无机材料学报 ›› 2025, Vol. 40 ›› Issue (8): 849-859.DOI: 10.15541/jim20250001 CSTR: 32189.14.jim20250001
收稿日期:2025-01-02
修回日期:2025-02-07
出版日期:2025-08-20
网络出版日期:2025-02-19
通讯作者:
吴成铁, 研究员. E-mail: chengtiewu@mail.sic.ac.cn作者简介:张洪健(1996-), 男, 博士. E-mail: zhanghongjian@mail.sic.ac.cn
基金资助:
ZHANG Hongjian1( ), ZHAO Ziyi1,2, WU Chengtie1,2(
), ZHAO Ziyi1,2, WU Chengtie1,2( )
)
Received:2025-01-02
Revised:2025-02-07
Published:2025-08-20
Online:2025-02-19
Contact:
WU Chengtie, professor. E-mail: chengtiewu@mail.sic.ac.cnAbout author:ZHANG Hongjian (1996-), male, PhD. E-mail: zhanghongjian@mail.sic.ac.cn
Supported by:摘要:
基于神经在组织再生中的关键作用, 开发具有神经诱导活性的组织工程支架引起了研究者们的广泛关注。近年来, 无机生物材料因具有高度可控的化学组成、微/纳拓扑结构及优异的理化性能, 在调控神经细胞功能及神经化组织再生中得到广泛应用。本文首先介绍了常用于神经调控的无机生物材料, 主要包括生物陶瓷材料和电活性材料, 接着阐述了无机生物材料通过调控细胞行为、调节免疫微环境和构建电活性微环境等途径对神经细胞活性及生物学功能的增强作用, 重点阐述了无机生物材料在脊髓、周围神经、皮肤、骨骼肌、海绵体等组织神经化再生中的最新研究进展, 最后讨论了无机生物材料在调控神经细胞活性及神经化组织再生中存在的难题及未来发展前景。
中图分类号:
