
Journal of Inorganic Materials ›› 2024, Vol. 39 ›› Issue (8): 911-919.DOI: 10.15541/jim20240025
Special Issue: 【能源环境】燃料电池(202506); 【能源环境】钙钛矿(202506)
• RESEARCH ARTICLE • Previous Articles Next Articles
PAN Jianlong( ), MA Guanjun, SONG Lemei, HUAN Yu(
), MA Guanjun, SONG Lemei, HUAN Yu( ), WEI Tao(
), WEI Tao( )
)
Received:2024-01-11
Revised:2024-03-08
Published:2024-08-20
Online:2024-03-30
Contact:
HUAN Yu, associate professor. E-mail: mse_huany@ujn.edu.cn;About author:PAN Jianlong (1998-), male, Master candidate. E-mail: pjl2812054@163.com
Supported by:CLC Number:
PAN Jianlong, MA Guanjun, SONG Lemei, HUAN Yu, WEI Tao. High Stability/Catalytic Activity Co-based Perovskite as SOFC Anode: In-situ Preparation by Fuel Reducing Method[J]. Journal of Inorganic Materials, 2024, 39(8): 911-919.
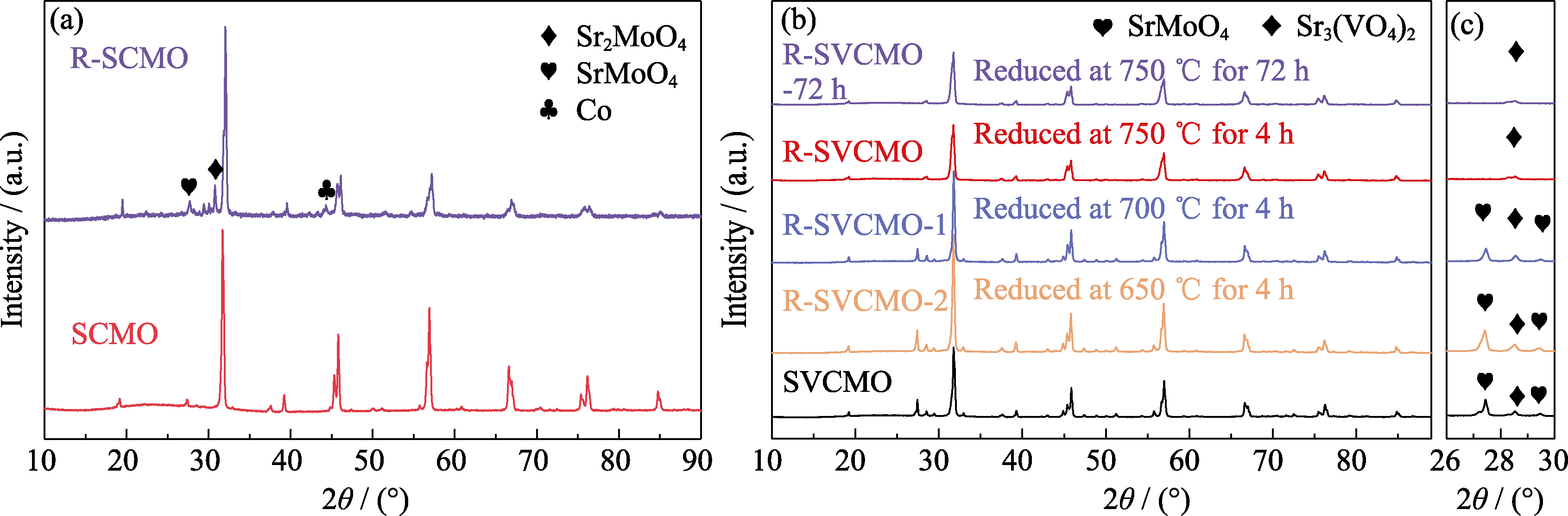
Fig. 1 XRD patterns of SCMO and SVCMO before and after calcination under different conditions (a) SCMO powders before and after H2 reduction at 750 ℃ for 4 h; (b) SVCMO powders before and after H2 reduction at different temperatures for 4 and 72 h; (c) Enlarged patterns of 2θ=26°-30° in (b)
| Parameter | SCMO | R-SVCMO |
|---|---|---|
| Space group | I4/m | I4/m |
| a=b/Å | 5.6374 | 5.6218 |
| c/Å | 7.9128 | 7.8823 |
| α/(º) | 90 | 90 |
| β/(º) | 90 | 90 |
| γ/(º) | 90 | 90 |
| Sr-O/Å | 2.8130 | 2.8071 |
| Co-O/Å | 2.0765 | 2.0532 |
| V-O/Å | — | 1.7758 |
| Mo-O/Å | 1.8945 | 1.9191 |
Table 1 Lattice parameters of SCMO and R-SVCMO obtained by XRD Rietveld refinement
| Parameter | SCMO | R-SVCMO |
|---|---|---|
| Space group | I4/m | I4/m |
| a=b/Å | 5.6374 | 5.6218 |
| c/Å | 7.9128 | 7.8823 |
| α/(º) | 90 | 90 |
| β/(º) | 90 | 90 |
| γ/(º) | 90 | 90 |
| Sr-O/Å | 2.8130 | 2.8071 |
| Co-O/Å | 2.0765 | 2.0532 |
| V-O/Å | — | 1.7758 |
| Mo-O/Å | 1.8945 | 1.9191 |
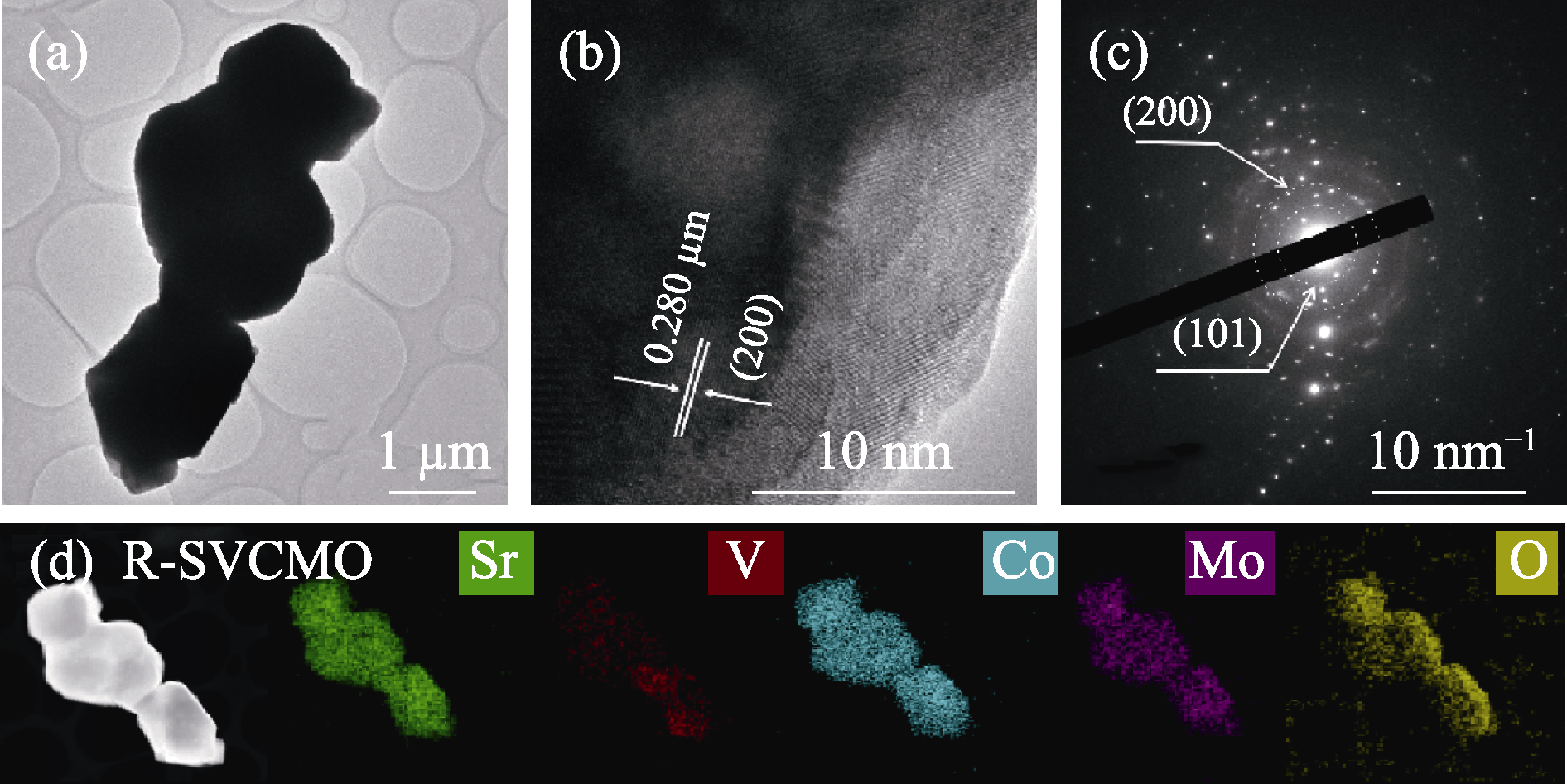
Fig. 2 Microstructure of R-SVCMO sample (a) TEM image; (b) HRTEM image and (c) corresponding SAED pattern; (d) STEM image and corresponding EDX elemental mappings
