
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (9): 959-971.DOI: 10.15541/jim20190548
Special Issue: 封面文章; MXene材料专辑(2020~2021); 【虚拟专辑】层状MAX,MXene及其他二维材料
• REVIEW • Next Articles
YANG Liuxin1,2( ),LUO Wenhua2,WANG Changan1,XU Chen2(
),LUO Wenhua2,WANG Changan1,XU Chen2( )
)
Received:2019-10-28
Revised:2019-12-04
Published:2020-09-20
Online:2020-04-05
Supported by:CLC Number:
YANG Liuxin,LUO Wenhua,WANG Changan,XU Chen. Novel Inorganic Two-dimensional Materials for Gas Separation Membranes[J]. Journal of Inorganic Materials, 2020, 35(9): 959-971.
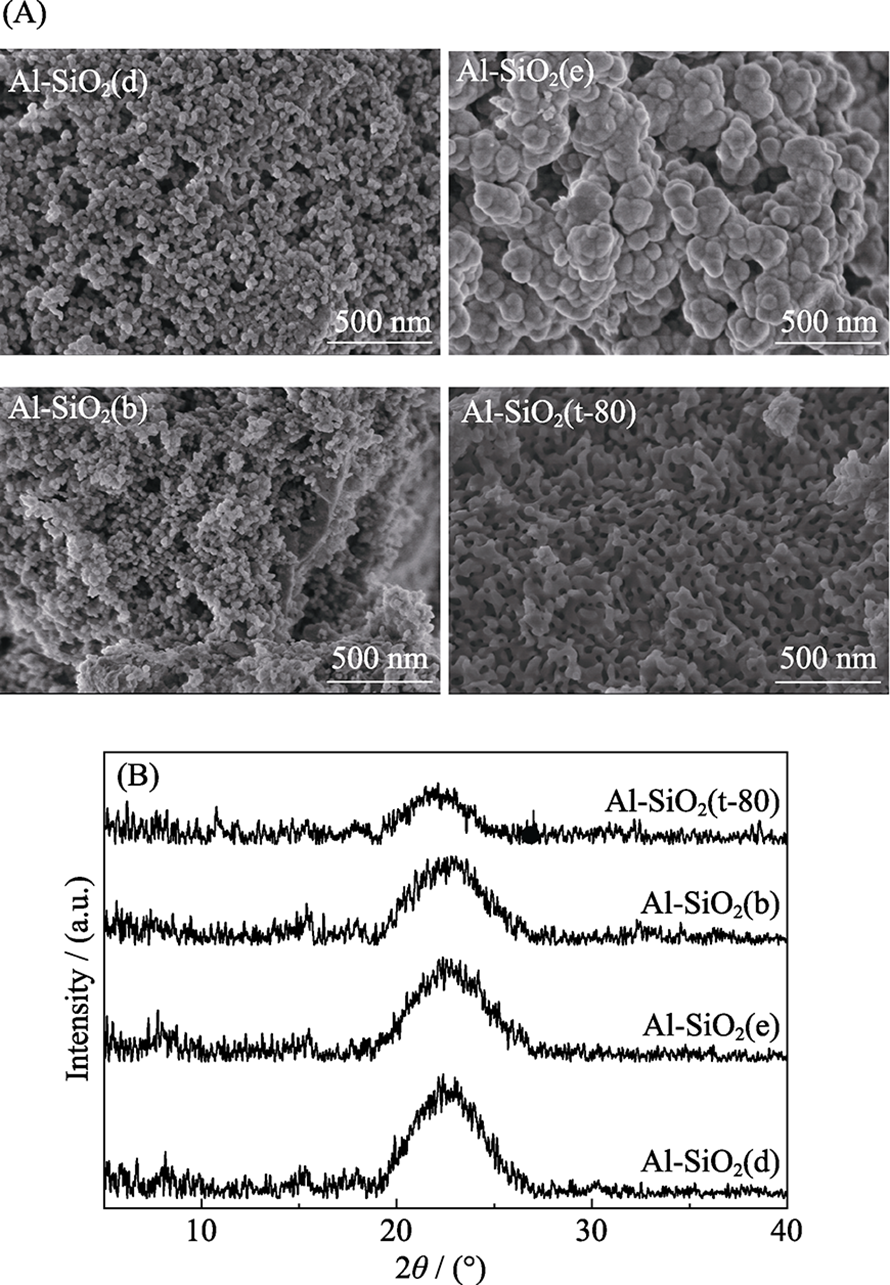
Fig. 1 Schematic representation of transport mechanisms in membranes, where Dc is the location of carbon centers, and the pore diameter is defined as Dp = Dc - Dvdw/$\sqrt{2}$, where Dvdw is the van der Waals diameter of a carbon atom[1,10,11,14-17]
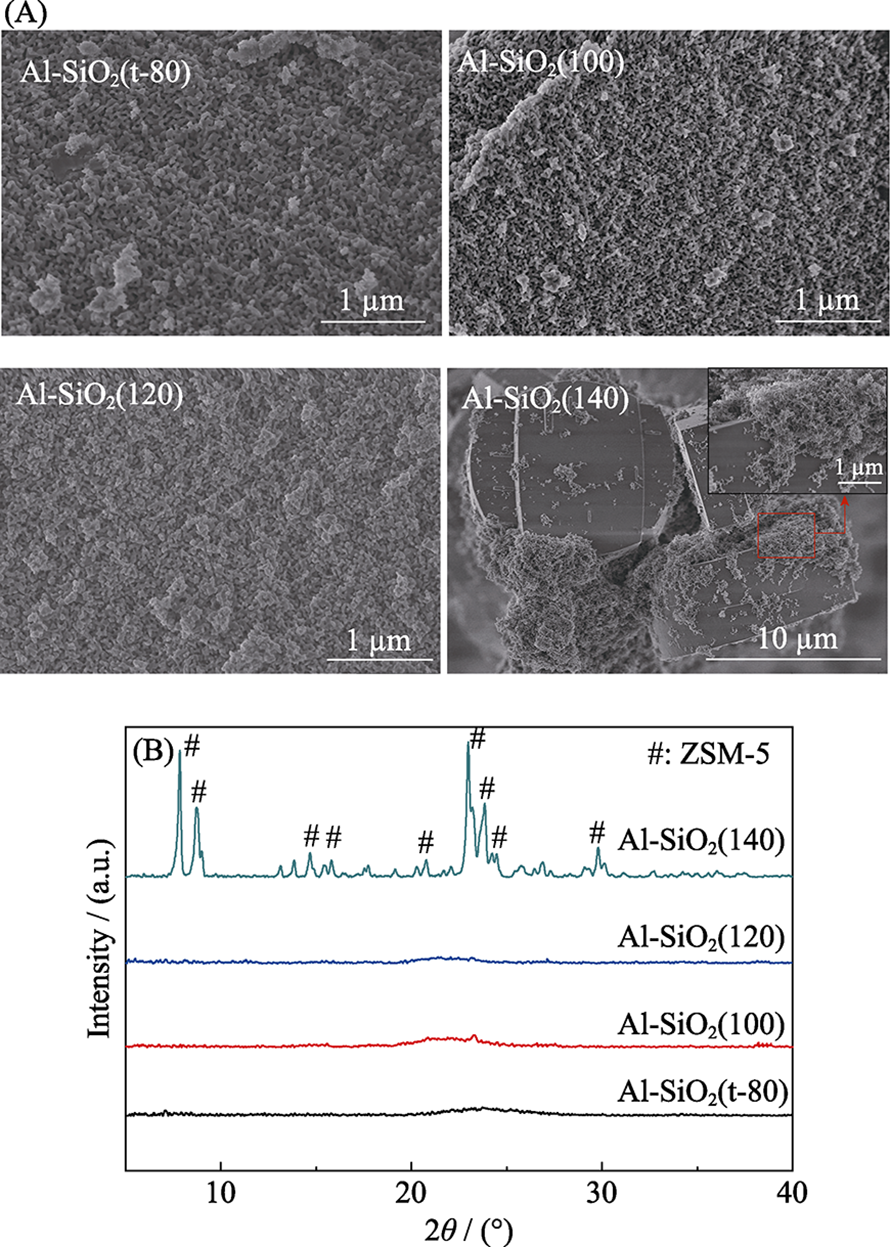
Fig. 2 Schematic of pores in graphene sheet (a-b) and pore electron density isosurfaces of porous graphene (c-d)[28] (a) Creation of a nitrogen-functionalized pore within a graphene sheet; (b) An all-hydrogen passivated pore in graphene; (c) Pore electron density isosurface of the nitrogen-functionalized porous graphene; (d) The all-hydrogen passivated porous grapheme Color code: C-black; N-green; H-cyan (isovalue is at 0.2 e/nm)
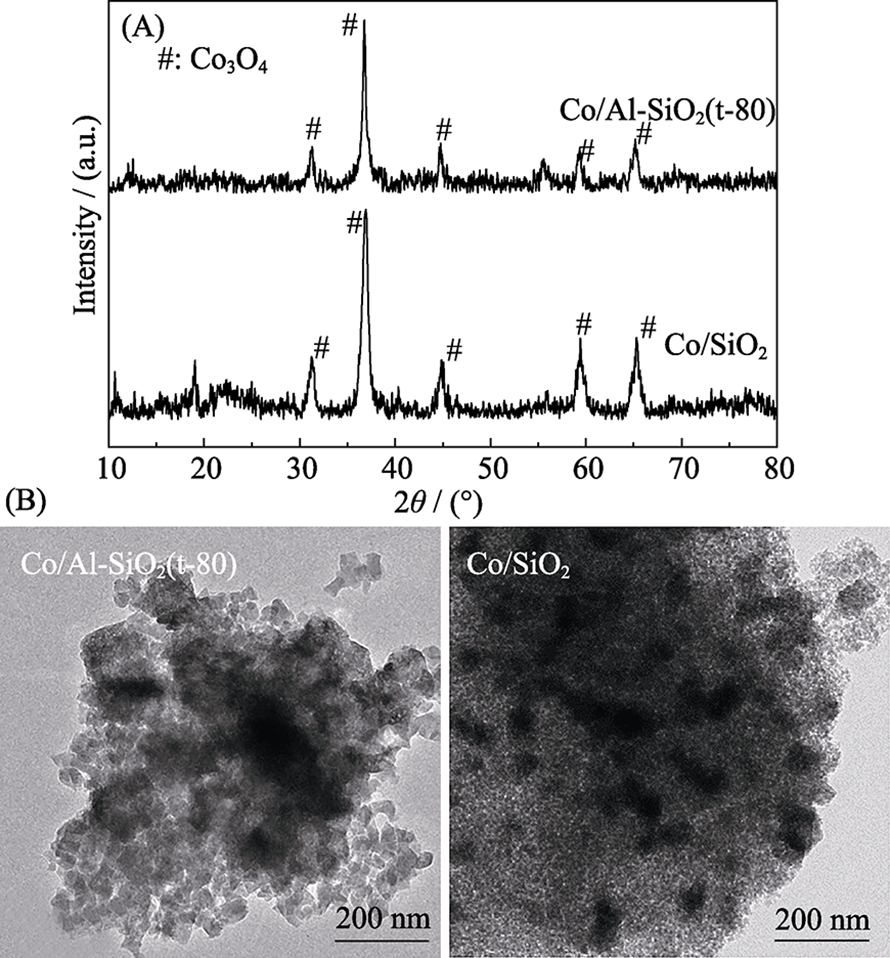
Fig. 4 Schematic of fabrication of a large-area graphene membrane by the nanoporous carbon film-assisted transfer method (a) and high-resolution TEM images of the intrinsic defects in graphene lattice (b)[33]
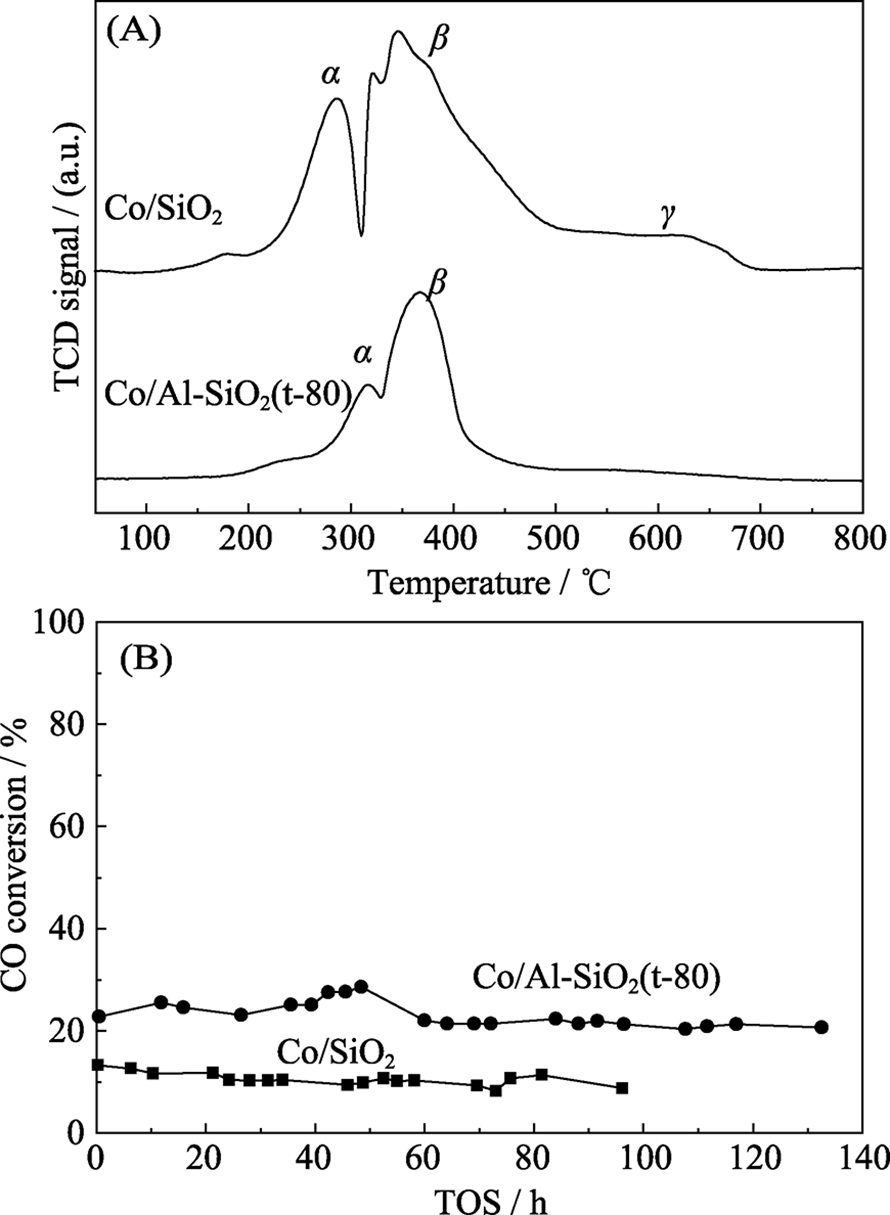
Fig. 5 Photo of a 1-μm-thick GO film (a), electron micrograph of the film’s cross section (b), schematic view for possible permeation through the laminates (c), and examples of He-leak measurements for a freestanding submicrometer-thick GO membrane and a reference PET film (d)[40] (1 mbar=100 Pa)