张洪健, 赵梓壹, 吴成铁. 无机生物材料调控神经细胞功能及神经化组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 849-859.
ZHANG Hongjian, ZHAO Ziyi, WU Chengtie. Inorganic Biomaterials on Regulating Neural Cell Function and Innervated Tissue Regeneration: A Review[J]. Journal of Inorganic Materials, 2025, 40(8): 849-859.
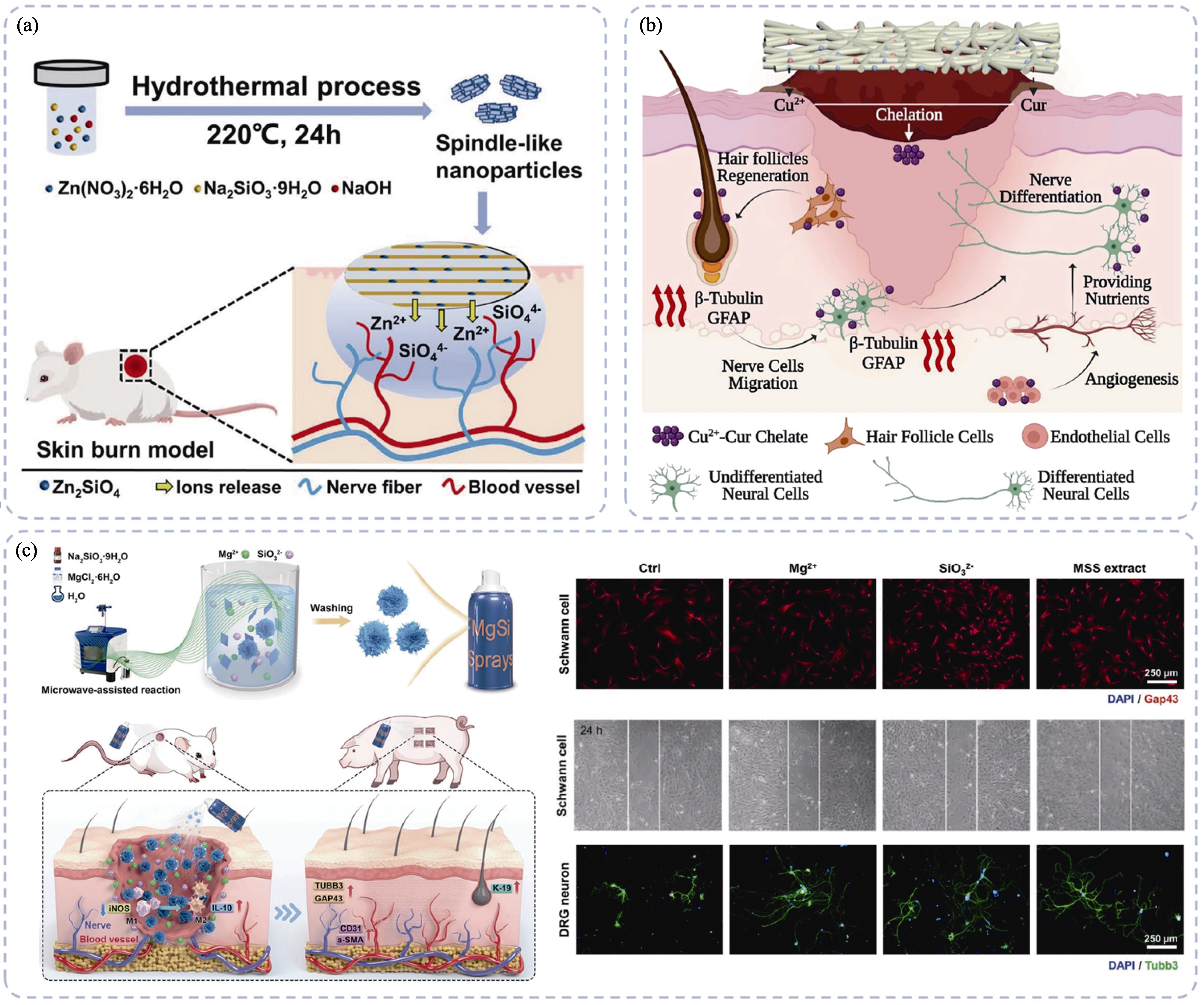
图1 无机生物材料通过增强细胞功能促进神经化皮肤再生[57-58,60]
Fig. 1 Inorganic biomaterials promoting innervated skin regeneration through enhancing cellular functions[57-58,60] (a) Spindle zinc silicate nanoparticles promoting neuro-vascularized wound healing via releasing bioactive Zn and Si ions[57]; (b) Cu-CS containing wound dressing promoting cutaneous neural networks reconstructions and wound healing[58]; (c) Sprayable magnesium silicate particles containing hydrogel promoting vascularized and innervated skin regeneration[60]. Colorful figures are available on website
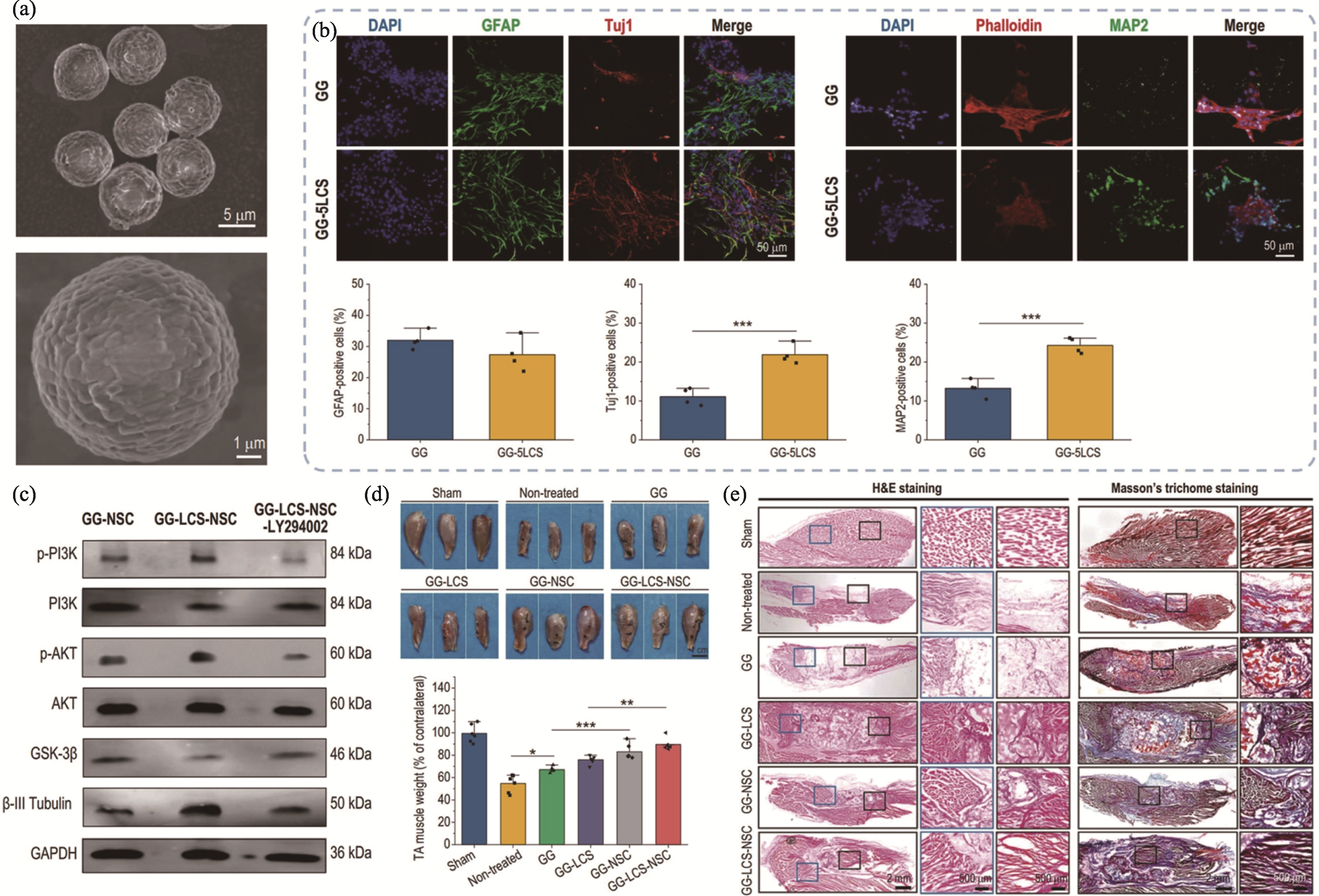
图2 生物3D打印锂-钙-硅生物陶瓷神经构建体用于神经化组织再生[62]
