无机材料学报 ›› 2025, Vol. 40 ›› Issue (8): 888-900.DOI: 10.15541/jim20250007 CSTR: 32189.14.jim20250007
马文平1,2( ), 韩雅卉1,2, 吴成铁1,2(
), 韩雅卉1,2, 吴成铁1,2( ), 吕宏旭1,2(
), 吕宏旭1,2( )
)
收稿日期:2025-01-07
修回日期:2025-02-17
出版日期:2025-08-20
网络出版日期:2025-04-02
通讯作者:
吴成铁, 研究员. E-mail: chengtiewu@mail.sic.ac.cn;作者简介:马文平(1997-), 女, 博士研究生. E-mail: 15737197919@163.com
基金资助:
MA Wenping1,2( ), HAN Yahui1,2, WU Chengtie1,2(
), HAN Yahui1,2, WU Chengtie1,2( ), LÜ Hongxu1,2(
), LÜ Hongxu1,2( )
)
Received:2025-01-07
Revised:2025-02-17
Published:2025-08-20
Online:2025-04-02
Contact:
WU Chengtie, professor. E-mail: chengtiewu@mail.sic.ac.cn;About author:About author:MA Wenping (1997-), female, PhD candidate. E-mail: 15737197919@163.com
Supported by:摘要:
类器官作为模拟相应组织/器官结构和功能的体外三维(3D)模型, 在生物医学领域显示出广阔的应用前景。类器官的构建需要对干细胞行为以及多细胞相互作用进行调控, 而无机活性材料具有良好的生物相容性和生物活性, 可以调节细胞行为、细胞-细胞和细胞-基质之间的相互作用, 在疾病诊疗和再生医学等领域得到了广泛研究, 有望用于调控类器官的构建、生长和发育。本文综述了无机活性材料在类器官研究中的作用, 强调了其在类器官培养和实际应用方面的研究进展。首先概述了类器官构建策略的基本步骤, 介绍了代表性无机活性材料的生物学功能, 特别是与类器官构建关键步骤相适配的功能; 重点阐释了无机活性材料促进类器官生长和发育的关键作用机制, 包括调控关键信号通路、基质材料以及细胞能量代谢等。此外, 还探讨了类器官作为辅助工具在促进无机活性材料研究和应用方面的作用; 最后展望了利用无机活性材料提供多种物理和生化调控信号的特性进一步推进类器官基础研究和应用研究的策略。
中图分类号:
马文平, 韩雅卉, 吴成铁, 吕宏旭. 无机活性材料在类器官研究领域的应用[J]. 无机材料学报, 2025, 40(8): 888-900.
MA Wenping, HAN Yahui, WU Chengtie, LÜ Hongxu. Application of Inorganic Bioactive Materials in Organoid Research[J]. Journal of Inorganic Materials, 2025, 40(8): 888-900.
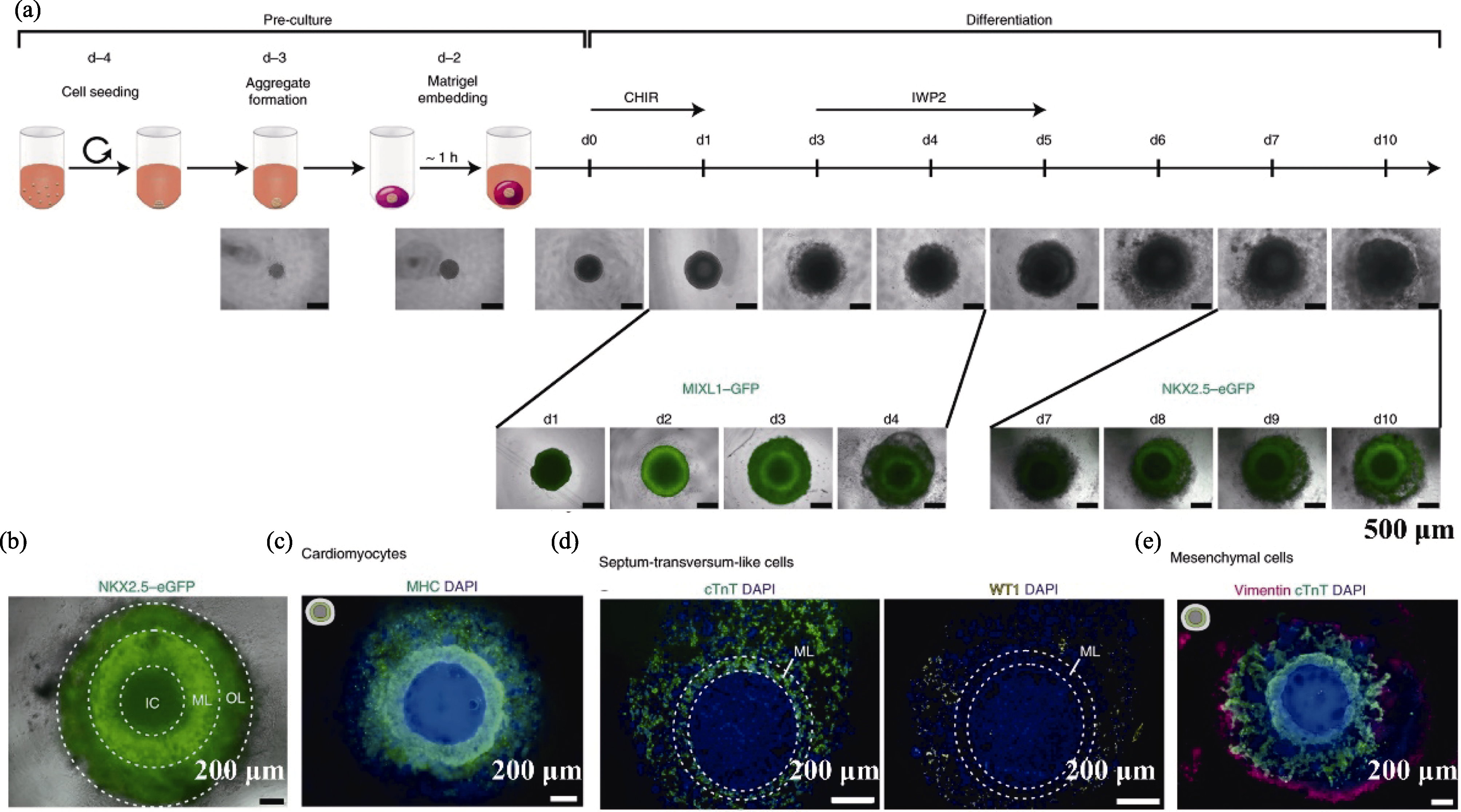
图1 iPSC衍生的心脏类器官构建策略[31]