Fig. 6 Schematic illustration of two different GO coating methods (Method one and Method two) (a), schematic illustration of possible gas transport through graphene membrane (b), AFM images of GO membrane surfaces with insert images showing depth profiles of GO membrane surfaces: (c) method one [root mean square roughness (Rq) = 0.8 nm, average roughness (Ra) = 0.614 nm] and (d) method two (Rq = 0.608 nm, Ra = 0.467 nm)[19]
Fig. 7 Schematic representation of the assembly of GO nanosheets in polymeric environment (a), digital photographs of the membrane with 0.1wt% GO (b), overview (the yellow dashed lines are eye-guiding lines indicating the GO laminates in these regions) (c) and expanded TEM image of the cross section of GO-1 membrane (d)[17]
Fig. 8 Force analysis for one 2D channel unit consisting of GO nanosheets and polymer chain (a), schematic illustration of intrinsic force induced disordered structure (left) and highly ordered laminar structures (right) driven by introduced synergistic external forces (b)[54]
Fig. 9 Schematic illustration of the gas solution-diffusion transport pathway through a GO-SILM (a), molecular structures of [BMIM][BF4] IL confined in the GO nanochannels (b), the cross section of the GO membrane (c) and GO-SILM (d), respectively[55]
| Materialsa | Preparation method | Feed condition | Selectivity | Permeate rate/ permeance/permeability | Ref. |
|---|---|---|---|---|---|
| Porous graphene monolayer | Simulation All-H passivation | H2/CH4 | 1023 | 10-20 mol?s-1?Pa-1 | [ |
| Porous graphene bilayer | Ultraviolet-induced oxidative etching | H2/CH4 | 104 | 4.5×10-23 mol?s-1?Pa-1 | [ |
| Porous graphene bilayer | Focused ion beam perforation | H2/CO2 | 4.6 | 5.0×103 mol?m-2?s-1?Pa-1 | [ |
| Porous graphene | Ozone functionalization-based pore-etching | H2/CH4 | 25 | 4.1×10-7 mol?m-2?s-1?Pa-1 | [ |
| GO/PES | Spin coating | H2/CO2 | 30 | 4.02×10-17 mol?m?m-2?s-1?Pa-1 | [ |
| Dip and spin coating | H2/CO2 | 20 | 5.69×10-17 mol?m?m-2?s-1?Pa-1 | ||
| GO/AAO | Vacuum filtration | H2/CO2 | 3400 | 10-7 mol?m-2?s-1?Pa-1 | [ |
