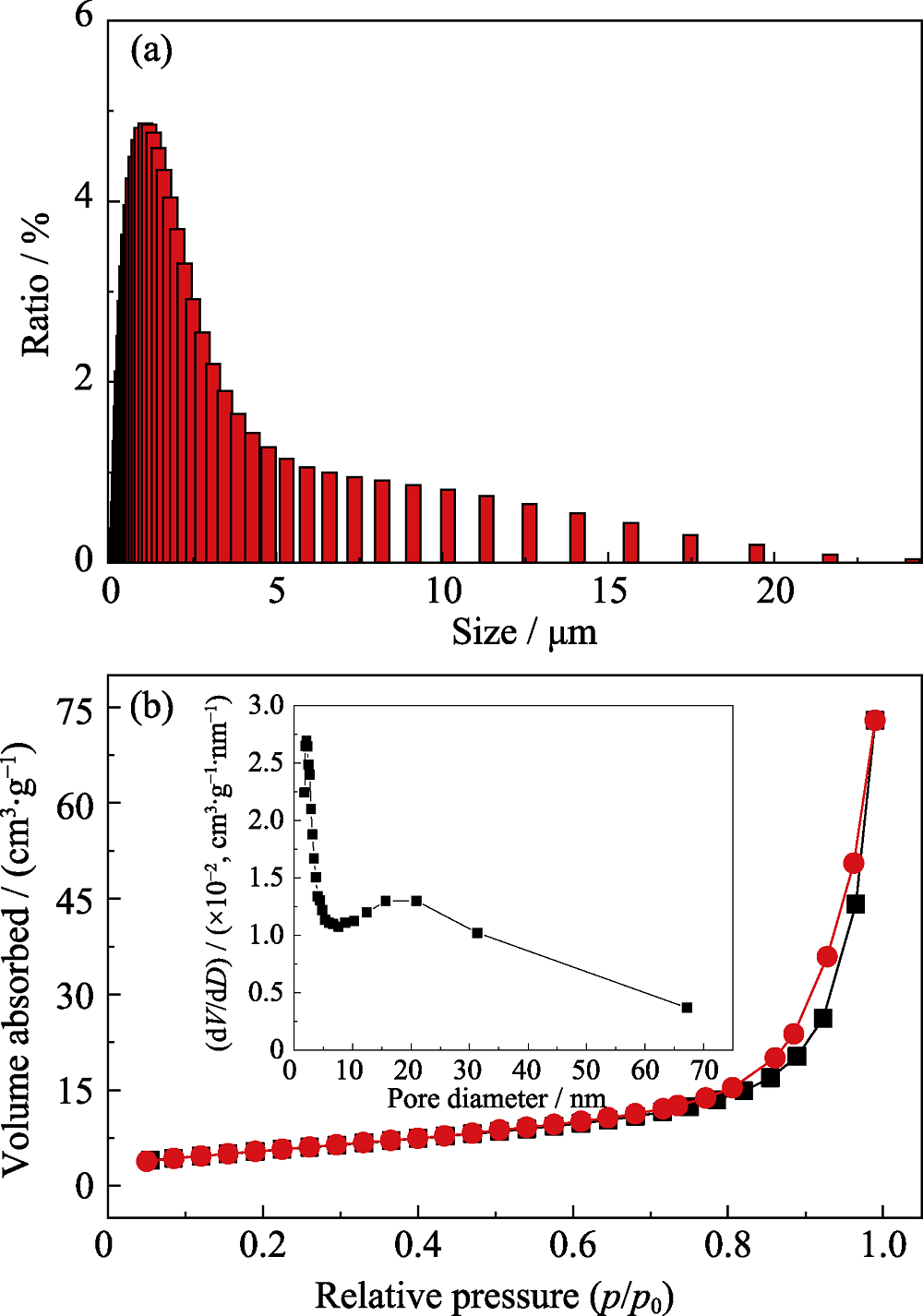
图5 在优化工艺条件下制备的Bi2Mn4O10的(a)粒度分布柱状图和(b)氮气吸脱附曲线以及孔径分布图
Fig. 5 (a) Particle size distribution and (b) N2 adsorption and desorption curves of Bi2Mn4O10 obtained at optimized conditions with inset in (b) showing the pore size distribution curve
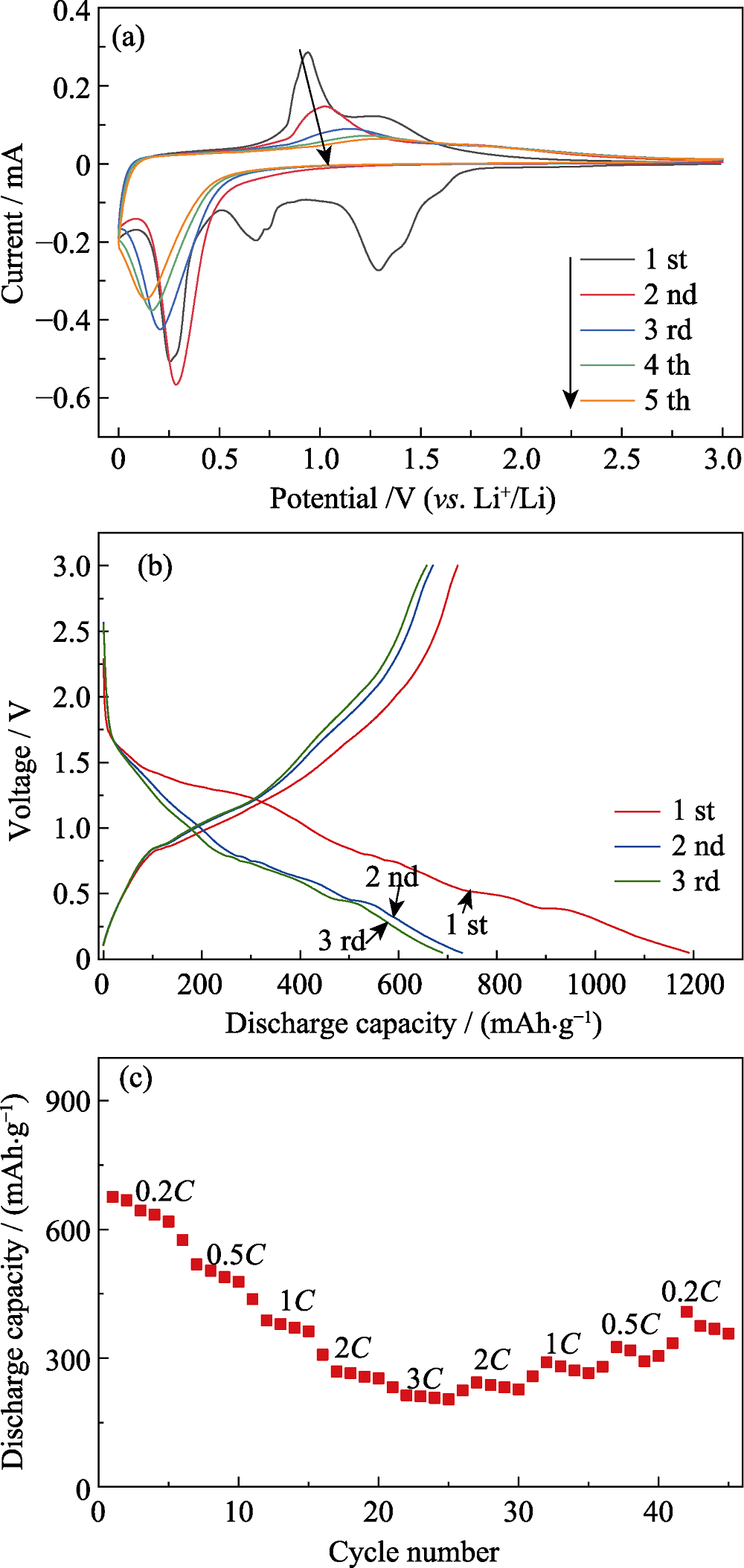
图6 优化工艺条件下制备的Bi2Mn4O10的(a)循环伏安曲线, (b)在0.1C时的电压-比容量曲线和(c)倍率性能图
Fig. 6 (a) CV curves, (b) voltage-specific capacity curves at 0.1C and (c) rate performance curve of Bi2Mn4O10 obtained at optimized conditions
| [1] |
GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: a perspective. Journal of the American Chemical Society, 2013,135(4):1167-1176.
DOI URL PMID |
| [2] |
FU X M, SUN H, XIE S L, et al. A fiber-shaped solar cell showing a record power conversion efficiency of 10%. Journal of Materials Chemistry A, 2018,6(1):45-51.
DOI URL |
| [3] |
GONG S Q, JIANG Z J, SHI P H, et al. Noble-metal-free heterostructure for efficient hydrogen evolution in visible region: molybdenum nitride/ultrathin graphitic carbon nitride. Applied Catalysis B-Environmental, 2018,238:318-327.
DOI URL |
| [4] |
DENG Y F, WAN L N, XIE Y, et al. Recent advances in Mn-based oxides as anode materials for lithium ion batteries. RSC Advances, 2014,4(45):23914-23935.