Fig. 2 3D bioprinted Li-Ca-Si bioceramics containing neural constructs for innervated tissue regeneration[62] (a) SEM images of the Li-Ca-Si bioceramic microspheres; (b) Immunofluorescence images and semi-quantitative analysis of Li-Ca-Si bioceramic microspheres inducing the neuronal differentiation and maturation of neural stem cells (NSCs); (c) Li-Ca-Si bioceramic microspheres inducing the neuronal differentiation and maturation of NSCs through activating PI3K-AKT signal pathway; (d) Optical images and their weight of the regenerated muscles; (e) Representative images of H&E and Masson staining assays. Colorful figures are available on website
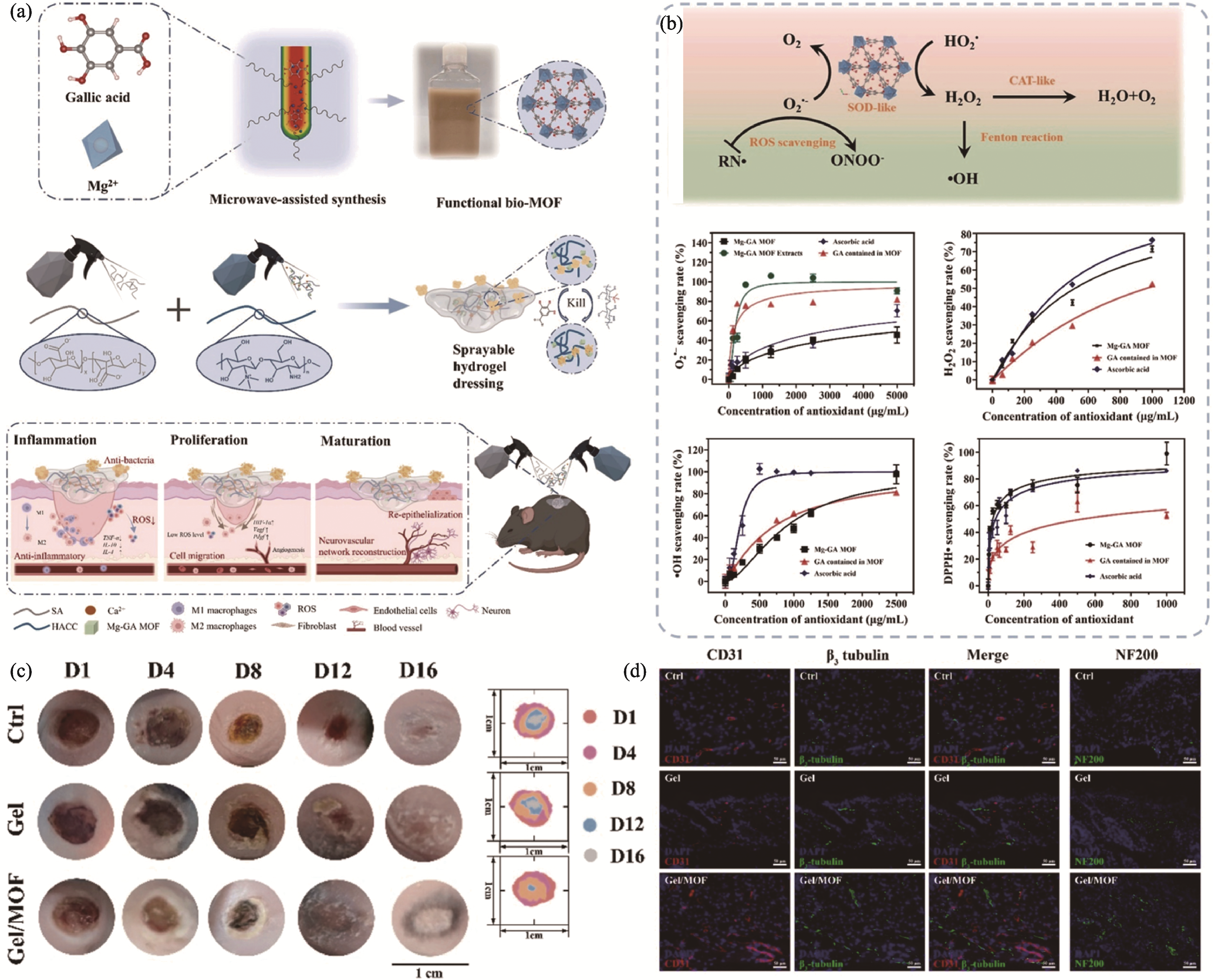
图3 可喷涂镁-没食子酸酯金属有机框架颗粒复合水凝胶通过免疫调节促进神经血管化糖尿病创面再生[67]
Fig. 3 Sprayable Mg-gallate metal-organic framework-based hydrogel promoting innervated and vascularized wound healing through immune-modulatory approaches[67] (a) Schematic illustrations of synthesis and application of sprayable hydrogel; (b) Enzyme-catalytic