| GO/AAO | Vacuum filtration | H2/CO2 | 22.5 | 1.14×10-7 mol?m-2?s-1?Pa-1 | [ |
| TU-GOF | Hydrothermal Self-assembly synthesis | H2/CO2 | 225 | 10-7 mol?m-2?s-1?Pa-1 | [ |
| GO/AAO | Spin coating | H2/CO2 | 240 | 3.4×10-7 mol?m-2?s-1?Pa-1 | [ |
| PEBA-GO | Drop casting | CO2/N2 | 91 | 3.35×10-14 mol?m?m-2?s-1?Pa-1 | [ |
| SPEEK/S-GO | Drop casting | CO2/CH4 | 72.2 | 4.44×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| EFDA-GO | Vaccum-spin | H2/CO2 | 29-33 | 2.8-4.0×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| GO-[BMIM][BF4] | Vacuum filtration | CO2/ H2 | 24 | 2.29×10-8 mol?m-2?s-1?Pa-1 | [ |
| CO2/ CH4 | 234 | ||||
| CO2/N2 | 382 |
Table 1 Graphene-based membranes for gas separation
| Materialsa | Preparation method | Feed condition | Selectivity | Permeate rate/ permeance/permeability | Ref. |
|---|---|---|---|---|---|
| Porous graphene monolayer | Simulation All-H passivation | H2/CH4 | 1023 | 10-20 mol?s-1?Pa-1 | [ |
| Porous graphene bilayer | Ultraviolet-induced oxidative etching | H2/CH4 | 104 | 4.5×10-23 mol?s-1?Pa-1 | [ |
| Porous graphene bilayer | Focused ion beam perforation | H2/CO2 | 4.6 | 5.0×103 mol?m-2?s-1?Pa-1 | [ |
| Porous graphene | Ozone functionalization-based pore-etching | H2/CH4 | 25 | 4.1×10-7 mol?m-2?s-1?Pa-1 | [ |
| GO/PES | Spin coating | H2/CO2 | 30 | 4.02×10-17 mol?m?m-2?s-1?Pa-1 | [ |
| Dip and spin coating | H2/CO2 | 20 | 5.69×10-17 mol?m?m-2?s-1?Pa-1 | ||
| GO/AAO | Vacuum filtration | H2/CO2 | 3400 | 10-7 mol?m-2?s-1?Pa-1 | [ |
| GO/AAO | Vacuum filtration | H2/CO2 | 22.5 | 1.14×10-7 mol?m-2?s-1?Pa-1 | [ |
| TU-GOF | Hydrothermal Self-assembly synthesis | H2/CO2 | 225 | 10-7 mol?m-2?s-1?Pa-1 | [ |
| GO/AAO | Spin coating | H2/CO2 | 240 | 3.4×10-7 mol?m-2?s-1?Pa-1 | [ |
| PEBA-GO | Drop casting | CO2/N2 | 91 | 3.35×10-14 mol?m?m-2?s-1?Pa-1 | [ |