Fig. 1 Strategy for constructing cardiac organoids derived from iPSC[31] (a) Formation scheme of cardiac organoids; (b) Cardiac organoids forming three layers; (c) Fluorescent staining of myocardial marker MHC; (d) Staining images of cTnT and WT1; (e) Staining image of mesenchymal cell marker vimentin CHIR: laduviglusib (CHIR99021); MHC: myosin heavy chain; cTnT: cardiac troponin T; WT1: Wilms’ tumor 1

图2 LCS生物陶瓷调控NSCs的行为[51]
Fig. 2 LCS bioceramics regulating the behaviors of NSCs[51] (a) Schematic diagram of preparation and application of neural platform based on LCS; (b) SEM images of LCS; (c) Representative immunofluorescence staining images of Nestin and MAP2 expression in NSCs; (d) Representative immunofluorescence staining images of GFAP and Tuj1 protein expression in NSCs; (e) Representative dual immunofluorescence staining images of GFAP and Tuj1 in NSCs under different culture conditions; (f) Representative immunofluorescence staining images of matural neuron marker MAP. MAP2: microtubule-associated protein 2; GFAP: glial fibrillary acidic protein; Tuj1: neuronal class III β-tubulin clone
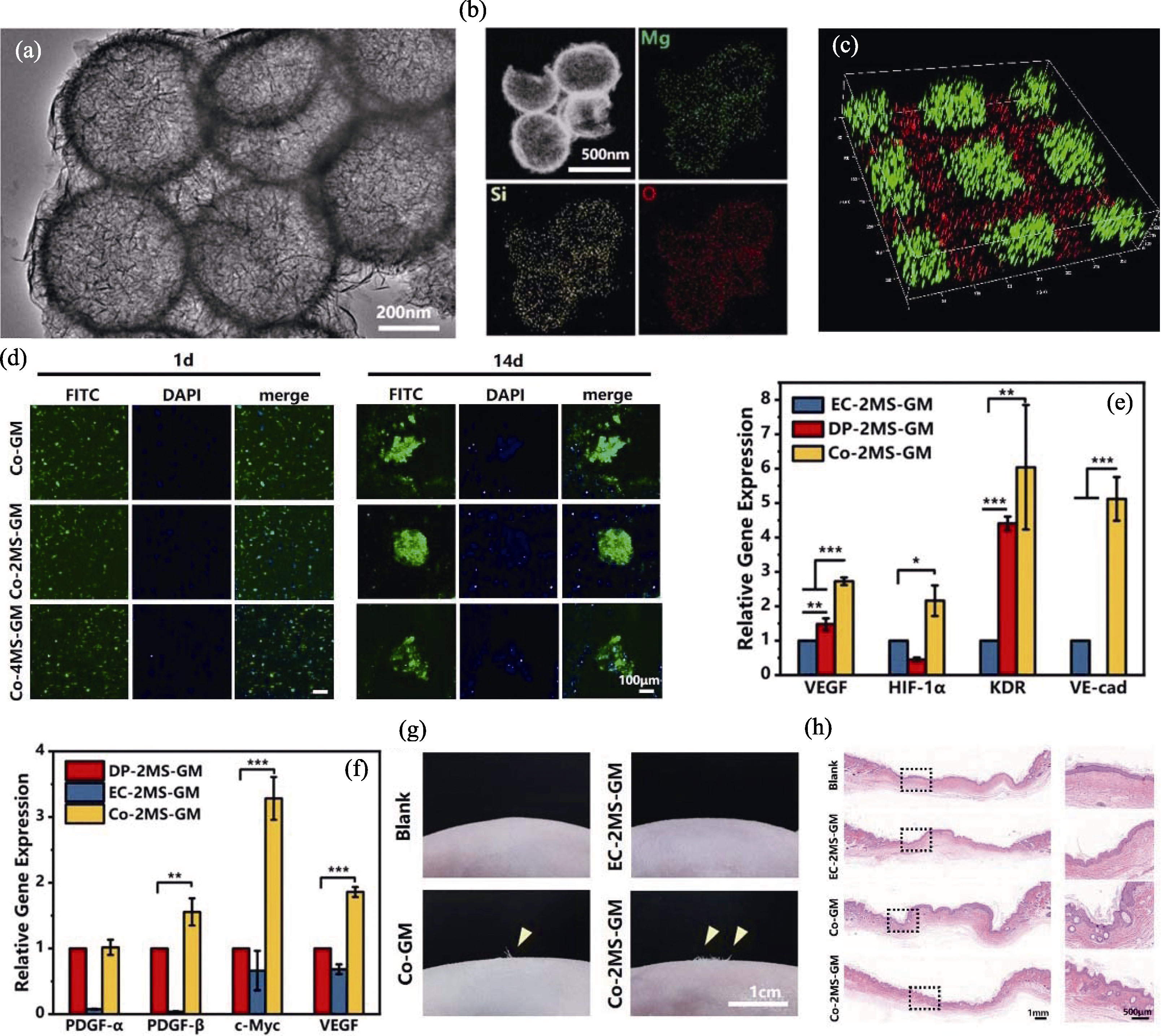
图3 MS纳米球调控内皮细胞和真皮细胞相互作用[55]
Fig. 3 MS nanospheres regulating the interaction between endothelial cells and dermal cells[55] (a) TEM image of MS nanospheres; (b) EDS mappings of elements including Mg, Si and O in MS nanospheres; (c) Fluorescence image displaying the biomimetic spatial distributions of human umbilical vascular endothelial cells (HUVECs, red fluorescence) and human hair dermal papilla cells (HHDPCs, green fluorescence); (d) Stained images of cell clusters during co-culture; (e, f) Expression of genes related to angiogenesis (e) and hair follicle formation (f) in co-culture and single culture systems; (g) Microscopic observation of hair growth