behaviors of hydrogel to scavenging ROS; (c) In vivo representative images of the wounds; (d) Representative immunofluorescent images of the regenerated nerves and vessels in the wound area. Colorful figures are available on website
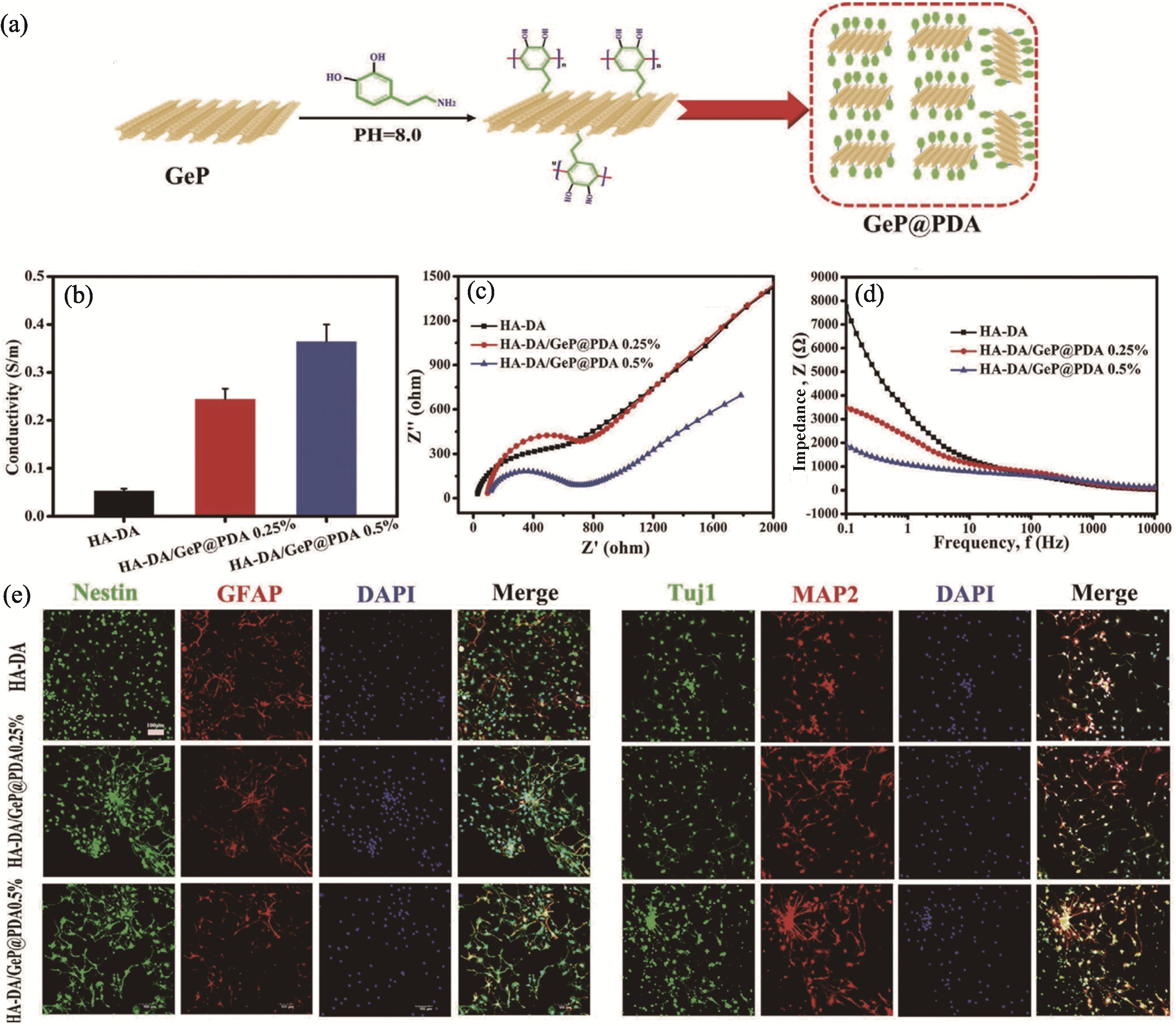
图4 含磷化镉纳米片导电水凝胶诱导神经干细胞分化[69]
Fig. 4 GeP nanosheet-based conductive hydrogel inducing the neuronal differentiation of NSCs[69] (a) Preparation of GeP@PDA nanosheets; (b-d) Conductivity (b), Nyquist curves (c) and impedance spectra (d) of hydrogel; (e) Representative immunostaining images of NSCs under different treatments. Colorful figures are available on website
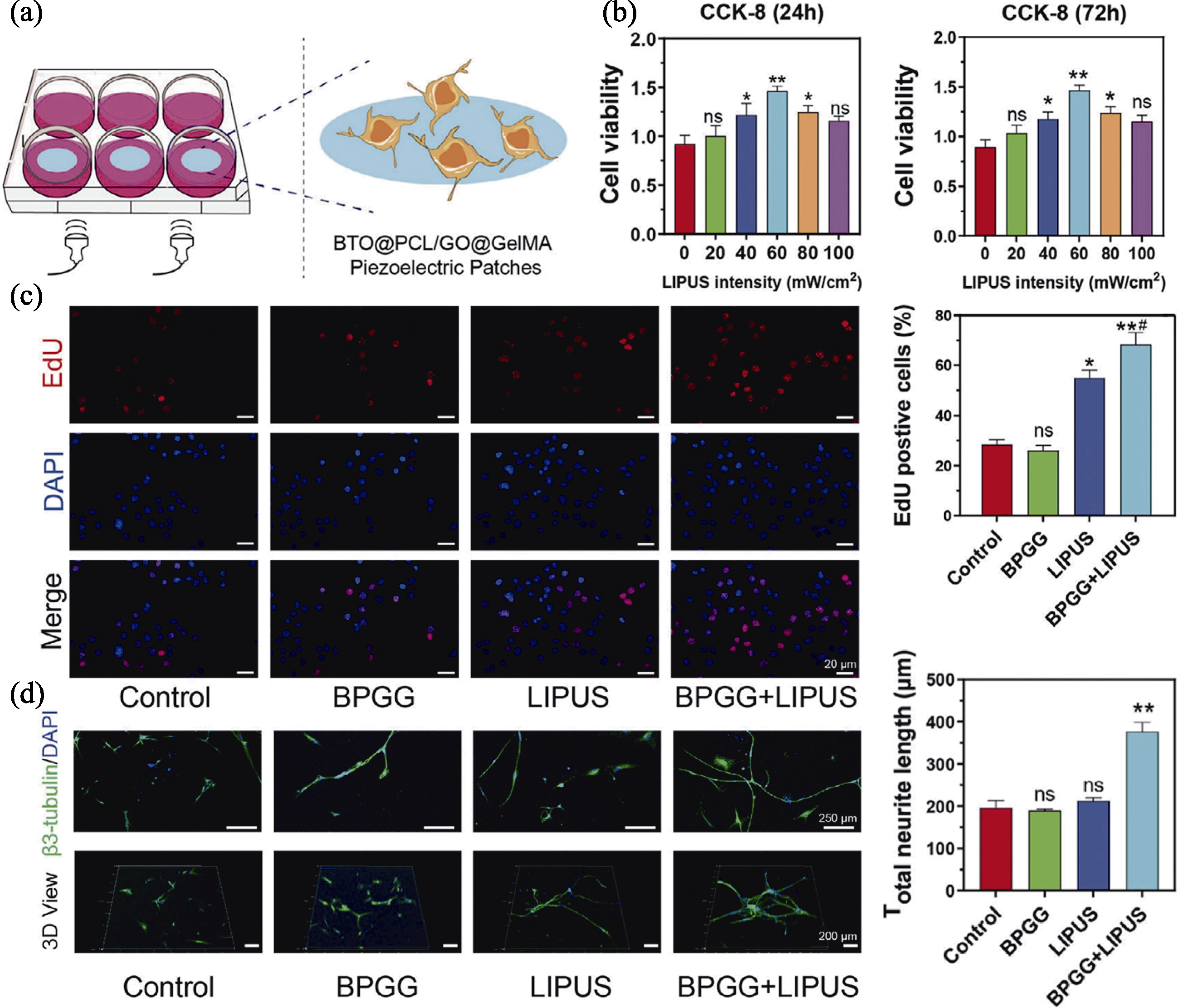
图5 超声触发无线电刺激纳米贴片促进神经分化[75]
Fig. 5 Ultrasound-triggered wireless electrical stimulation nanopatch promoting neural differentiation[75] (a) Scheme of the nanopatch of stimulating neural cells; (b) Cell viability of nanopatch under different LIPUS stimulation intensities; (c) EdU