| SPEEK/S-GO | Drop casting | CO2/CH4 | 72.2 | 4.44×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| EFDA-GO | Vaccum-spin | H2/CO2 | 29-33 | 2.8-4.0×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| GO-[BMIM][BF4] | Vacuum filtration | CO2/ H2 | 24 | 2.29×10-8 mol?m-2?s-1?Pa-1 | [ |
| CO2/ CH4 | 234 | ||||
| CO2/N2 | 382 |
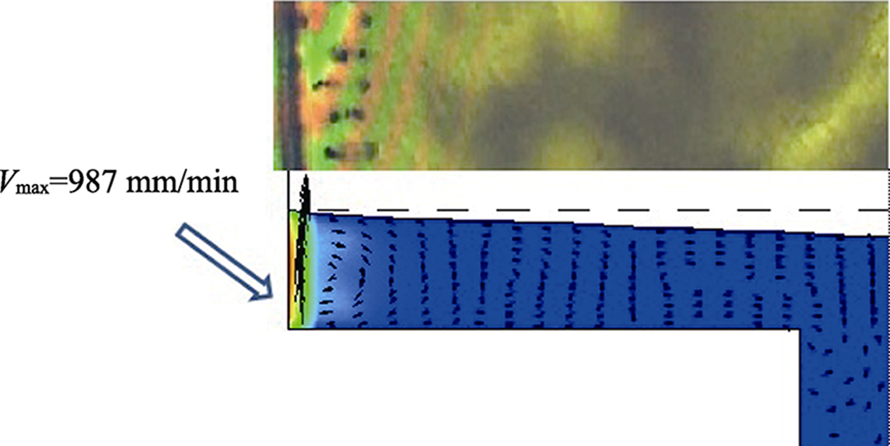
Fig.10 Schematic of gas permeation pathway across MoS2 membranes before and after heating at 160 ℃(a)[63], cross section SEM images of the membrane (b-e)[64], and synthesis process for MoS2-SILM (f)[69](b) GO membrane; (c) MoS2 membrane; (d) GO-MoS2 (50/50) hybrid membrane; (e) GO-MoS2 (75/25) hybrid membrane
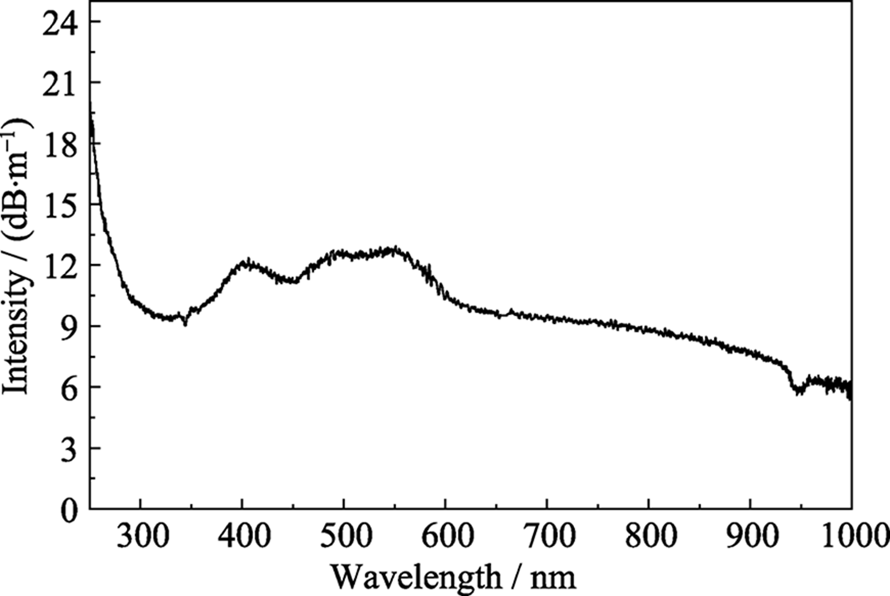
Fig. 11 SEM image of the delaminated MXene nanosheets on porous anodic aluminum oxide (AAO) (a) with insert showing the Tyndall scattering effect in MXene colloidal solution in water, cross-sectional SEM image of the MXene membrane (b) with insert showing a tweezer bent membrane, AFM image of the Mxene nanosheet on cleaved mica (c), illustration of the spacing between the neighboring MXene nanosheets in the membrane (d)[77], structures and gas transport of H2-selective and CO2-selective Mxene nanofilms (e)[78]
| Materialsa | Preparation method | Feed Condition | Selectivity | Permeate rate/permeance/ permeability | Ref. |
|---|---|---|---|---|---|
| MoS2/AAO | Vacuum filtration | H2/CO2 | 3.4 | 9.19×10-6 mol?m-2?s-1?Pa-1 | [ |
| MoS2 | Vacuum filtration | H2/CO2 | 8.29 | 3.94×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| GO/MoS2 | Vacuum filtration | H2/CO2 | 26.7 | 8.04×10-7 mol m-2 s-1 Pa-1 | [ |
| MoS2-Pebax | Spin coating | CO2/N2 | 93 | 2.14×10-14 mol?m?m-2?s-1?Pa-1 | [ |
| MoS2-[BMIM][BF4] | Vacuum filtration and drop | CO2/N2 | 131.42 | 1.60×10-8 mol?m-2?s-1?Pa-1 | [ |
| WS2-[BMIM][BF4] | Vacuum filtration and spin-coating | CO2/N2 | 153.21 | 1.58×10-8 mol?m-2?s-1?Pa-1 | [ |
| Ti2C3Tx | Vacuum filtration | H2/CO2 | 160 | 7.37×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| Ti2C3Tx/borate-PEI | Vacuum filtration and spin-coating | CO2/CH4 | 15.3 | 1.17×10-7 mol?m-2?s-1?Pa-1 | [ |