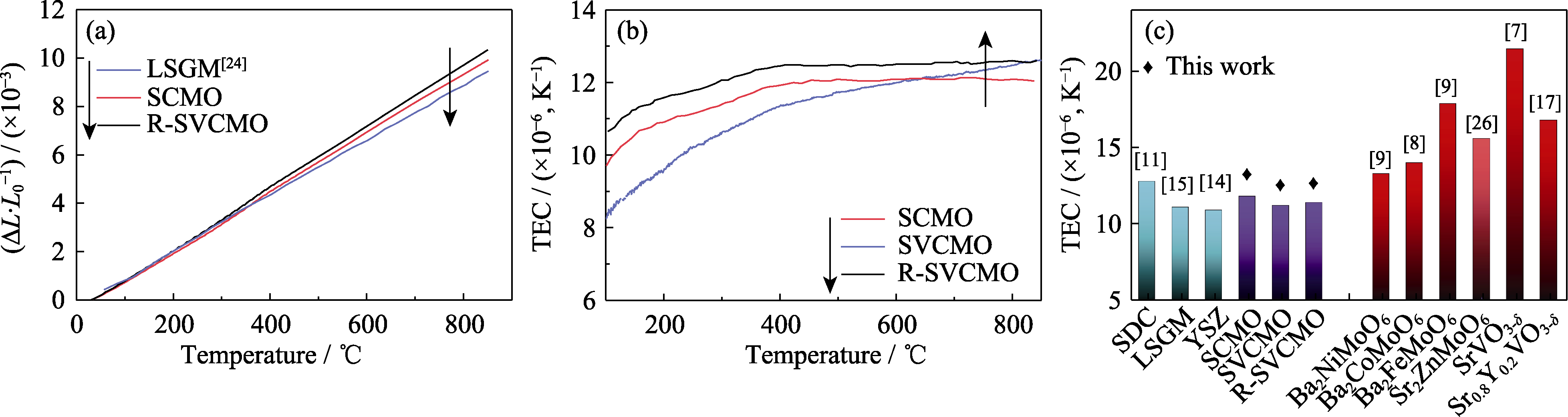
Fig. 3 Thermal expansion of different materials (a) Thermal expansion (ΔL/L0) curves of electrolyte LSGM, anodes SCMO and R-SVCMO from room temperature to 850 ℃; (b) TEC curves of SCMO, SVCMO and R-SVCMO in the range of 100−850 ℃; (c) Comparison of TEC of SCMO, SVCMO and R-SVCMO with conventional electrolytes and other perovskite anode materials[7⇓-9,11,14 -15,17,26]
| Sample | Valence ratio/% | ||||||
|---|---|---|---|---|---|---|---|
| Mo | O | Co | |||||
| Mo5+ | Mo6+ | Olatt. | Oad. | H2O | Co2+ | Co3+ | |
| SCMO | 34.1 | 65.9 | 31.4 | 60.5 | 8.2 | 74.0 | 26.0 |
| R-SVCMO | 58.5 | 41.5 | 31.1 | 65.5 | 3.4 | 62.9 | 37.1 |
Table 2 Area content percentages of Mo and Co elements in different valences, and different O types for SCMO and R-SVCMO samples based on XPS data
| Sample | Valence ratio/% | ||||||
|---|---|---|---|---|---|---|---|
| Mo | O | Co | |||||
| Mo5+ | Mo6+ | Olatt. | Oad. | H2O | Co2+ | Co3+ | |
| SCMO | 34.1 | 65.9 | 31.4 | 60.5 | 8.2 | 74.0 | 26.0 |
| R-SVCMO | 58.5 | 41.5 | 31.1 | 65.5 | 3.4 | 62.9 | 37.1 |
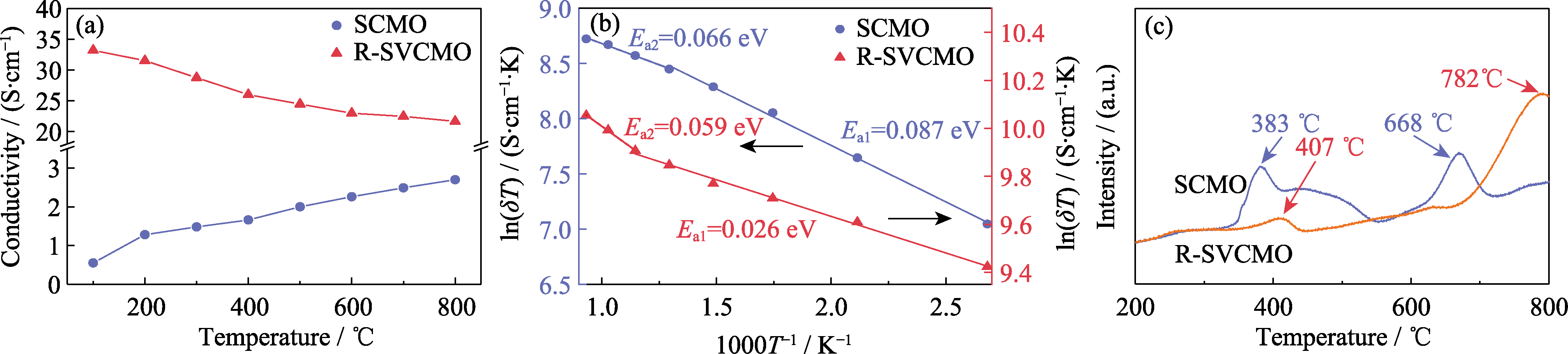
Fig. 5 Conductivity and H2-TPR curves for SCMO and R-SVCMO in testing H2 (a) Temperature dependence of conductivity curves; (b) Arrhenius curves; (c) H2-TPR curves; Colorful figures are available on website
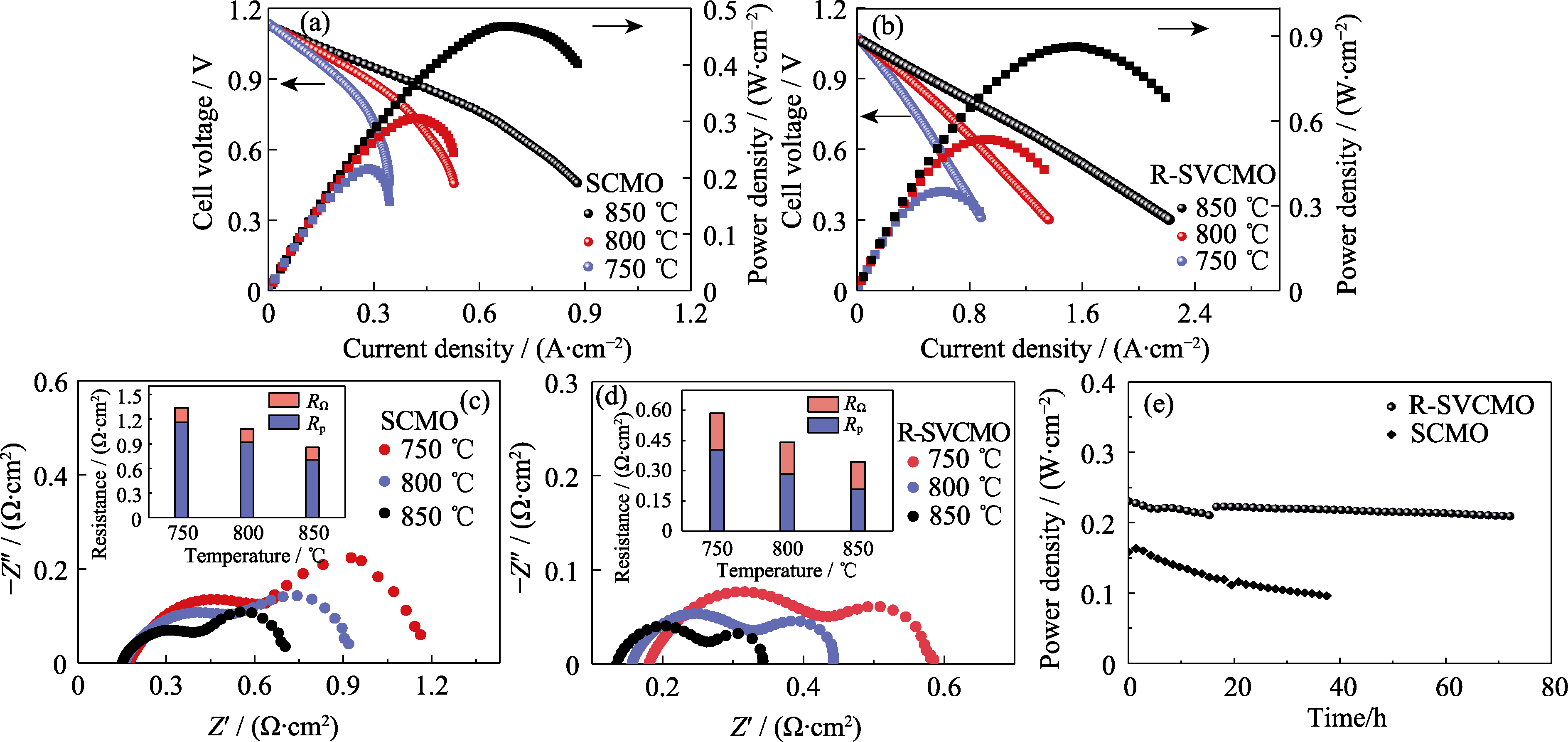
Fig. 6 Electrochemical performance of single cells with SCMO and R-SVCMO as anode materials (a, b) I-V-P curves for single cells with (a) SCMO and (b) R-SVCMO as anodes obtained under H2 at different temperatures; (c, d) Electrochemical Impedance spectra for (c) SCMO and (d) R-SVCMO single cells under H2 at different temperatures; (e) Durability of single cells with SCMO and R-SVCMO as anodes under 0.7 V at 750 ℃; Colorful figures are available on website
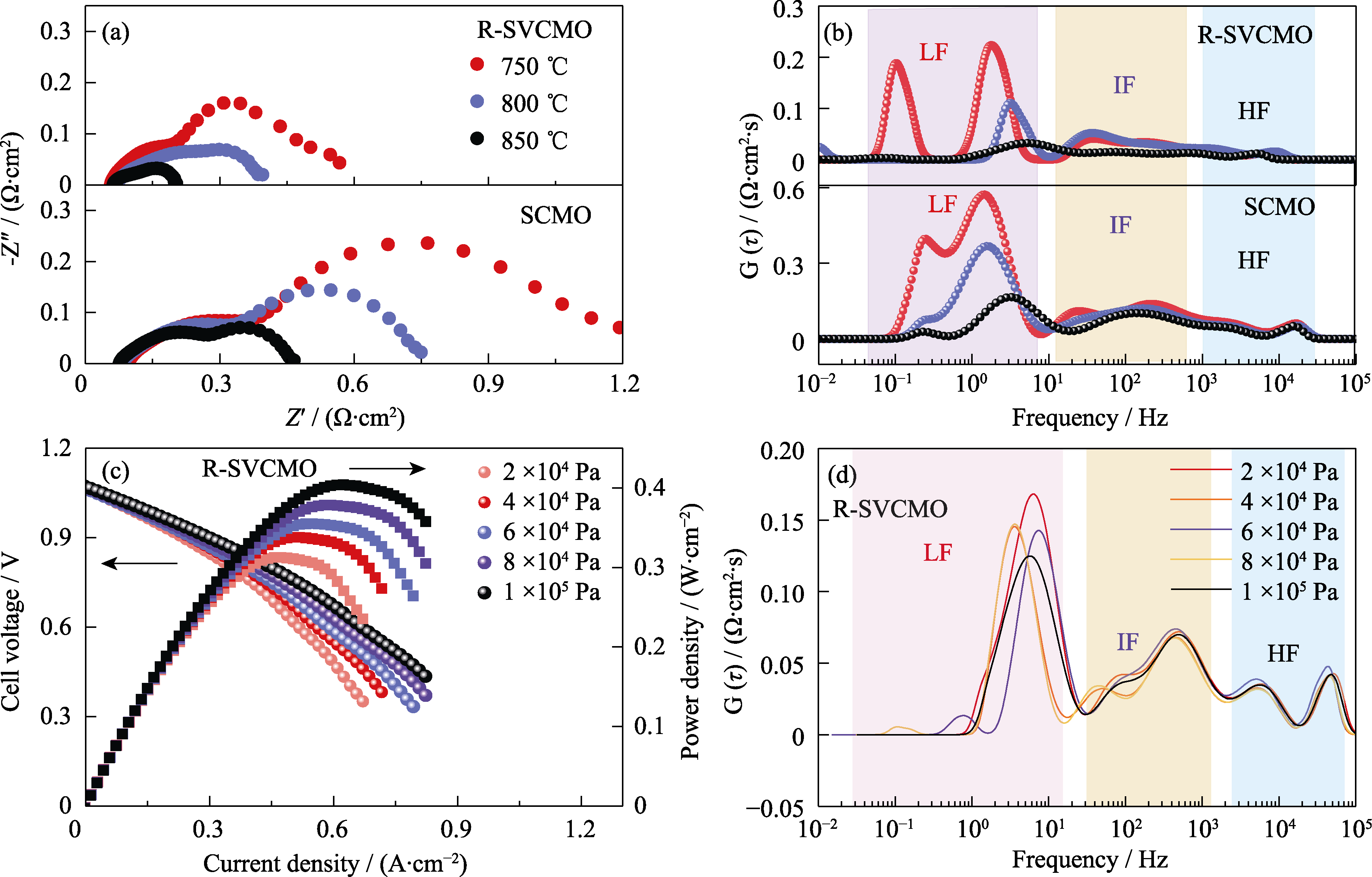