fluorescence images of cells treated with different groups and corresponding statistical analysis; (d) Representative immunostaining images of β3-tubulin of neurons and corresponding statistical analysis of neurite length. Colorful figures are available on website
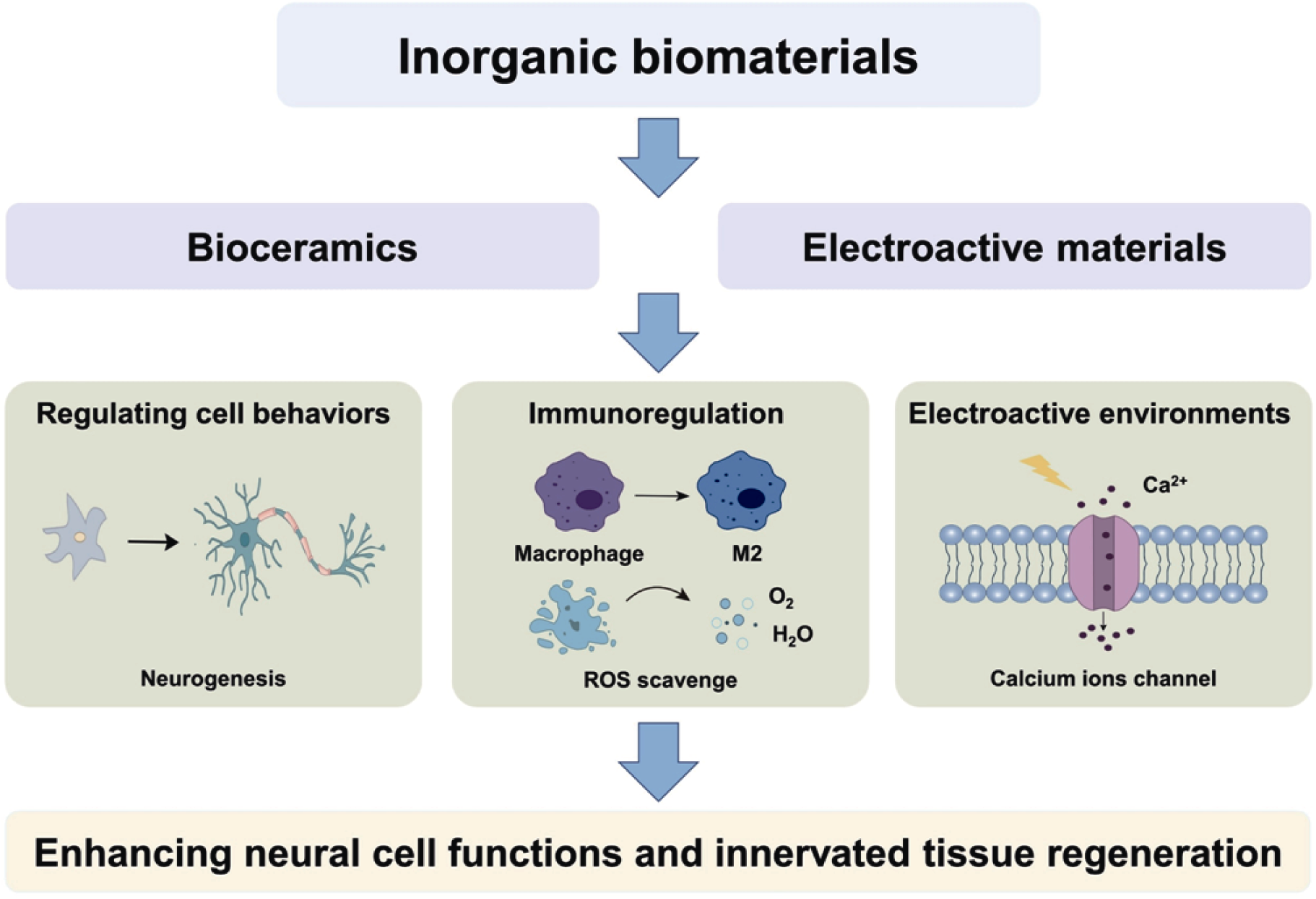
图6 无机生物材料通过调控细胞行为、调节免疫微环境、构建电活性微环境等途径增强神经细胞功能及神经化组织再生
Fig. 6 Inorganic biomaterials enhancing neural cell functions and innervated tissue regeneration through modulating cellular behaviors, regulating immune microenvironment and building electroactive microenvironment
| [1] | WAN Q Q, QIN W P, MA Y X, et al. Crosstalk between bone and nerves within bone. Advanced Science, 2021, 8(7): 2003390. |
| [2] | WANG X D, LI S Y, ZHANG S J, et al. The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach. Theranostics, 2020, 10(11): 4839. |
| [3] |
ELEFTERIOU F. Impact of the autonomic nervous system on the skeleton. Physiological Reviews, 2018, 98(3): 1083.