in newborn skin area of nude mice; (h) Distribution of mature hair follicles in co-culture group. VEGF: vascular endothelial growth factor; HIF-1α: hypoxia-inducible factor 1-α; KDR: kinase insert domain receptor; VE-cad: Vascular endothelial cadherin; PDGF-α: platelet-derived growth factor receptor α; PDGF-β: platelet-derived growth factor receptor β; c-Myc: cellular myelocytomatosis oncogene; *, ** and *** indicate p<0.05, 0.01 and 0.001, respectively
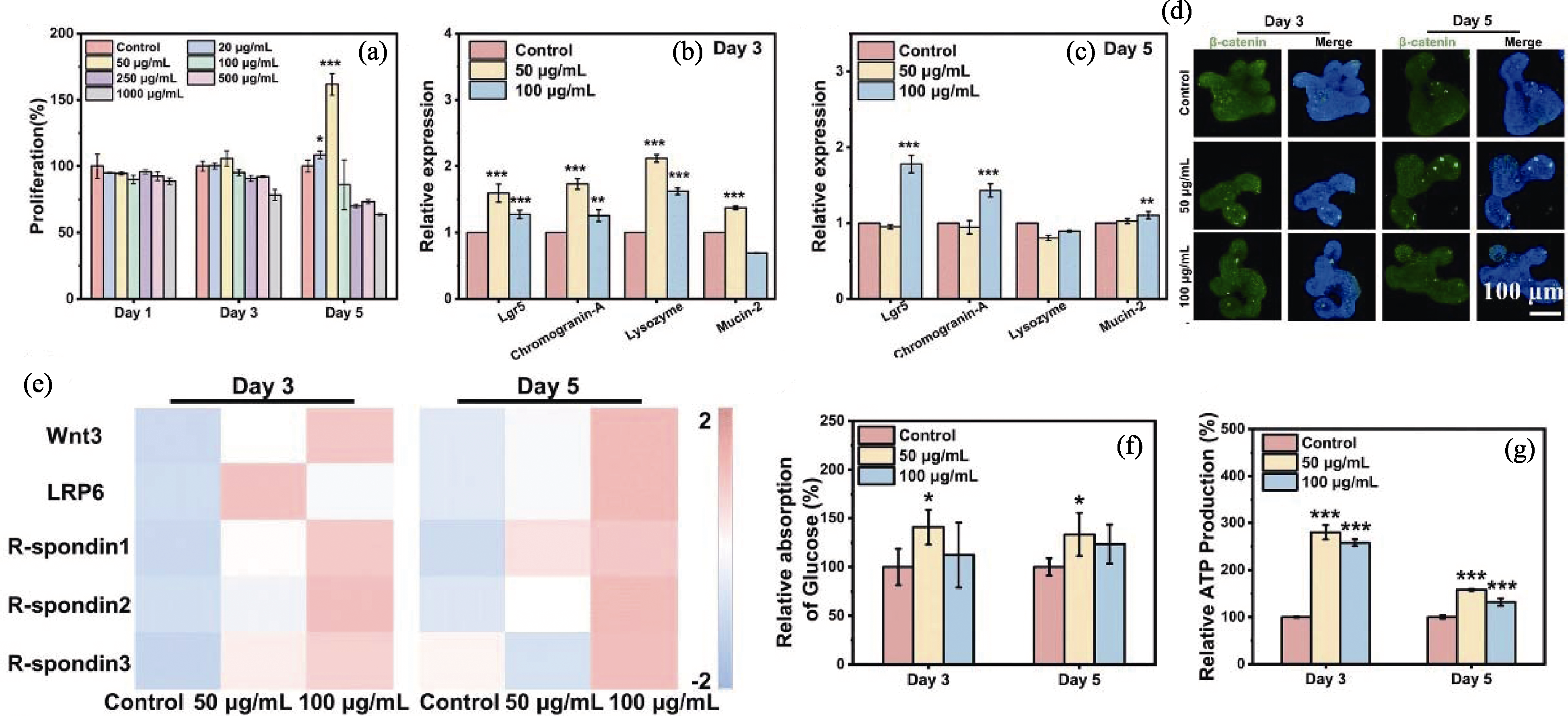
图4 CS纳米线释放的生物活性离子用于促进肠道类器官发育[47]
Fig. 4 Bioactive ions released by CS nanowires for promoting intestinal organoid development[47] (a) Proliferation of intestinal organoids treated with CS nanowires of different concentrations in culture medium; (b, c) Gene expression of four differentiated cell types treated with 0, 50 and 100 μg/mL CS nanowires for 3 (b) and 5 days (c); (d) Immunofluorescence staining of β-catenin in intestinal organoids treated with CS nanowires for 3 and 5 days; (e) Heat maps of gene expression related to Wnt/β-catenin signaling pathway in intestinal organoids after 3 and 5 days of CS nanowire treatment; (f) Relative glucose absorption of different groups; (g) Relative ATP production of different groups. Wnt3: Wingless-type MMTV integration site family, member 3; LRP6: Low-density lipoprotein receptor-related protein 6; *, ** and *** indicate p<0.05, 0.01 and 0.001, respectively

图5 CNT促进肠道类器官发育[61]