| MgAl-CO3 LDH | Electrophoretic deposition | CO2/N2 | 1.53 | 2.34×10-7 mol?m-2?s-1?Pa-1 | [ |
| MgAl-CO3 LDH | Vacuum-suction | CO2/N2 | 34.4 | 5.5×10-10 mol?m-2?s-1?Pa-1 | [ |
| NiAl-CO3 LDH | In situ growth | H2/CH4 | 80 | 4.5×10-8 mol?m-2?s-1?Pa-1 | [ |
| h-BN-XTR | Drop casting | H2/CH4 | 24.1 | 7.03×10-14 mol?m?m-2?s-1?Pa-1 | [ |
| mica-[BMIM][BF4] | Vacuum filtration and drop-casting | CO2/N2 | 87 | 2.68×10-8 mol?m-2?s-1?Pa-1 | [ |
Table 2 Other novel inorganic 2DMs for membrane gas separation
| Materialsa | Preparation method | Feed Condition | Selectivity | Permeate rate/permeance/ permeability | Ref. |
|---|---|---|---|---|---|
| MoS2/AAO | Vacuum filtration | H2/CO2 | 3.4 | 9.19×10-6 mol?m-2?s-1?Pa-1 | [ |
| MoS2 | Vacuum filtration | H2/CO2 | 8.29 | 3.94×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| GO/MoS2 | Vacuum filtration | H2/CO2 | 26.7 | 8.04×10-7 mol m-2 s-1 Pa-1 | [ |
| MoS2-Pebax | Spin coating | CO2/N2 | 93 | 2.14×10-14 mol?m?m-2?s-1?Pa-1 | [ |
| MoS2-[BMIM][BF4] | Vacuum filtration and drop | CO2/N2 | 131.42 | 1.60×10-8 mol?m-2?s-1?Pa-1 | [ |
| WS2-[BMIM][BF4] | Vacuum filtration and spin-coating | CO2/N2 | 153.21 | 1.58×10-8 mol?m-2?s-1?Pa-1 | [ |
| Ti2C3Tx | Vacuum filtration | H2/CO2 | 160 | 7.37×10-13 mol?m?m-2?s-1?Pa-1 | [ |
| Ti2C3Tx/borate-PEI | Vacuum filtration and spin-coating | CO2/CH4 | 15.3 | 1.17×10-7 mol?m-2?s-1?Pa-1 | [ |
| MgAl-CO3 LDH | Electrophoretic deposition | CO2/N2 | 1.53 | 2.34×10-7 mol?m-2?s-1?Pa-1 | [ |
| MgAl-CO3 LDH | Vacuum-suction | CO2/N2 | 34.4 | 5.5×10-10 mol?m-2?s-1?Pa-1 | [ |
| NiAl-CO3 LDH | In situ growth | H2/CH4 | 80 | 4.5×10-8 mol?m-2?s-1?Pa-1 | [ |
| h-BN-XTR | Drop casting | H2/CH4 | 24.1 | 7.03×10-14 mol?m?m-2?s-1?Pa-1 | [ |
| mica-[BMIM][BF4] | Vacuum filtration and drop-casting | CO2/N2 | 87 | 2.68×10-8 mol?m-2?s-1?Pa-1 | [ |
| [1] |
LIU G, JIN W, XU N. Graphene-based membranes. Chemical Society Reviews, 2015,44(15):5016-5030.