DOI PMID |
| [4] | BROKESH A M, GAHARWAR A K. Inorganic biomaterials for regenerative medicine. ACS Applied Materials & Interfaces, 2020, 12(5): 5319. |
| [5] |
PEI Z, LEI H, CHENG L. Bioactive inorganic nanomaterials for cancer theranostics. Chemical Society Reviews, 2023, 52(6): 2031.
DOI PMID |
| [6] | ZOU Y, HUANG B, CAO L, et al. Tailored mesoporous inorganic biomaterials: assembly, functionalization, and drug delivery engineering. Advanced Materials, 2021, 33(2): 2005215. |
| [7] | QIN C, WU C. Inorganic biomaterials-based bioinks for three-dimensional bioprinting of regenerative scaffolds. VIEW, 2022, 3(4): 20210018. |
| [8] |
ZHAO C, LIU W, ZHU M, et al. Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: a review. Bioactive Materials, 2022, 18: 383.
DOI PMID |
| [9] | ZHOU Y, WU C, CHANG J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Materials Today, 2019, 24: 41. |
| [10] | GOU Y, QI K, WEI Y, et al. Advances of calcium phosphate nanoceramics for the osteoinductive potential and mechanistic pathways in maxillofacial bone defect repair. Nano TransMed, 2024, 3: 100033. |
| [11] | ZHANG Y, XU J, RUAN Y C, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone- fracture healing in rats. Nature Medicine, 2016, 22(10): 1160. |
| [12] | YANG Y, WANG H, YANG H, et al. Magnesium-based whitlockite bone mineral promotes neural and osteogenic activities. ACS Biomaterials Science & Engineering, 2020, 6(10): 5785. |
| [13] |
XU Y, XU C, HE L, et al. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioactive Materials, 2022, 16: 271.
DOI PMID |
| [14] | MA Y X, JIAO K, WAN Q Q, et al. Silicified collagen scaffold induces semaphorin 3A secretion by sensory nerves to improve in-situ bone regeneration. Bioactive Materials, 2022, 9: 475. |
| [15] | MEI P, JIANG S, MAO L, et al. In situ construction of flower-like nanostructured calcium silicate bioceramics for enhancing bone regeneration mediated via FAK/p38 signaling pathway. Journal of Nanobiotechnology, 2022, 20(1): 162. |
| [16] | LI T, ZHAI D, MA B, et al. 3D printing of hot dog-like biomaterials with hierarchical architecture and distinct bioactivity. Advanced Science, 2019, 6(19): 1901146. |
| [17] | MA W P, YANG Z B, LU M X, et al. Hierarchically structured biomaterials for tissue regeneration. Microstructures, 2024, 4(2): 2024014. |
| [18] | YANG Z, XUE J, SHI Z, et al. Naturally derived flexible bioceramics: biomass recycling approach and advanced function. Matter, 2024, 7(3): 1275. |
| [19] | LIU Z, WAN X, WANG Z L, et al. Electroactive biomaterials and systems for cell fate determination and tissue regeneration: design and applications. Advanced Materials, 2021, 33(32): 2007429. |
| [20] | QIAN Y, LIN H, YAN Z, et al. Functional nanomaterials in peripheral nerve regeneration: scaffold design, chemical principles and microenvironmental remodeling. Materials Today, 2021, 51: 165. |
| [21] |
ZHANG M, ZHAI X, MA T, et al. Sequential therapy for bone regeneration by cerium oxide-reinforced 3D-printed bioactive glass scaffolds. ACS Nano, 2023, 17(5): 4433.
DOI PMID |
| [22] | KIM J W, MAHAPATRA C, HONG J Y, et al. Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Advanced Science, 2017, 4(10): 1700034. |
| [23] |
ZHENG Y, WU J, ZHU Y, et al. Inorganic-based biomaterials for rapid hemostasis and wound healing. Chemical Science, 2022, 14(1): 29.
DOI PMID |
| [24] | MA J, WU C. Bioactive inorganic particles-based biomaterials for skin tissue engineering. Exploration, 2022, 2(5): 20210083. |
| [25] | HAN D, LIU X, WU S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chemical Society Reviews, 2022, 51(16): 7138. |
| [26] | 张兴栋, WILLIAMS D. 二十一世纪生物材料定义. 北京: 科学出版社, 2021: 25. |
| [27] | WU C T, CHANG J. Silicate bioceramics for bone tissue regeneration. Journal of Inorganic Materials, 2013, 28(1): 29. |
| [28] | WU H, HUA Y, WU J, et al. The morphology of hydroxyapatite nanoparticles regulates clathrin-mediated endocytosis in melanoma cells and resultant anti-tumor efficiency. Nano Research, 2022, 15(7): 6256. |
| [29] | ZHU D, LU B, YANG Q, et al. Lanthanum-doped mesoporous bioglasses/chitosan composite scaffolds enhance synchronous osteogenesis and angiogenesis for augmented osseous regeneration. Chemical Engineering Journal, 2021, 405: 127077. |
| [30] |
GUPTA B, PAPKE J B, MOHAMMADKHAH A, et al. Effects of chemically doped bioactive borate glass on neuron regrowth and regeneration. Annals of Biomedical Engineering, 2016, 44(12): 3468.