Fig. 5 CNT for promoting intestinal organoid development[61] (a) Typical images of epithelial subpopulations in intestinal organoids treated with CNT (50 μg/mL) at different time periods; (b) Representative SEM images showing degradation of the matrix over time during development of intestinal organoids; (c) Statistical analysis of changes over time in phosphorylated p38 mitogen activated protein kinase (P-p38 MAPK), p38 mitogen activated protein kinase (p38 MAPK), yes-related protein (YAP), and phosphorylated yes-related protein (P-YAP) in intestinal organoids treated with CNT (50 μg/mL); (d) Quantification of YAP nuclear translocation over time in intestinal organoids treated with CNT (50 μg/mL); (e) Representative confocal images of mitochondrial membrane potential and relative mitochondrial activity in intestinal organoids over time; (f) Effects of different amino acids on the proliferation and differentiation of intestinal organoids; (g) Proposed mechanism of CNT induced intestinal organoid development. SWCNTs: single-walled carbon nanotubes; MWCNTs: multiwalled carbon nanotubes; L-FABP: liver fatty acid-binding protein; ISC: intestinal stem cell; *, ** and *** indicate p<0.05, 0.01 and 0.001, respectively
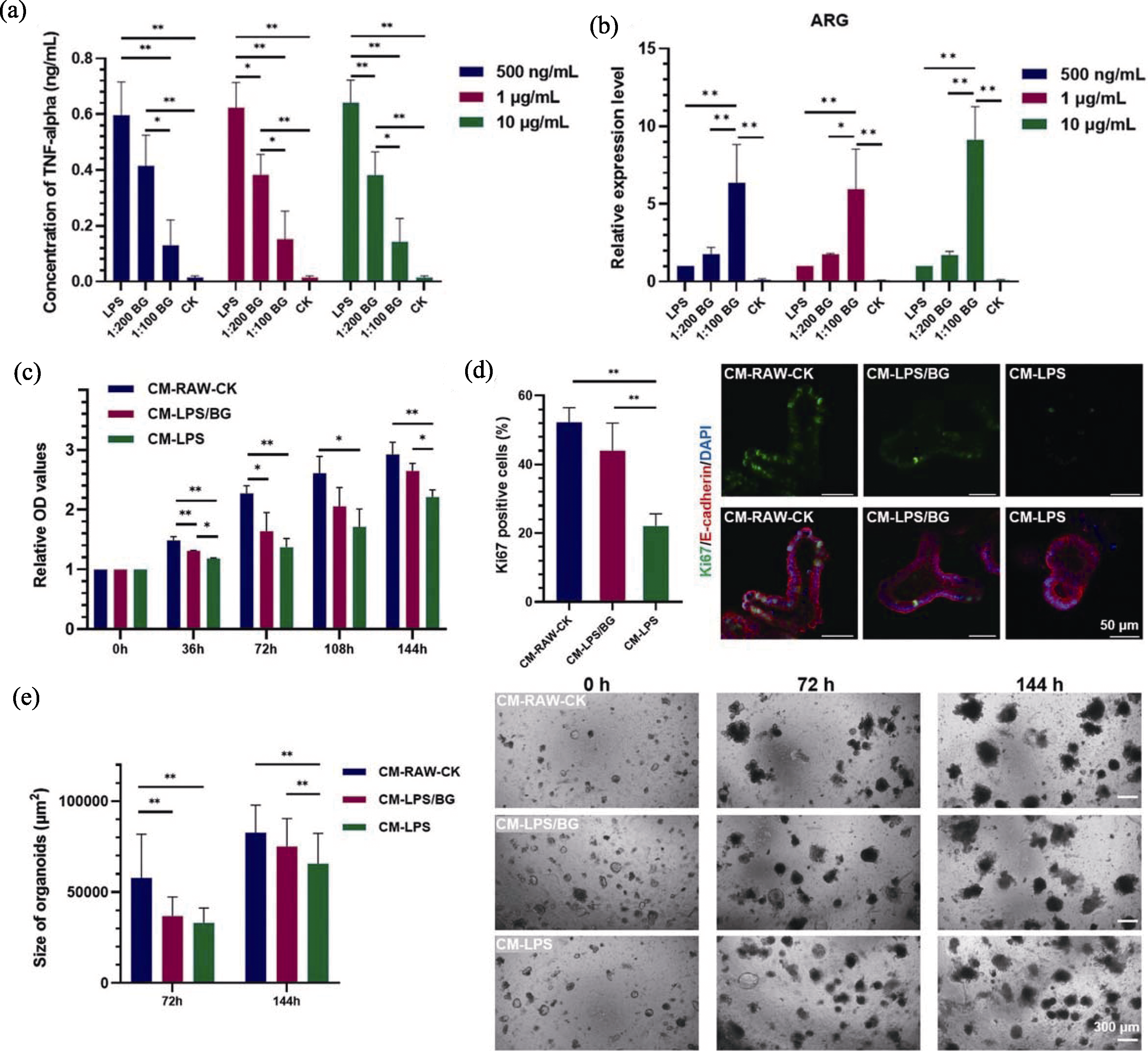
图6 肠道类器官在探究口服生物玻璃微球减少肠道炎症损伤中的应用[71]
Fig. 6 Application of organoids in exploring reduction of intestinal inflammatory damage by bioactive glass microspheres[71] (a) Concentration of TNF-α in macrophage culture medium after different treatments; (b) Expression of anti-inflammatory gene ARG in macrophage culture after different treatments; (c) CCK-8 testing of intestinal organoids cultured under different media conditions; (d) Immunofluorescence staining of Ki67 in intestinal organoids cultured under different conditions for 144 h; (e) Morphology and size of intestinal organoids