Fig. 7 Electrochemical performance of single cells or symmetric cells with SCMO and R-SVCMO as anodes or symmetric electrodes (a) EIS and (b) DRT spectra of SCMO and R-SVCMO symmetric cells under H2 at different temperatures; (c) I-V-P curves and (d) DRT spectra of R-SVCMO single cell under different H2 partial pressures at 750 ℃; Colorful figures are available on website
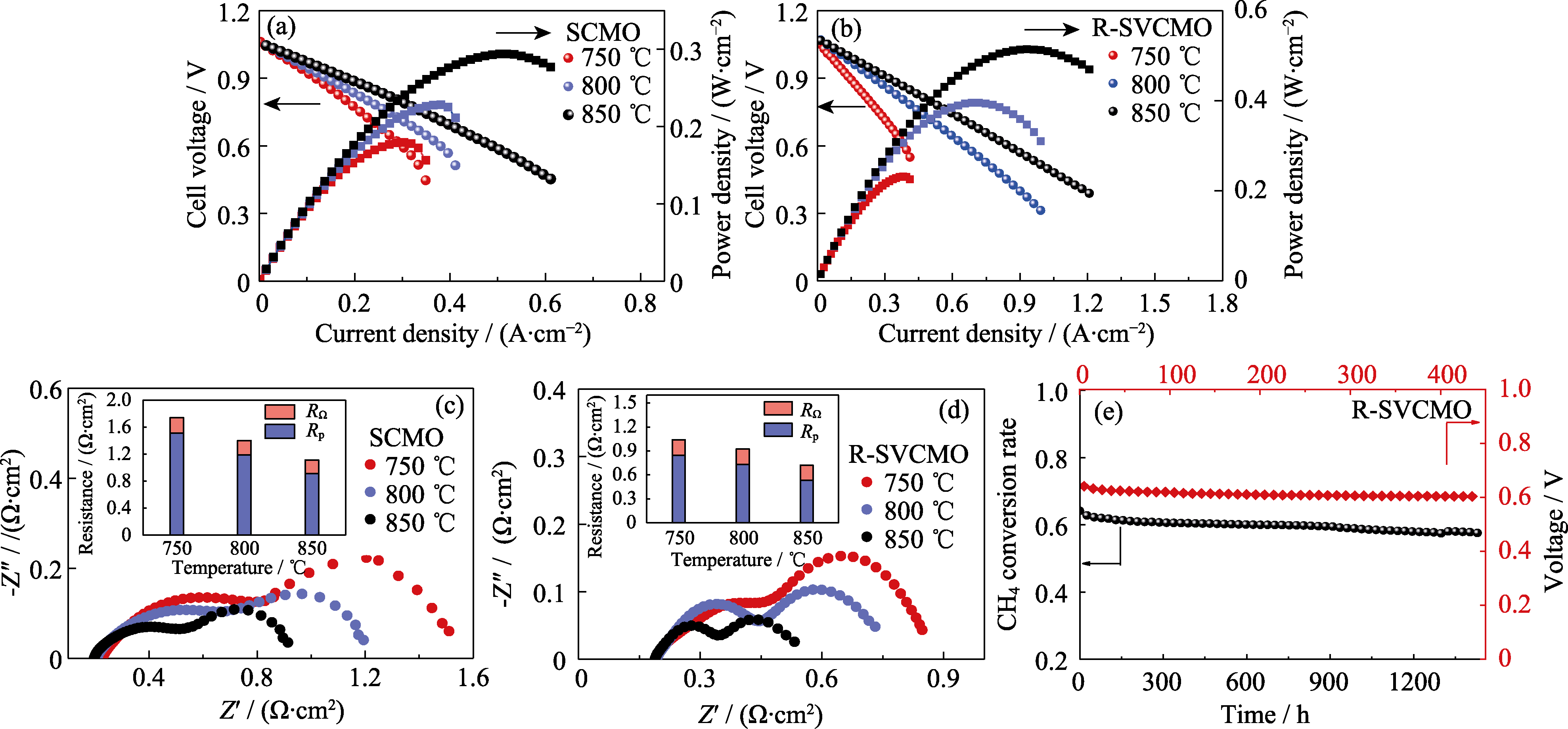
Fig. 8 Electrochemical performance of single cells with SCMO and R-SVCMO as anodes in CH4 atmosphere (a, b) I-V-P curves of (a) SCMO and (b) R-SVCMO based SOFC with humidified CH4 as fuel gas at different temperatures; (c, d) EIS spectra of (c) SCMO and (d) R-SVCMO based SOFC in humidified CH4 at different temperatures; (e) CH4 conversion rate of R-SVCMO catalyst for CH4 reforming and R-SVCMO based single cell working at 0.7 V under humidified CH4 at 750 ℃ as a function of testing time; Colorful figures are available on website
| [1] | DWIVEDI S. Solid oxide fuel cell: materials for anode, cathode and electrolyte. International Journal of Hydrogen Energy, 2020, 45(44): 23988. |
| [2] | PAN K, HUSSAIN A M, HUANG Y L, et al. High performance SrFe0.2Co0.4Mo0.4O3-δ ceramic anode supported low-temperature SOFCs. Journal of the Electrochemical Society, 2021, 168(11): 114503. |
| [3] | BILAL H M, MOTOLA M, QAYYUM S, et al. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chemical Engineering Journal, 2022, 428: 132603. |
| [4] | ZHOU S M, MIAO X B, ZHAO X, et al. Engineering electrocatalytic activity in nanosized perovskite cobaltite through surface spin-state transition. Nature Communications, 2016, 7: 11510. |
| [5] | NIU B, JIN F, FU R, et al. Pd-impregnated Sr1.9VMoO6-δ double perovskite as an efficient and stable anode for solid-oxide fuel cells operating on sulfur-containing syngas. Electrochimica Acta, 2018, 274: 91. |
| [6] | ZHENG K, ŚWIERCZEK K. Physicochemical properties of rock salt-type ordered Sr2MMoO6 (M=Mg, Mn, Fe, Co, Ni) double perovskites. Journal of the European Ceramic Society, 2014, 34(16): 4273. |