PMID |
| [31] | HUANG J, HUANG J, ZHANG X, et al. A bioactive multifunctional dressing with simultaneous visible monitoring of pH values and H2O2 concentrations for promoting diabetic wound healing. Materials Horizons, 2025, 12: 267. |
| [32] | HUANG J, ZHENG Y, NIU H, et al. A multifunctional hydrogel for simultaneous visible H2O2 monitoring and accelerating diabetic wound healing. Advanced Healthcare Materials, 2024, 13(3): 2302328. |
| [33] | ZHANG Z, KLAUSEN L H, CHEN M, et al. Electroactive scaffolds for neurogenesis and myogenesis: graphene-based nanomaterials. Small, 2018, 14(48): 1801983. |
| [34] | 宁成云, 毛传斌. 电活性生物材料. 北京: 科学出版社, 2017: 1. |
| [35] |
SHIN M, LIM J, PARK Y, et al. Carbon-based nanocomposites for biomedical applications. RSC Advances, 2024, 14(10): 7142.
DOI PMID |
| [36] | ZHENG Y, HONG X, WANG J, et al. 2D nanomaterials for tissue engineering and regenerative nanomedicines: recent advances and future challenges. Advanced Healthcare Materials, 2021, 10(7): 2001743. |
| [37] | WANG X, HAN X, LI C, et al. 2D materials for bone therapy. Advanced Drug Delivery Reviews, 2021, 178: 113970. |
| [38] | GU G, CUI Z, DU X, et al. Recent advances in biomacromolecule-reinforced 2D material (2DM) hydrogels: from interactions, synthesis, and functionalization to biomedical applications. Advanced Functional Materials, 2024, 34(48): 2408367. |
| [39] | WANG Z L. Progress in piezotronics and piezo-phototronics. Advanced Materials, 2012, 24(34): 4632. |
| [40] | KAPAT K, SHUBHRA Q T H, ZHOU M, et al. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Advanced Functional Materials, 2020, 30(44): 1909045. |
| [41] | KIM W, LEE H, LEE J, et al. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials, 2020, 230: 119632. |
| [42] | HE J, HAO M, DUAN J, et al. Synergistic effect of endocellular calcium ion release and nanotopograpy of one-dimensional hydroxyapatite nanomaterials for accelerating neural stem cell differentiation. Composites Part B-Engineering, 2021, 219: 108944. |
| [43] | DONG X, LIU S, YANG Y, et al. Aligned microfiber-induced macrophage polarization to guide Schwann-cell-enabled peripheral nerve regeneration. Biomaterials, 2021, 272: 120767. |
| [44] | HAO M, ZHANG Z, LIU C, et al. Hydroxyapatite nanorods function as safe and effective growth factors regulating neural differentiation and neuron development. Advanced Materials, 2021, 33(33): 2100895. |
| [45] | DAI H, FAN Q, WANG C. Recent applications of immunomodulatory biomaterials for disease immunotherapy. Exploration, 2022, 2(6): 20210157. |
| [46] | LI L, XIAO B, MU J, et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano, 2019, 13(12): 14283. |
| [47] |
SUN Y, ZHANG H, ZHANG Y, et al. Li-Mg-Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioactive Materials, 2023, 28: 227.
DOI PMID |
| [48] |
LEVIN M. Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell, 2021, 184(8): 1971.
DOI PMID |
| [49] | MURILLO G, BLANQUER A, VARGAS-ESTEVEZ C, et al. Electromechanical nanogenerator-cell interaction modulates cell activity. Advanced Materials, 2017, 29(24): 1605048. |
| [50] | ZHANG X, WANG T, ZHANG Z, et al. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Materials Today, 2023, 68: 177. |
| [51] | KHARE D, BASU B, DUBEY A K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials, 2020, 258: 120280. |
| [52] | SEBASTIAN A, VOLK S W, HALAI P, et al. Enhanced neurogenic biomarker expression and reinnervation in human acute skin wounds treated by electrical stimulation. Journal of Investigative Dermatology, 2017, 137(3): 737. |
| [53] | FAN L, XIAO C, GUAN P, et al. Extracellular matrix-based conductive interpenetrating network hydrogels with enhanced neurovascular regeneration properties for diabetic wounds repair. Advanced Healthcare Materials, 2022, 11(1): 2101556. |
| [54] | TAN M H, XU X H, YUAN T J, et al. Self-powered smart patch promotes skin nerve regeneration and sensation restoration by delivering biological-electrical signals in program. Biomaterials, 2022, 283: 121413. |
| [55] | GAO J, YU X, WANG X, et al. Biomaterial-related cell microenvironment in tissue engineering and regenerative medicine. Engieering, 2022, 13: 31. |
| [56] |
ZHANG H, WU C. 3D printing of biomaterials for vascularized and innervated tissue regeneration. International Journal of Bioprinting, 2023, 9(3): 706.