cultured under different conditions for 72 and 144 h. BG: bioactive glass; LPS: lipopolysaccharide; CK: non-treated control; ARG: arginase; Conditioned media were collected from untreated RAW “CM-RAW-CK”, LPS-activated RAW “CM-LPS”, and LPS-activated RAW after pretreatment of BG extract liquids (1 : 100 dilution ratio) “CM-LPS/BG”; *, ** and *** indicate p<0.05, 0.01 and 0.001, respectively
| [1] | HOFER M, LUTOLF M P. Engineering organoids. Nature Reviews Materials, 2021, 6(5): 402. |
| [2] |
KRATOCHVIL M J, SEYMOUR A J, LI T L, et al. Engineered materials for organoid systems. Nature Reviews Materials, 2019, 4(9): 606.
DOI |
| [3] | SATO T, VRIES R G, SNIPPERT H J, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 2009, 459(7244): 262. |
| [4] | BARKER N, HUCH M, KUJALA P, et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell, 2010, 6(1): 25. |
| [5] | CHO A N, JIN Y, AN Y, et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nature Communications, 2021, 12: 4730. |
| [6] |
MORIZANE R, LAM A Q, FREEDMAN B S, et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nature Biotechnology, 2015, 33(11): 1193.
PMID |
| [7] | TAO T T, DENG P W, WANG Y Q, et al. Microengineered multi-organoid system from hiPSCs to recapitulate human liver-islet axis in normal and type 2 diabetes. Advanced Science, 2022, 9(5): 2103495. |
| [8] | LEWIS-ISRAELI Y R, WASSERMAN A H, GABALSKI M A, et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nature Communications, 2021, 12: 5142. |
| [9] |
RICHARDS D J, LI Y, KERR C M, et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nature Biomedical Engineering, 2020, 4(4): 446.