| [7] | ZHANG Q, WEI T, HUANG Y H. Electrochemical performance of double-perovskite Ba2MMoO6 (M=Fe, Co, Mn, Ni) anode materials for solid oxide fuel cells. Journal of Power Sources, 2012, 198: 59. |
| [8] | SUBOTIĆ V, BALDINELLI A, BARELLI L, et al. Applicability of the SOFC technology for coupling with biomass-gasifier systems: short- and long-term experimental study on SOFC performance and degradation behaviour. Applied Energy, 2019, 256: 113904 |
| [9] | SHIRATORI Y. YSZ-MgO composite electrolyte with adjusted thermal expansion coefficient to other SOFC components. Solid State Ionics, 2003, 164(1/2): 27. |
| [10] | SUN K, ZHANG J, JIANG T, et al. Flash-sintering and characterization of La0.8Sr0.2Ga0.8Mg0.2O3-δ electrolytes for solid oxide fuel cells. Electrochimica Acta, 2016, 196: 487. |
| [11] | ZHANG J, PAYDAR S, AKBAR N, et al. Electrical properties of Ni-doped Sm2O3 electrolyte. International Journal of Hydrogen Energy, 2021, 46(15): 9758. |
| [12] | HOU N, YAO T, LI P, et al. A-site ordered double perovskite with in situ exsolved core-shell nanoparticles as anode for solid oxide fuel cells. ACS Applied Materials & Interfaces, 2019, 11(7): 6995. |
| [13] | XU K, ZHANG H, DENG W, et al. Self-hydrating of a ceria-based catalyst enables efficient operation of solid oxide fuel cells on liquid fuels. Science Bulletin, 2023, 68(21): 2574. |
| [14] | SONG L, CHEN D, PAN J, et al. B-site super-excess design Sr2V0.4Fe0.9Mo0.7O6-δ-Ni0.4 as a highly active and redox-stable solid oxide fuel cell anode. ACS Applied Materials & Interfaces, 2023, 15(41): 48296. |
| [15] | YAREMCHENKO A A, BRINKMANN B, JANSSEN R, et al. Electrical conductivity, thermal expansion and stability of Y- and Al-substituted SrVO3 as prospective SOFC anode material. Solid State Ionics, 2013, 247: 86. |