DOI PMID |
| [57] | ZHANG H, MA W, MA H, et al. Spindle-like zinc silicate nanoparticles accelerating innervated and vascularized skin burn wound healing. Advanced Healthcare Materials, 2022, 10: 2102359. |
| [58] | ZHANG Z, CHANG D, ZENG Z, et al. CuCS/Cur composite wound dressings promote neuralized skin regeneration by rebuilding the nerve cell “factory” in deep skin burns. Materials Today Bio, 2024, 26: 101075. |
| [59] | KANG Y, LIU K, CHEN Z, et al. Healing with precision: a multi- functional hydrogel-bioactive glass dressing boosts infected wound recovery and enhances neurogenesis in the wound bed. Journal of Controlled Release, 2024, 370: 210. |
| [60] | XU S, ZHANG Y, DAI B, et al. Green-prepared magnesium silicate sprays enhance the repair of burn-skin wound and appendages regeneration in rats and minipigs. Advanced Functional Materials, 2024, 34(9): 2307439. |
| [61] | SAMANDARI M, QUINT J, RODRIGUEZ-DELAROSA A, et al. Bioinks and bioprinting strategies for skeletal muscle tissue engineering. Advanced Materials, 2022, 34(12): 2105883. |
| [62] | ZHANG H, QIN C, SHI Z, et al. Bioprinting of inorganic- biomaterial/neural-stem-cell constructs for multiple tissue regeneration and functional recovery. National Science Review, 2024, 11(4): nwae035. |
| [63] | ZHU Y, ZHANG X, CHANG G, et al. Bioactive glass in tissue regeneration: unveiling recent advances in regenerative strategies and applications. Advanced Materials, 2025, 37(2): 2312964. |
| [64] |
WOLTERINK R G J K, WU G S, CHIU I M, et al. Neuroimmune interactions in peripheral organs. Annual Review of Neuroscience, 2022, 45: 339.
DOI PMID |
| [65] | LU Y Z, NAYER B, SINGH S K, et al. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature, 2024, 628: 604. |
| [66] | CUI X, WANG L, GAO X, et al. Self-assembled silk fibroin injectable hydrogels based on layered double hydroxides for spinal cord injury repair. Matter, 2024, 7(2): 620. |
| [67] |
LIAN C, LIU J, WEI W, et al. Mg-gallate metal-organic framework-based sprayable hydrogel for continuously regulating oxidative stress microenvironment and promoting neurovascular network reconstruction in diabetic wounds. Bioactive Materials, 2024, 38: 181.
DOI PMID |
| [68] | PENG L H, XU X H, HUANG Y F, et al. Self-adaptive all-in-one delivery chip for rapid skin nerves regeneration by endogenous mesenchymal stem cells. Advanced Functional Materials, 2020, 30(40): 2001751. |
| [69] | XU C, CHANG Y, WU P, et al. Two-dimensional-germanium phosphide-reinforced conductive and biodegradable hydrogel scaffolds enhance spinal cord injury repair. Advanced Functional Materials, 2021, 31(41): 2104440. |
| [70] | YU Q, JIN S, WANG S, et al. Injectable, adhesive, self-healing and conductive hydrogels based on MXene nanosheets for spinal cord injury repair. Chemical Engineering Journal, 2023, 452: 139252. |
| [71] |
AN G, GUO F, LIU X, et al. Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1α-expressing stem cells. Nature Communications, 2020, 11: 2687.
DOI PMID |
| [72] | WANG S, WANG Z, YANG W, et al. In situ-sprayed bioinspired adhesive conductive hydrogels for cavernous nerve repair. Advanced Materials, 2024, 36(19): 2311264. |
| [73] | PI W, CHEN H, LIU Y, et al. Flexible sono-piezo patch for functional sweat gland repair through endogenous microenvironmental remodeling. ACS Nano, 2024, 18(31): 20283. |
| [74] | CHEN P, XU C, WU P, et al. Wirelessly powered electrical- stimulation based on biodegradable 3D piezoelectric scaffolds promotes the spinal cord injury. ACS Nano, 2022, 16(10): 16513. |
| [75] | LIU Y, ZHANG Z, ZHAO Z, et al. An easy nanopatch promotes peripheral nerve repair through wireless ultrasound-electrical stimulation in a band-aid-like way. Advanced Functional Materials, 2024, 34(44): 2407411. |
| [76] |
WANG L, DU J, LIU Q, et al. Wrapping stem cells with wireless electrical nanopatches for traumatic brain injury therapy. Nature Communications, 2024, 15: 7223.
DOI PMID |
| [77] |
VARADARAJAN S G, HUNYARA J L, HAMILTON N R, et al. Central nervous system regeneration. Cell, 2022, 185(1): 77.
DOI PMID |
| [1] | 余升阳, 苏海军, 姜浩, 余明辉, 姚佳彤, 杨培鑫. 激光增材制造超高温氧化物陶瓷孔隙缺陷形成及抑制研究进展[J]. 无机材料学报, 2025, 40(9): 944-956. |
| [2] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [3] | 肖晓琳, 王玉祥, 谷佩洋, 朱圳荣, 孙勇. 二维无机材料调控病损皮肤组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 860-870. |
| [4] | 马景阁, 吴成铁. 无机生物材料用于毛囊和毛发再生的研究[J]. 无机材料学报, 2025, 40(8): 901-910. |
| [5] | 艾敏慧, 雷波. 微纳米生物活性玻璃: 功能化设计与血管化皮肤再生[J]. 无机材料学报, 2025, 40(8): 921-932. |
| [6] | 王宇彤, 常江, 徐合, 吴成铁. 硅酸盐生物陶瓷/玻璃促创面修复的研究进展:作用、机制和应用方式[J]. 无机材料学报, 2025, 40(8): 911-920. |
| [7] | 马文平, 韩雅卉, 吴成铁, 吕宏旭. 无机活性材料在类器官研究领域的应用[J]. 无机材料学报, 2025, 40(8): 888-900. |
| [8] | 罗晓民, 乔志龙, 刘颍, 杨晨, 常江. 无机生物活性材料调控心肌再生的研究进展[J]. 无机材料学报, 2025, 40(8): 871-887. |
| [9] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [10] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [11] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [12] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [13] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [14] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [15] | 陈曦, 袁媛, 谭业强, 刘昌胜. 无机非金属生物材料发展战略研究[J]. 无机材料学报, 2025, 40(5): 449-456. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||