DOI PMID |
| [10] |
DUTTA D, HEO I, CLEVERS H. Disease modeling in stem cell-derived 3D organoid systems. Trends in Molecular Medicine, 2017, 23(5): 393.
DOI PMID |
| [11] | CRUZ-ACUÑA R, QUIRÓS M, FARKAS A E, et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nature Cell Biology, 2017, 19(11): 1326. |
| [12] |
VLACHOGIANNIS G, HEDAYAT S, VATSIOU A, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science, 2018, 359(6378): 920.
DOI PMID |
| [13] | BROKESH A M, GAHARWAR A K.Inorganic biomaterials for regenerative medicine. ACS Applied Materials & Interfaces, 2020, 12(5): 5319. |
| [14] | ERMAKOV A, DAKS A, FEDOROVA O, et al. Ca2+-depended signaling pathways regulate self-renewal and pluripotency of stem cells. Cell Biology International, 2018, 42(9): 1086. |
| [15] | TAWFIK I, GOTTSCHALK B, JARC A, et al. T3-induced enhancement of mitochondrial Ca2+ uptake as a boost for mitochondrial metabolism. Free Radical Biology and Medicine, 2022, 181: 197. |
| [16] |
HAN P P, WU C T, XIAO Y. The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomaterials Science, 2013, 1(4): 379.
DOI PMID |
| [17] |
AISENBREY E A, MURPHY W L. Synthetic alternatives to matrigel. Nature Reviews Materials, 2020, 5(7): 539.
DOI PMID |
| [18] |
ROSSI G, MANFRIN A, LUTOLF M P. Progress and potential in organoid research. Nature Reviews Genetics, 2018, 19(11): 671.
DOI PMID |
| [19] |
ROOKMAAKER M B, SCHUTGENS F, VERHAAR M C, et al. Development and application of human adult stem or progenitor cell organoids. Nature Reviews Nephrology, 2015, 11(9): 546.
DOI PMID |
| [20] | LUKONIN I, SERRA D, MEYLAN L C, et al. Phenotypic landscape of intestinal organoid regeneration. Nature, 2020, 586(7828): 275. |
| [21] | BOUFFI C, WIKENHEISER-BROKAMP K A, CHATURVEDI P, et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nature Biotechnology, 2023, 41(6): 824. |
| [22] | SORRENTINO G, REZAKHANI S, YILDIZ E, et al. Mechano- modulatory synthetic niches for liver organoid derivation. Nature Communications, 2020, 11: 3416. |
| [23] |
CLEVERS H. Modeling development and disease with organoids. Cell, 2016, 165(7): 1586.
DOI PMID |
| [24] | BARTFELD S, BAYRAM T, VAN DE WETERING M,et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology, 2015, 148(1): 126. |
| [25] | XU J S, PATTON D, JACKSON S K, et al. In-vitro maintenance and functionality of primary renal tubules and their application in the study of relative renal toxicity of nephrotoxic drugs. Journal of Pharmacological and Toxicological Methods, 2013, 68(2): 269. |
| [26] |
YU J Y, VODYANIK M A, SMUGA-OTTO K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science, 2007, 318(5858): 1917.
DOI PMID |
| [27] | LEE J, SUTANI A, KANEKO R, et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nature Communications, 2020, 11: 4283. |
| [28] |
ROMITTI M, TOURNEUR A, DA FONSECA B D F,et al. Transplantable human thyroid organoids generated from embryonic stem cells to rescue hypothyroidism. Nature Communications, 2022, 13: 7057.
DOI PMID |
| [29] |
WANG S Y, WANG X, TAN Z L, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Research, 2019, 29(12): 1009.
DOI PMID |
| [30] |
TAKAHASHI K, TANABE K, OHNUKI M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007, 131(5): 861.
DOI PMID |
| [31] |
DRAKHLIS L, BISWANATH S, FARR C M, et al. Human heart-forming organoids recapitulate early heart and foregut development. Nature Biotechnology, 2021, 39(6): 737.
DOI PMID |
| [32] | FRENZ-WIESSNER S, FAIRLEY S D, BUSER M, et al. Generation of complex bone marrow organoids from human induced pluripotent stem cells. Nature Methods, 2024, 21(5): 868. |
| [33] | YOON S J, ELAHI L S, PAȘCA A M, et al. Reliability of human cortical organoid generation. Nature Methods, 2019, 16(1): 75. |
| [34] | GIGER S, HOFER M, MILJKOVIC-LICINA M, et al. Microarrayed human bone marrow organoids for modeling blood stem cell dynamics. APL Bioengineering, 2022, 6(3): 036101. |
| [35] |
PRONDZYNSKI M, BERKSON P, TREMBLEY M A, et al. Efficient and reproducible generation of human iPSC-derived cardiomyocytes and cardiac organoids in stirred suspension systems. Nature Communications, 2024, 15: 5929.
DOI PMID |
| [36] | BERGENHEIM F, FREGNI G, BUCHANAN C F, et al. A fully defined 3D matrix for ex vivo expansion of human colonic organoids from biopsy tissue. Biomaterials, 2020, 262: 120248. |
| [37] |
HUCH M, KNOBLICH J A, LUTOLF M P, et al. The hope and the hype of organoid research. Development, 2017, 144(6): 938.