| [16] | WANG F Y, ZHONG G B, LUO S, et al. Porous Sr2MgMo1-xVxO6-δ ceramics as anode materials for SOFCs using biogas fuel. Catalysis Communications, 2015, 67: 108. |
| [17] | DOS SANTOS-GÓMEZ L, LEÓN-REINA L, PORRAS-VÁZQUEZ J M, et al. Chemical stability and compatibility of double perovskite anode materials for SOFCs. Solid State Ionics, 2013, 239: 1. |
| [18] | MA G J, CHEN D Z, JI S J, et al. Medium-entropy SrV1/3Fe1/3Mo1/3O3 with high conductivity and strong stability as SOFCs- high-performance anode. Materials, 2022, 15(6): 2298. |
| [19] | DEWA M, YU W, DALE N, et al. Recent progress in integration of reforming catalyst on metal-supported SOFC for hydrocarbon and logistic fuels. International Journal of Hydrogen Energy, 2021, 46(67): 33523. |
| [20] | FARES A, BARAMA A, BARAMA S, et al. Synthesis and characterization of Ba0.5Sr0.5NixCo0.8-xFe0.2O3-δ (x=0 and 0.2) perovskites as electro-catalysts for methanol oxidation in alkaline media. Electroanalysis, 2017, 29(10): 2323. |
| [21] | XIA W W, LI Q, SUN L P, et al. Electrochemical performance of Sn-doped Bi0.5Sr0.5FeO3-δ perovskite as cathode electrocatalyst for solid oxide fuel cells. Journal of Alloys and Compounds, 2020, 835: 155406. |
| [22] | LI K, LI X, LI J, et al. Structural stability of Ni-Fe supported solid oxide fuel cells based on stress analysis. Journal of Inorganic Materials, 2019, 34(6): 611. |
| [23] | MORI M, SAMMES N M J S S I. Sintering and thermal expansion characterization of Al-doped and Co-doped lanthanum strontium chromites synthesized by the Pechini method. Solid State Ionics, 2002, 146(3/4): 301. |
| [24] | LI Y, YIN B, FAN Y, et al. Achieving high mechanical-strength CH4-based SOFCs by low-temperature sintering (1100 ℃). International Journal of Hydrogen Energy, 2020, 45(4): 3086. |
| [25] | FLORES-LASLUISA J X, HUERTA F, CAZORLA-AMORÓS D, et al. Structural and morphological alterations induced by cobalt substitution in LaMnO perovskites. Journal of Colloid and Interface Science, 2019, 556: 658. |