URL PMID |
| [2] |
SHOLL D S, LIVELY R P. Seven chemical separations to change the world. Nature, 2016,532(7600):435-437.
DOI URL PMID |
| [3] |
LI P, WANG Z, QIAO Z, et al. Recent developments in membranes for efficient hydrogen purification. Journal of Membrane Science, 2015. 495:130-168.
DOI URL |
| [4] | JEON Y W, LEE D H. Gas membranes for CO2/CH4 (biogas) separation: a review. Environmental Engineering Science, 2015,32(2):71-85. |
| [5] | DALANE K, DAI Z, MOGSETH G, et al. Potential applications of membrane separation for subsea natural gas processing: a review. Journal of Natural Gas Science and Engineering, 2017,39:101-117. |
| [6] | HIMMA N F, WARDANI A K, PRASETYA N, et al. Recent progress and challenges in membrane-based O2/N2 separation. Reviews in Chemical Engineering, 2019,35(5):591-625. |
| [7] | ZHU J, HOU J, ULIANA A, et al. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. Journal of Materials Chemistry A, 2018,6(9):3773-3792. |
| [8] |
GIN D L, NOBLE R D. Designing the next generation of chemical separation membranes. Science, 2011,332(6030):674.
URL PMID |
| [9] | QIU T, KUANG C, ZHENG X, et al. On the research and application trends of global gas membrane separation technology—— based on analysis of SCI articles and patents in recent 20 years. Chemical Industry & Engineering Progress, 2016,35(7):2299-2308. |
| [10] | YAMPOLSKII Y. Polymeric gas separation membranes. Macromolecules, 2012,45(8):3298-3311. |
| [11] | PROZOROVSKA L, KIDAMBI P R. State-of-the-art and future prospects for atomically thin membranes from 2D materials. Advanced Materials, 2018,30(52):1801179. |
| [12] | LIU M, GURR P A, FU Q, et al. Two-dimensional nanosheet-based gas separation membranes. Journal of Materials Chemistry A, 2018,6(46):23169-23196. |
| [13] |
LIU G, JIN W, XU N. Two-dimensional-material membranes: a new family of high-performance separation membranes. Angewandte Chemie International Edition, 2016,55(43):13384-13397.
URL PMID |
| [14] |
WANG L, BOUTILIER M S H, KIDAMBI P R, et al. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nature Nanotechnology, 2017,12:509.
DOI URL PMID |
| [15] | WIJMANS J G, BAKER R W J. The solution-diffusion model: a review. Journal of Membrane Science, 1995,107:1-21. |
| [16] | LI C, MECKLER S M, SMITH Z P, et al. Engineered transport in microporous materials and membranes for clean energy technologies. Advanced Materials, 2018,30(8):1704953. |
| [17] | SHEN J, LIU G, HUANG K, et al. Membranes with fast and selective gas-transport channels of laminar graphene oxide for efficient CO2 capture. Angewandte Chemie International Edition, 2015,127(2):588-592. |
| [18] |
DRAHUSHUK L W, WANG L, KOENIG S P, et al. Analysis of time-varying, stochastic gas transport through graphene membranes. ACS Nano, 2016,10(1):786-795.
URL PMID |
| [19] |
KIM H W, YOON H W, YOON S M, et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science, 2013,342(6154):91-95.
URL PMID |
| [20] |
LI H, SONG Z, ZHANG X, et al. Ultrathin, molecular-sieving graphene oxide membranes for selective hydrogen separation. Science, 2013,342(6154):95-98.
URL PMID |
| [21] |
WANG Z, WANG D, ZHANG S, et al. Interfacial design of mixed matrix membranes for improved gas separation performance. Advanced Materials, 2016,28(17):3399-3405.
URL PMID |
| [22] |
ZHU X, TIAN C, DO-THANH C L, et al. Two‐dimensional materials as prospective scaffolds for mixed‐matrix membrane‐based CO2 separation. ChemSusChem, 2017,10(17):3304-3316.
DOI URL PMID |
| [23] |
GEIM A K, NOVOSELOV K S. The rise of graphene. Nature Materials, 2007,6:183.
DOI URL PMID |
| [24] |
PARTOENS B, PEETERS F. From graphene to graphite: electronic structure around the K point. Phys. Rev. B, 2006,74(7):075404.
DOI URL |
| [25] |
BUNCH J S, VERBRIDGE S S, ALDEN J S, et al. Impermeable atomic membranes from graphene sheets. Nano Letters, 2008,8(8):2458-2462.
URL PMID |
| [26] |
BERRY V. Impermeability of graphene and its applications. Carbon, 2013,62:1-10.
DOI URL |
| [27] |
LEE C, WEI X, KYSAR J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science, 2008,321(5887):385-388.
URL PMID |
| [28] |
JIANG D E, COOPER V, DAI S. Porous graphene as the ultimate membrane for gas separation. Nano Letters, 2009,9:4019-4024.
URL PMID |
| [29] | WANG S, DAI S, JIANG D E. Continuously tunable pore size for gas separation via a bilayer nanoporous graphene membrane. ACS Applied Nano Materials, 2019,2(1):379-384. |
| [30] |
KOENIG S P, WANG L, PELLEGRINO J, et al. Selective molecular sieving through porous graphene. Nature Nanotechnology, 2012,7(11):728-732.
URL PMID |
| [31] |
BELL D, LEMME M, STERN L, et al. Precision cutting and patterning of graphene with helium ions. Nanotechnology, 2009,20:455301.
DOI URL PMID |
| [32] |
CELEBI K, BUCHHEIM J, WYSS R M, et al. Ultimate permeation across atomically thin porous graphene. Science, 2014,344(6181):289-292.
URL PMID |
| [33] |
HUANG S, DAKHCHOUNE M, LUO W, et al. Single-layer graphene membranes by crack-free transfer for gas mixture separation. Nature Communications, 2018,9(1):2632-2632.
DOI URL PMID |
| [34] |
FISCHBEIN M D, DRNDIĆ M. Electron beam nanosculpting of suspended graphene sheets. Applied Physics Letters, 2008,93(11):113107.