DOI PMID |
| [38] | LIU H T, WANG Y Q, CUI K L, et al. Advances in hydrogels in organoids and organs-on-a-chip. Advanced Materials, 2019, 31(50): 1902042. |
| [39] | POLING H M, WU D, BROWN N, et al. Mechanically induced development and maturation of human intestinal organoids in vivo. Nature Biomedical Engineering, 2018, 2(6): 429. |
| [40] | HOMAN K A, GUPTA N, KROLL K T, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nature Methods, 2019, 16(3): 255. |
| [41] |
DE BOER J, SIDDAPPA R, GASPAR C, et al. Wnt signaling inhibits osteogenic differentiation of human mesenchymal stem cells. Bone, 2004, 34(5): 818.
PMID |
| [42] | WEI P F, JING W, YUAN Z Y, et al. Vancomycin- and strontium-loaded microspheres with multifunctional activities against bacteria, in angiogenesis, and in osteogenesis for enhancing infected bone regeneration. ACS Applied Materials & Interfaces, 2019, 11(34): 30596. |
| [43] | CHEN L, DENG C J, LI J Y, et al. 3D printing of a lithium- calcium-silicate crystal bioscaffold with dual bioactivities for osteochondral interface reconstruction. Biomaterials, 2019, 196: 138. |
| [44] | WANG S, HASHEMI S, STRATTON S, et al. The effect of physical cues of biomaterial scaffolds on stem cell behavior. Advanced Healthcare Materials, 2021, 10(3): 2001244. |
| [45] | WANG P Y, CLEMENTS L R, THISSEN H, et al. Screening mesenchymal stem cell attachment and differentiation on porous silicon gradients. Advanced Functional Materials, 2012, 22(16): 3414. |
| [46] | GJOREVSKI N, SACHS N, MANFRIN A, et al. Designer matrices for intestinal stem cell and organoid culture. Nature, 2016, 539(7630): 560. |
| [47] |
MA W P, ZHENG Y, YANG G Z, et al. A bioactive calcium silicate nanowire-containing hydrogel for organoid formation and functionalization. Materials Horizons, 2024, 11(12): 2957.
DOI PMID |
| [48] | DAS S R, UZ M, DING S W, et al. Electrical differentiation of mesenchymal stem cells into schwann-cell-like phenotypes using inkjet-printed graphene circuits. Advanced Healthcare Materials, 2017, 6(7): 1601087. |
| [49] | SHENG R Y Y, MU J, CHERNOZEM R V, et al. Fabrication and characterization of piezoelectric polymer composites and cytocompatibility with mesenchymal stem cells. ACS Applied Materials & Interfaces, 2023, 15(3): 3731. |
| [50] | LIU Z R, WAN X Y, WANG Z L, et al. Electroactive biomaterials and systems for cell fate determination and tissue regeneration: design and applications. Advanced Materials, 2021, 33(32): 2007429. |
| [51] | ZHANG H J, QIN C, SHI Z, et al. Bioprinting of inorganic- biomaterial/neural-stem-cell constructs for multiple tissue regeneration and functional recovery. National Science Review, 2024, 11(4): nwae035. |
| [52] | DU L, QIN C, ZHANG H J, et al. Multicellular bioprinting of biomimetic inks for tendon-to-bone regeneration. Advanced Science, 2023, 10(21): 2301309. |
| [53] | HE D, LI H Y. Biomaterials affect cell-cell interactions in vitro in tissue engineering. Journal of Materials Science & Technology, 2021, 63: 62. |
| [54] |
GAUTAM V, NAUREEN S, SHAHID N, et al. Engineering highly interconnected neuronal networks on nanowire scaffolds. Nano Letters, 2017, 17(6): 3369.
DOI PMID |
| [55] |
MA J G, QIN C, WU J F, et al. 3D multicellular micropatterning biomaterials for hair regeneration and vascularization. Materials Horizons, 2023, 10(9): 3773.
DOI PMID |
| [56] | WANG X Y, GAO L, HAN Y, et al. Silicon-enhanced adipogenesis and angiogenesis for vascularized adipose tissue engineering. Advanced Science, 2018, 5(11): 1800776. |
| [57] |
GAO C D, PENG S P, FENG P, et al. Bone biomaterials and interactions with stem cells. Bone Research, 2017, 5: 17059.
DOI PMID |
| [58] | LUO J J, WALKER M, XIAO Y B, et al. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix-a review. Bioactive Materials, 2022, 15: 145. |
| [59] | CHEN W C, TIAN B X, LIANG J Q, et al. Matrix stiffness regulates the interactions between endothelial cells and monocytes. Biomaterials, 2019, 221: 119362. |
| [60] | ZHANG Z, GAO S, HU Y N, et al. Ti3C2TxMXene composite 3D hydrogel potentiates mTOR signaling to promote the generation of functional hair cells in cochlea organoids. Advanced Science, 2022, 9(32): 2203557. |
| [61] |
BAO L, CUI X J, WANG X Y, et al. Carbon nanotubes promote the development of intestinal organoids through regulating extracellular matrix viscoelasticity and intracellular energy metabolism. ACS Nano, 2021, 15(10): 15858.