| [26] | QIN M X, XIAO Y, YANG H Y, et al. Ru/Nb co-doped perovskite anode: achieving good coking resistance in hydrocarbon fuels via core-shell nanocatalysts exsolution. Applied Catalysis B-Environmental, 2021, 299: 120613. |
| [27] | LING Y H, LI X W, CHUANG T C, et al. Double perovskite Sr2CoFeO5+δ: preparation and its performance as cathode material for intermediate-temperature solid oxide fuel cells. Journal of Inorganic Materials, 2023, 39(3): 337. |
| [28] | XU C M, SUN W, REN R Z, et al. A highly active and carbon-tolerant anode decorated with grown cobalt nano-catalyst for intermediate-temperature solid oxide fuel cells. Applied Catalysis B-Environmental, 2021, 282: 119553. |
| [29] | ZHAO H L, XU N S, CHENG Y F, et al. Investigation of mixed conductor BaCo0.7Fe0.3-xYxO3-δ with high oxygen permeability. Journal of Physical Chemistry C, 2010, 114: 17975. |
| [30] | HUAN Y, LI Y, YIN B, et al. High conductive and long-term phase stable anode materials for SOFCs: A2FeMoO6 (A = Ca, Sr, Ba). Journal of Power Sources, 2017, 359: 384. |
| [31] | SEREDA V V, TSVETKOV D S, SEDNEV A L, et al. Thermodynamics of Sr2NiMoO6 and Sr2CoMoO6 and their stability under reducing conditions. Physical Chemistry Chemical Physics, 2018, 20(30): 20108. |
| [32] | ALVAREZ M, LÓPEZ T, ODRIOZOLA J A, et al. 2, 4-Dichlorophenoxyacetic acid (2,4-D) photodegradation using an Mn+/ZrO2 photocatalyst: XPS, UV-Vis, XRD characterization. Applied Catalysis B: Environmental, 2007, 73(1/2): 34. |
| [33] | LUO L H, HU J X, CHENG L, XU X, et al. Performance of the composite cathode Ba0.5Sr0.5Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2-δ for medium- low temperature solid oxide fuel cell. Journal of Inorganic Materials, 2018, 33(4): 441. |
| [34] | XIA J, WANG C, WANG X F, et al. A perspective on DRT applications for the analysis of solid oxide cell electrodes. Electrochimica Acta, 2020, 349: 136328. |