DOI URL |
| [5] | ETACHERI V, MAROM R, ELsAZARI R, et al. Challenges in the development of advanced Li-ion batteries: a review. Energy & Environmental Science, 2011,4(9):3243-3262. |
| [6] |
ZHANG L H, ZHU S Q, CAO H, et al. Hierarchical porous ZnMn2O4 hollow nanotubes with enhanced lithium storage toward lithium-ion batteries. Chemistry-A European Journal, 2015,21(30):10771-10777.
DOI URL |
| [7] |
CHEN J, ZHAN J, ZHANG Y M, et al. Construction of a novel ZnCo2O4/Bi2O3 heterojunction photocatalyst with enhanced visible light photocatalytic activity. Chinese Chemical Letters, 2019,30(3):735-738.
DOI URL |
| [8] |
CHEN J, ZHAN J, LI Q H. Exploration and crystal phase engineering from bismuthinite ore to visible-light responsive photocatalyst of Bi2O3. Journal of Environmental Chemical Engineering, 2019,7(5):103375.
DOI URL |
| [9] |
CHEN J, ZHAN J, DING F H, et al. Novel synthesis method of sheet-like agglomerates beta-Bi2O3 with high photocatalytic activity. Journal of Inorganic Materials, 2018,33(8):919-923.
DOI URL |
| [10] |
LU Y, YU Y, LOU X W. Nanostructured conversion-type anode materials for advanced lithium-ion batteries. Chem, 2018,4(5):972-996.
DOI URL |
| [11] |
REDDY M V, RAO G V S, CHOWDARI B V R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chemical Reviews, 2013,113(7):5364-5457.
DOI URL PMID |
| [12] |
CABANA J, MONCONDUIT L, LARCHER D, et al. Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Advanced Materials, 2010,22(35):E170-E192.
DOI URL PMID |
| [13] |
ZHOU L, ZHAO D Y, LOU X W. Double-shelled CoMn2O4 hollow microcubes as high-capacity anodes for lithium-ion batteries. Advanced Materials, 2012,24(6):745-748.
DOI URL PMID |
| [14] |
ZHANG G Q, YU L, WU H B, et al. Formation of ZnMn2O4 ball-in-ball hollow microspheres as a high-performance anode for lithium-ion batteries. Advanced Materials, 2012,24(34):4609-4613.
DOI URL PMID |
| [15] |
LI J F, XIONG S L, LIU Y R, et al. High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for Li-ion batteries. ACS Applied Materials & Interfaces, 2013,5(3):981-988.
DOI URL PMID |
| [16] |
SONG Z H, ZHANG H Z, FENG K, et al. Bi2Mn4O10: a new mullite-type anode material for lithium-ion batteries. Dalton Transactions, 2018,47(23):7739-7746.
DOI URL PMID |
| [17] |
ZHAN J, LONG Y Y. Synthesis of Bi2Mn4O10 nanoparticles and its anode properties for LIB. Ceramics International, 2018,44(12):14891-14895.
DOI URL |
| [18] | WANG Z, ZHANG C, ZHAN J, et al. Preparation and characterization of ultrafine Bi2Mn4O10 powders. Journal of Central South University (Science and Technology), 2018,49(10):2398-2404. |
| [19] | ZHENG Y P, GAO W J, ZHA Y, et al. Preparation of LaxSr1-xMO3 nanopowders by polyacrylamide Sol-Gel method. Journal of Anhui Normal University (Natural Science), 2008,31(6):552-555. |
| [20] |
XIAN T, YANG H, SHEN X, et al. Synthesis of BiFeO3 nanoparticles by a polyacrylamide gel route. Journal of Inorganic Materials, 2010,25(3):251-254.
DOI URL |
| [21] | ZHENG Y, GAO W, ZHA Y, et al. Synthesis and properties of intermediate-temperature solid electrolyte La0.9Sr0.1Ga0.8Mg0.2O3-δ from polyacrylamidesol-gelprecursor. Journal of Southeast University (Natural Science Edition), 2008, (5):902-906. |
| [22] |
CHANG J, HUANG X, ZHOU G, et al. Multilayered Si nanoparticle/ reduced graphene oxide hybrid as a high-performance lithium-ion battery anode. Advanced Materials, 2014,26(5):758-764.
DOI URL |
| [23] |
LI M, YIN Y X, LI C, et al. Well-dispersed bi-component-active CoO/CoFe2O4 nanocomposites with tunable performances as anode materials for lithium-ion batteries. Chemical Communications, 2012,48(3):410-412.