DOI URL |
| [35] |
LU N, WANG J, FLORESCA H C, et al. In situ studies on the shrinkage and expansion of graphene nanopores under electron beam irradiation at temperatures in the range of 400-1200 ℃. Carbon, 2012,50(8):2961-2965.
DOI URL |
| [36] |
GARAJ S, HUBBARD W, REINA A, et al. Graphene as a subnanometre trans-electrode membrane. Nature, 2010,467:190.
DOI URL PMID |
| [37] | MERCHANT C. DNA translocation through graphene nanopores. Biophysical Journal, 2011,100(3):521a. |
| [38] |
HUMMERS JR W S, OFFEMAN R E. Preparation of graphitic oxide. Journal of the American Chemical Society, 1958,80(6):1339.
DOI URL |
| [39] |
DIMIEV A, TOUR J. Mechanism of graphene oxide formation. ACS Nano, 2014,8(3):3060-3067.
URL PMID |
| [40] |
NAIR R R, WU H A, JAYARAM P N, et al. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science, 2012,335(6067):442-444.
DOI URL PMID |
| [41] |
IBRAHIM A, LIN Y S. Gas permeation and separation properties of large-sheet stacked graphene oxide membranes. Journal of Membrane Science, 2018,550:238-245.
DOI URL |
| [42] | YANG J, GONG D, LI G, et al. Self-assembly of thiourea-crosslinked graphene oxide framework membranes toward separation of small molecules. Advanced Materials, 2018,30(16):1705775. |
| [43] |
ABRAHAM J, VASU K S, WILLIAMS C D,et al. Tunable sieving of ions using graphene oxide membranes. Nature Nanotechnology, 2017,12(6):546-550.
URL PMID |
| [44] |
CHI C, WANG X, PENG Y, et al. Facile preparation of graphene oxide membranes for gas separation. Chemistry of Materials, 2016,28(9):2921-2927.
DOI URL |
| [45] | YANG E, HAM M H, PARK H B, et al. Tunable semi-permeability of graphene-based membranes by adjusting reduction degree of laminar graphene oxide layer. Journal of Membrane Science, 2018,547:73-79. |
| [46] |
SU Y, KRAVETS V G, WONG S L, et al. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nature Communications, 2014,5(1):4843.
DOI URL PMID |
| [47] |
SUN P, WANG K, ZHU H. Recent developments in graphene-based membranes: structure, mass-transport mechanism and potential applications. Advanced Materials, 2016,28(12):2287-2310.
DOI URL PMID |
| [48] |
ZHANG Y, ZHANG S, CHUNG T S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environmental Science & Technology, 2015,49(16):10235-10242.
DOI URL PMID |
| [49] |
BURRESS J W, GADIPELLI S, FORD J, et al. Graphene oxide framework materials: theoretical predictions and experimental results. Angewandte Chemie International Edition, 2010,49(47):8902-8904.
URL PMID |
| [50] | KARUNAKARAN M, VILLALOBOS L F, KUMAR M, et al. Graphene oxide doped ionic liquid ultrathin composite membranes for efficient CO2 capture. Journal of Materials Chemistry A, 2017,5(2):649-656. |
| [51] | WANG S, XIE Y, HE G, et al. Graphene oxide membranes with heterogeneous nanodomains for efficient CO2 separations. Angewandte Chemie, 2017,129(45):14434-14439. |
| [52] | HUANG G, ISFAHANI A P, MUCHTAR A, et al. Pebax/ionic liquid modified graphene oxide mixed matrix membranes for enhanced CO2 capture. Journal of Membrane Science, 2018,565:370-379. |
| [53] | XIN Q, MA F, ZHANG L, et al. Interface engineering of mixed matrix membrane via CO2-philic polymer brush functionalized graphene oxide nanosheets for efficient gas separation. Journal of Membrane Science, 2019,586:23-33. |
| [54] |
SHEN J, LIU G, HUANG K, et al. Subnanometer two-dimensional graphene oxide channels for ultrafast gas sieving. ACS Nano, 2016,10(3):3398-3409.
DOI URL PMID |
| [55] |
YING W, CAI J, ZHOU K, et al. Ionic liquid selectively facilitates CO2 transport through graphene oxide membrane. ACS Nano, 2018,12(6):5385-5393.
URL PMID |
| [56] |
SCHMIDT H, WANG S, CHU L, et al. Transport properties of monolayer MoS2 grown by chemical vapor deposition. Nano Letters, 2014,14(4):1909-1913.
DOI URL PMID |
| [57] |
NICOLOSI V, CHHOWALLA M, G KANATZIDIS M et al. Liquid exfoliation of layered materials. Science, 2013,340:1226419.
DOI URL |
| [58] |
ZHANG W, HUANG J K, CHEN C H, et al. High-gain phototransistors based on a CVD MoS2 monolayer. Advanced Materials, 2013,25(25):3456-3461.
DOI URL PMID |
| [59] |
WANG H, FENG H, LI J. Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small, 2014,10(11):2165-2181.
DOI URL PMID |
| [60] | ZENG Z, SUN T, ZHU J, et al. An effective method for the fabrication of few-layer-thick inorganic nanosheets. Angewandte Chemie International Edition, 2012,124(36):9186-9190. |
| [61] | GEE M A, FRINDT R F, JOENSEN P, et al. Inclusion compounds of MoS2. Materials Research Bulletin, 1986,21(5):543-549. |
| [62] |
WANG D, WANG Z, WANG L, et al. Ultrathin membranes of single- layered MoS2 nanosheets for high-permeance hydrogen separation. Nanoscale, 2015,7(42):17649-17652.
DOI URL PMID |
| [63] | ACHARI A, SAHANA S, ESWARAMOORTHY M. High performance MoS2 membranes: effects of thermally driven phase transition on CO2 separation efficiency. Energy & Environmental Science, 2016,9(4):1224-1228. |
| [64] | OSTWAL M, SHINDE D B, WANG X, et al. Graphene oxide- molybdenum disulfide hybrid membranes for hydrogen separation. Journal of Membrane Science, 2018,550:145-154. |
| [65] | ZHAO S, XUE J, KANG W. Gas adsorption on MoS2 monolayer from first-principles calculations. Chemical Physics Letters, 2014, 595-596:35-42. |
| [66] |
HE Q, ZENG Z, YIN Z, et al. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small, 2012,8(19):2994-2999.