| [35] | SHI N, SU F, HUAN D, et al. Performance and DRT analysis of P-SOFCs fabricated using new phase inversion combined tape casting technology. Journal of Materials Chemistry A, 2017, 5(37): 19664. |
| [1] | CHAI Runyu, ZHANG Zhen, WANG Menglong, XIA Changrong. Preparation of Ceria Based Metal-supported Solid Oxide Fuel Cells by Direct Assembly Method [J]. Journal of Inorganic Materials, 2025, 40(7): 765-771. |
| [2] | QU Jifa, WANG Xu, ZHANG Weixuan, ZHANG Kangzhe, XIONG Yongheng, TAN Wenyi. Enhanced Sulfur-resistance for Solid Oxide Fuel Cells Anode via Doping Modification of NaYTiO4 [J]. Journal of Inorganic Materials, 2025, 40(5): 489-496. |
| [3] | XUE Ke, CAI Changkun, XIE Manyi, LI Shuting, AN Shengli. Pr1+xBa1-xFe2O5+δ Cathode Materials for Solid Oxide Fuel Cells: Preparation and Electrochemical Performance [J]. Journal of Inorganic Materials, 2025, 40(4): 363-371. |
| [4] | ZHANG Jinghui, LU Xiaotong, MAO Haiyan, TIAN Yazhou, ZHANG Shanlin. Effect of Sintering Additives on Sintering Behavior and Conductivity of BaZr0.1Ce0.7Y0.2O3-δ Electrolytes [J]. Journal of Inorganic Materials, 2025, 40(1): 84-90. |
| [5] | YE Zibin, ZOU Gaochang, WU Qiwen, YAN Xiaomin, ZHOU Mingyang, LIU Jiang. Preparation and Performances of Tubular Cone-shaped Anode-supported Segmented-in-series Direct Carbon Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2024, 39(7): 819-827. |
| [6] | ZHANG Kun, WANG Yu, ZHU Tenglong, SUN Kaihua, HAN Minfang, ZHONG Qin. LaNi0.6Fe0.4O3 Cathode Contact Material: Electrical Conducting Property Manipulation and Its Effect on SOFC Electrochemical Performance [J]. Journal of Inorganic Materials, 2024, 39(4): 367-373. |
| [7] | CHEN Zhengpeng, JIN Fangjun, LI Mingfei, DONG Jiangbo, XU Renci, XU Hanzhao, XIONG Kai, RAO Muming, CHEN Chuangting, LI Xiaowei, LING Yihan. Double Perovskite Sr2CoFeO5+δ: Preparation and Performance as Cathode Material for Intermediate-temperature Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2024, 39(3): 337-344. |