DOI PMID |
| [62] | WANG J, WU Y, LI G F, et al. Engineering large-scale self-mineralizing bone organoids with bone matrix-inspired hydroxyapatite hybrid bioinks. Advanced Materials, 2024, 36(30): 2309875. |
| [63] | TAN Y, COYLE R C, BARRS R W, et al. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Science Advances, 2023, 9(31): eadf2898. |
| [64] | TIAN M, WEI J S, LV E G, et al. Drug evaluation platform based on non-destructive and real-time in situ organoid fate state monitoring by graphene field-effect transistor. Chemical Engineering Journal, 2024, 498: 155355. |
| [65] |
ROTH J G, BRUNEL L G, HUANG M S, et al. Spatially controlled construction of assembloids using bioprinting. Nature Communications, 2023, 14: 4346.
DOI PMID |
| [66] |
POON W, ZHANG Y N, OUYANG B, et al. Elimination pathways of nanoparticles. ACS Nano, 2019, 13(5): 5785.
DOI PMID |
| [67] |
ZHANG R, LI D, ZHAO R B, et al. Spike structure of gold nanobranches induces hepatotoxicity in mouse hepatocyte organoid models. Journal of Nanobiotechnology, 2024, 22(1): 92.
DOI PMID |
| [68] |
BAEK A, KWON I H, LEE D H, et al. Novel organoid culture system for improved safety assessment of nanomaterials. Nano Letters, 2024, 24(3): 805.
DOI PMID |
| [69] | WEISS P, TAYLOR A C. Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation. Proceedings of the National Academy of Scinences of the United States of America, 1960, 46(9): 1177. |
| [70] | MA P Q, CHEN Y, LAI X Y, et al. The translational application of hydrogel for organoid technology: challenges and future perspectives. Macromolecular Bioscience, 2021, 21(10): 2100191. |
| [71] | ZHU Y L, WANG Y W, XIA G G, et al. Oral delivery of bioactive glass-loaded core-shell hydrogel microspheres for effective treatment of inflammatory bowel disease. Advanced Science, 2023, 10(18): 2207418. |
| [72] | ZHANG H, LU Y, ZHANG R, et al. Synthesis of multifunctional plasmonic nanodarts through one-end deposition on gold nanobipyramids for tumor organoid ablation and antimicrobial applications. Advanced Functional Materials, 2024, 34(44): 2405588. |
| [73] | ISSA R, LOZANO N, KOSTARELOS K, et al. Functioning human lung organoids model pulmonary tissue response from carbon nanomaterial exposures. Nano Today, 2024, 56: 102254. |
| [74] |
HEID S, BOCCACCINI A R. Advancing bioinks for 3D bioprinting using reactive fillers: a review. Acta Biomaterialia, 2020, 113: 1.
DOI PMID |
| [75] | LI R T, LIU K, HUANG X, et al. Bioactive materials promote wound healing through modulation of cell behaviors. Advanced Science, 2022, 9(10): 2105152. |
| [76] |
THUNDYIL J, PAVLOVSKI D, SOBEY C G, et al. Adiponectin receptor signalling in the brain. British Journal of Pharmacology, 2012, 165(2): 313.
DOI PMID |
| [77] | PUSCHHOF J, PLEGUEZUELOS-MANZANO C, CLEVERS H. Organoids and organs-on-chips: insights into human gut-microbe interactions. Cell Host & Microbe, 2021, 29(6): 867. |
| [1] | 余升阳, 苏海军, 姜浩, 余明辉, 姚佳彤, 杨培鑫. 激光增材制造超高温氧化物陶瓷孔隙缺陷形成及抑制研究进展[J]. 无机材料学报, 2025, 40(9): 944-956. |
| [2] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [3] | 肖晓琳, 王玉祥, 谷佩洋, 朱圳荣, 孙勇. 二维无机材料调控病损皮肤组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 860-870. |
| [4] | 马景阁, 吴成铁. 无机生物材料用于毛囊和毛发再生的研究[J]. 无机材料学报, 2025, 40(8): 901-910. |
| [5] | 张洪健, 赵梓壹, 吴成铁. 无机生物材料调控神经细胞功能及神经化组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 849-859. |
| [6] | 艾敏慧, 雷波. 微纳米生物活性玻璃: 功能化设计与血管化皮肤再生[J]. 无机材料学报, 2025, 40(8): 921-932. |
| [7] | 王宇彤, 常江, 徐合, 吴成铁. 硅酸盐生物陶瓷/玻璃促创面修复的研究进展:作用、机制和应用方式[J]. 无机材料学报, 2025, 40(8): 911-920. |
| [8] | 罗晓民, 乔志龙, 刘颍, 杨晨, 常江. 无机生物活性材料调控心肌再生的研究进展[J]. 无机材料学报, 2025, 40(8): 871-887. |
| [9] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [10] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [11] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [12] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [13] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [14] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [15] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||