URL PMID |
| [67] |
BEREAN K J, OU J Z, DAENEKE T, et al. 2D MoS2 pdms nanocomposites for NO2 separation. Small, 2015,11(38):5035-5040.
URL PMID |
| [68] |
SHEN Y, WANG H, ZHANG X, et al. MoS2 nanosheets functionalized composite mixed matrix membrane for enhanced CO2 capture via surface drop-coating method. ACS Applied Materials & Interfaces, 2016,8(35):23371-23378.
DOI URL PMID |
| [69] |
CHEN D K, YING W, GUO Y, et al. Enhanced gas separation through nanoconfined ionic liquid in laminated MoS2 membrane. ACS Applied Materials & Interfaces, 2017,9(50):44251-44257.
URL PMID |
| [70] | CHEN D, WANG W, YING W, et al. CO2-philic WS2 laminated membranes with a nanoconfined ionic liquid. Journal of Materials Chemistry A, 2018,6(34):16566-16573. |
| [71] | ALHABEB M, MALESKI K, ANASORI B, et al. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chemistry of Materials, 2017,29(18):7633-7644. |
| [72] |
WANG J T, CHEN P P, SHI B B, et al. A regularly channeled lamellar membrane for unparalleled water and organics permeation. Angewandte Chemie-International Edition, 2018,57(23):6814-6818.
DOI URL PMID |
| [73] |
DING L, WEI Y, WANG Y, et al. A two-dimensional lamellar membrane: MXene nanosheet stacks. Angewandte Chemie International Edition, 2017,56(7):1825-1829.
DOI URL PMID |
| [74] |
REN C E, HATZELL K B, ALHABEB M, et al. Charge- and size-selective ion sieving through Ti3C2Tx MXene membranes. The Journal of Physical Chemistry Letters, 2015,6(20):4026-4031.
URL PMID |
| [75] |
RASOOL K, MAHMOUD K A, JOHNSON D J, et al. Efficient antibacterial membrane based on two-dimensional Ti3C2Tx (MXene) nanosheets. Sci. Rep, 2017,7(1):1598.
DOI URL PMID |
| [76] |
RASOOL K, HELAL M, ALI A, et al. Antibacterial activity of Ti3C2Tx MXene. ACS Nano, 2016,10(3):3674-3684.
DOI URL PMID |
| [77] |
DING L, WEI Y, LI L, et al. MXene molecular sieving membranes for highly efficient gas separation. Nature Communications, 2018,9:155.
DOI URL PMID |
| [78] |
SHEN J, LIU G Z, JI Y F, et al. 2D MXene nanofilms with tunable gas transport channels. Advanced Functional Materials, 2018,28(31):1801511.
DOI URL |
| [79] |
LI L, ZHANG T, DUAN Y, et al. Selective gas diffusion in two-dimensional MXene lamellar membranes: insights from molecular dynamics simulations. Journal of Materials Chemistry A, 2018,6(25):11734-11742.
DOI URL |
| [80] |
WANG Q, O’HARE D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chemical Reviews, 2012,112(7):4124-4155.
URL PMID |
| [81] | LU P, LIU Y, ZHOU T, et al. Recent advances in layered double hydroxides (LDHs) as two-dimensional membrane materials for gas and liquid separations. Journal of Membrane Science, 2018,567:89-103. |
| [82] | KIM T W, SAHIMI M, TSOTSIS T T. Preparation of hydrotalcite thin films using an electrophoretic technique. Industrial & Engineering Chemistry Research, 2008,47(23):9127-9132. |
| [83] | KIM T W, SAHIMI M, TSOTSIS T T. The preparation and characterization of hydrotalcite thin films. Industrial & Engineering Chemistry Research, 2009,48(12):5794-5801. |
| [84] |
LIU Y, WANG N, CAO Z, et al. Molecular sieving through interlayer galleries. Journal of Materials Chemistry A, 2014,2(5):1235-1238.
DOI URL |
| [85] |
WANG Y, LOW Z X, KIM S, et al. Functionalized boron nitride nanosheets: a thermally rearranged polymer nanocomposite membrane for hydrogen separation. Angewandte Chemie International Edition, 2018,57(49):16056-16061.
URL PMID |
| [86] |
KIM S, HOU J, WANG Y, et al. Highly permeable thermally rearranged polymer composite membranes with a graphene oxide scaffold for gas separation. Journal of Materials Chemistry A, 2018,6(17):7668-7674.
DOI URL |
| [87] |
ZHANG X, HE Y, LI R, et al. 2D mica crystal as electret in organic field-effect transistors for multistate memory. Advanced Materials, 2016,28(19):3755-3760.
DOI URL PMID |
| [88] |
WANG D, YUAN G, HAO G, et al. All-inorganic flexible piezoelectric energy harvester enabled by two-dimensional mica. Nano Energy, 2018,43:351-358.
DOI URL |
| [89] |
YING W, HAN B, LIN H, et al. Laminated mica nanosheets supported ionic liquid membrane for CO2 separation. Nanotechnology, 2019,30(38):385705.
DOI URL PMID |
| [90] | ZHANG H P, HU W, DU A, et al. Doped phosphorene for hydrogen capture: a DFT study. Applied Surface Science, 2018,433:249-255. |
| [91] | ZHANG H P, DU A, SHI Q B, et al. Adsorption behavior of CO2 on pristine and doped phosphorenes: a dispersion corrected DFT study. Journal of CO2 Utilization, 2018,24:463-470. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | ZHOU Fan, TIAN Zhilin, LI Bin. Research Progress on Carbide Ultra-high Temperature Ceramic Anti-ablation Coatings for Thermal Protection System [J]. Journal of Inorganic Materials, 2025, 40(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||