| [8] | XUE Dingxi, YI Bingyao, LI Guojun, MA Shuai, LIU Keqin. Numerical Simulation of Thermal Stress in Solid Oxide Fuel Cells with Functional Gradient Anode [J]. Journal of Inorganic Materials, 2024, 39(11): 1189-1196. |
| [9] | GUO Tianmin, DONG Jiangbo, CHEN Zhengpeng, RAO Mumin, LI Mingfei, LI Tian, LING Yihan. Enhanced Compatibility and Activity of High-entropy Double Perovskite Cathode Material for IT-SOFC [J]. Journal of Inorganic Materials, 2023, 38(6): 693-700. |
| [10] | FAN Shuai, JIN Tian, ZHANG Shanlin, LUO Xiaotao, LI Chengxin, LI Changjiu. Effect of Li2O Sintering Aid on Sintering Characteristics and Electrical Conductivity of LSGM Electrolyte for Solid Oxide Fuel Cell [J]. Journal of Inorganic Materials, 2022, 37(10): 1087-1092. |
| [11] | CAO Dan,ZHOU Mingyang,LIU Zhijun,YAN Xiaomin,LIU Jiang. Fabrication and Characterization of Anode-supported Solid Oxide Fuel Cell Based on Proton Conductor Electrolyte [J]. Journal of Inorganic Materials, 2020, 35(9): 1047-1052. |
| [12] | XIA Tian, MENG Xie, LUO Ting, ZHAN Zhongliang. La 3+-substituted Sr2Fe1.5Ni0.1Mo0.4O6-δ as Anodes for Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2020, 35(5): 617-622. |
| [13] | Kai LI, Xiao LI, Jian LI, Jia-Miao XIE. Structural Stability of Ni-Fe Supported Solid Oxide Fuel Cells Based on Stress Analysis [J]. Journal of Inorganic Materials, 2019, 34(6): 611-617. |
| [14] | Wei WANG, Li-Li YUAN, Qian-Yuan QIU, Ming-Yang ZHOU, Mei-Lin LIU, Jiang LIU. A Direct Carbon Solid Oxide Fuel Cell Stack Based on a Single Electrolyte Plate Fabricated by Tape Casting Technique [J]. Journal of Inorganic Materials, 2019, 34(5): 509-514. |
| [15] | XIA Tian, MENG Xie, LUO Ting, ZHAN Zhong-Liang. Synthesis and Evaluation of Ca-doped Sr2Fe1.5Mo0.5O6-δ as Symmetrical Electrodes for High Performance Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2019, 34(10): 1109-1114. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||