DOI URL |
| [24] | LI Z W, YONG X, HUA F, et al. Effect of glucose on the perflormance of Li1.2Ni0.13Co0.13Mn0.54O2 synthesized by Sol-Gel method. Chinese Journal of Inorganic Chemistry, 2015,31(5):873-879. |
| [25] |
ZHENG Z M, CHENG Y L, YAN X B, et al. Enhanced electrochemical properties of graphene-wrapped ZnMn2O4 nanorods for lithium-ion batteries. Journal of Materials Chemistry A, 2014,2(1):149-154.
DOI URL |
| [26] |
LI Y L, TRUJILLO M A, FU E G, et al. Bismuth oxide: a new lithium-ion battery anode. Journal of Materials Chemistry A, 2013,1(39):12123-12127.
DOI URL |
| [27] |
DENG Z, LIU T T, CHEN T, et al. Enhanced electrochemical performances of Bi2O3/rGO nanocomposite via chemical bonding as anode materials for lithium ion batteries. ACS Applied Materials & Interfaces, 2017,9(14):12469-12477.
DOI URL PMID |
| [28] |
ETTE P M, GURUNATHAN P, RAMESHA K. Self-assembled lamellar alpha-molybdenum trioxide as high performing anode material for lithium-ion batteries. Journal of Power Sources, 2015,278:630-638.
DOI URL |
| [29] |
LI L, RAJI A R O, TOUR J M. Graphene-wrapped MnO2-graphene nanoribbons as anode materials for high-performance lithium ion batteries. Advanced Materials, 2013,25(43):6298-6302.
DOI URL PMID |
| [1] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [2] | 万俊池, 杜路路, 张永上, 李琳, 刘建德, 张林森. Na4FexP4O12+x/C钠离子电池正极材料的结构演变及其电化学性能[J]. 无机材料学报, 2025, 40(5): 497-503. |
| [3] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [4] | 刘鹏东, 王桢, 刘永锋, 温广武. 硅泥在锂离子电池中的应用研究进展[J]. 无机材料学报, 2024, 39(9): 992-1004. |
| [5] | 程节, 周月, 罗薪涛, 高美婷, 骆思妃, 蔡丹敏, 吴雪垠, 朱立才, 袁中直. 蛋黄壳结构FeF3·0.33H2O@N掺杂碳纳米笼正极材料的构筑及其电化学性能[J]. 无机材料学报, 2024, 39(3): 299-305. |
| [6] | 陈正鹏, 金芳军, 李明飞, 董江波, 许仁辞, 徐韩昭, 熊凯, 饶睦敏, 陈创庭, 李晓伟, 凌意瀚. 双钙钛矿Sr2CoFeO5+δ阴极材料的制备及其中温固体氧化物燃料电池性能研究[J]. 无机材料学报, 2024, 39(3): 337-344. |
| [7] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| [8] | 苏楠, 邱介山, 王治宇. 高容量氟掺杂碳包覆纳米硅负极材料: 气相氟化法制备及其储锂性能[J]. 无机材料学报, 2023, 38(8): 947-953. |
| [9] | 杨卓, 卢勇, 赵庆, 陈军. X射线衍射Rietveld精修及其在锂离子电池正极材料中的应用[J]. 无机材料学报, 2023, 38(6): 589-605. |
| [10] | 宿拿拿, 韩静茹, 郭印毫, 王晨宇, 石文华, 吴亮, 胡执一, 刘婧, 李昱, 苏宝连. 基于ZIF-8的三维网络硅碳复合材料锂离子电池性能研究[J]. 无机材料学报, 2022, 37(9): 1016-1022. |
| [11] | 王洋, 范广新, 刘培, 尹金佩, 刘宝忠, 朱林剑, 罗成果. 钾离子掺杂提高锂离子电池正极锰酸锂性能的微观机制[J]. 无机材料学报, 2022, 37(9): 1023-1029. |
| [12] | 朱河圳, 王选朋, 韩康, 杨晨, 万睿哲, 吴黎明, 麦立强. 超高镍LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2正极材料的储锂稳定性的提升机制[J]. 无机材料学报, 2022, 37(9): 1030-1036. |
| [13] | 冯锟, 朱勇, 张凯强, 陈长, 刘宇, 高彦峰. 勃姆石纳米片增强锂离子电池隔膜性能研究[J]. 无机材料学报, 2022, 37(9): 1009-1015. |
| [14] | 陈莹, 栾伟玲, 陈浩峰, 朱轩辰. 基于应力场的锂离子电池正极多尺度失效研究[J]. 无机材料学报, 2022, 37(8): 918-924. |
| [15] | 江依义, 沈旻, 宋半夏, 李南, 丁祥欢, 郭乐毅, 马国强. 双功能电解液添加剂对锂离子电池高温高电压性能的影响[J]. 无机材料学报, 2022, 37(7): 710-716. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||