|
|
Kindling the “Material Fire” in High-temperature Fuel Cells
LING Yihan
2025 Vol. 40 (12): 1309–1310
 Abstract
Abstract(
175 )
 HTML
HTML(
4)
 PDF
PDF(201KB)(
74
)
High-temperature fuel cells, as highly efficient and clean electrochemical energy conversion devices, represent a kind of key technology for achieving “dual carbon” energy strategies. Their core appeal lies in the broad fuel adaptability, high energy conversion efficiency and stable all-solid-state structure. Fuel cells can utilize not only hydrogen as fuel but also directly employ diverse fuels such as hydrocarbons (e.g., natural gas, methanol) and ammonia, demonstrating excellent application flexibility and energy compatibility. Compared to conventional internal combustion engine power generation, high-temperature fuel cells directly convert chemical energy into electricity through electrochemical reactions, achieving power generation efficiencies exceeding 60%. Furthermore, by utilizing waste heat from the reaction process for combined heat and power generation, overall energy utilization efficiency can be elevated to over 80%. However, commercialization of high-temperature fuel cell technology remains intrinsically tied to a core challenge—key component materials. Specifically, developing component materials capable of long-term stable operation under harsh conditions of high temperatures is currently the bottleneck that restricts advancement of high-temperature fuel cells. Breakthroughs in this area will lay cornerstone for development of high-temperature fuel cell technology in the future. Conventional high-temperature fuel cells rely on classic material systems such as yttria-stabilized zirconia (YSZ) electrolytes, nickel-YSZ metal-ceramic anodes, and lanthanum-strontium-manganese (LSM) oxide cathodes. These materials exhibit outstanding electrochemical activity and ionic conductivity under high-temperature conditions, providing a crucial foundation for achieving efficient energy conversion. However, as operating temperatures are reduced to the medium-to-low range (400-700 ℃), the oxygen ion migration rate and electrode reaction kinetics of conventional material systems significantly decline, markedly deteriorating the electrochemical performance of the cells. To enable the operation of high-temperature fuel cells at lower temperatures while maintaining efficiency and stability, researchers are actively developing novel electrolyte materials with high ionic conductivity and highly catalytically active electrode materials. This advancement aims to promote the widespread application of high-temperature fuel cell technology within next-generation energy systems. At the invitation of the editorial board of Journal of Inorganic Materials, I served as a guest editor to organize and compile this topical section on “Key Materials for High-temperature Fuel Cells”. Renowned domestic research groups from institutions including Harbin Institute of Technology, Xi’an Jiaotong University, Beijing Huairou Laboratory, and Wuhan Institute of Technology have dedicated themselves to analyzing the latest advances in fundamental research, preparation techniques, performance optimization, and mechanism studies concerning materials for high-temperature fuel cells. This topical section is intended to provide researchers with a deeper understanding of high-temperature fuel cells, serving as a window into the latest developments within the field, and actively promoting advancement of materials for high-temperature fuel cells as well as progress in the discipline. I extend my heartfelt gratitude to all experts who contributed to this topical section amidst their busy schedules. It is through their diligent efforts and generous support that this publication has come to fruition.

|
|
|
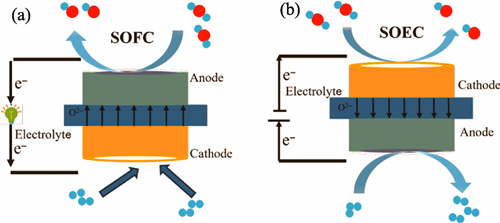
Research Progress on Preparation Technologies and Performance of Straight-pore Electrode Structures for Solid Oxide Cells
LING Yihan, GUO Sheng, CAO Zhiqiang, TIAN Yunfeng, LIU Fangsheng, JIN Fangjun, GAO Yuan
2025 Vol. 40 (12): 1311–1323
 Abstract
Abstract(
402 )
 HTML
HTML(
19)
 PDF
PDF(7121KB)(
899
)
Solid oxide cell (SOC) has attracted extensive attention in recent years due to its high-efficiency clean power generation capability in fuel cell (SOFC) mode and excellent hydrogen production and energy storage potential in electrolysis cell (SOEC) mode. Conventional SOC typically employs pore-forming agents such as graphite or carbon powder to fabricate porous electrode supports. This approach results in disordered pore distribution and complex pore structure, leading to high tortuosity factor. Particularly under conditions of dilute fuel or high current densities, these factors cause concentration polarization, limiting further performance improvements. To address these challenges, application of straight-pore structures has shown significant progress. The ordered pore channels in this structure enhance gas diffusion and transport, reduce concentration polarization, and improve the impregnation efficiency of electrode materials while increasing the utilization of active sites, thereby significantly boosting the electrochemical performance of SOC. This review systematically summarizes recent advancements in the preparation techniques for straight-pore structured SOC. It details the pore-forming mechanisms, process characteristics, and applications of key technologies (phase inversion, freeze-drying and alginate ion gelation) in both planar and tubular SOC configurations. Furthermore, it provides an in-depth analysis of how the straight-pore structure enhances performance in both SOFC mode (hydrogen and hydrocarbon fuel adaptability) and SOEC mode (conventional H2O/CO2 electrolysis and fuel-assisted electrolysis), elucidating the underlying mechanisms. Despite the great potential demonstrated by straight-pore structures in SOC, comprehensive reviews focusing on their fabrication techniques remain scarce. This paper aims to consolidate the latest progress in straight-pore SOC preparation technologies, analyze their technical advantages and existing challenges, and propose future research directions.
|
|
|
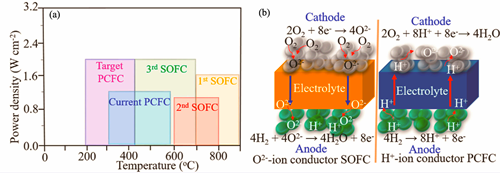
Research Progress on Proton-conducting Solid Oxide Fuel Cells with Hydrogen-containing Fuel
XUE Zixuan, YIN Chaofan, YAO Yuechao, WANG Yanmin, SUN Yueyue, LIU Zhengrong, ZHOU Yucun, ZHOU Jun, WU Kai
2025 Vol. 40 (12): 1324–1340
 Abstract
Abstract(
495 )
 HTML
HTML(
8)
 PDF
PDF(7194KB)(
4281
)
Driven by global energy transition and carbon neutrality goals, proton-conducting solid oxide fuel cells (P-SOFCs) have become a research hotspot in clean energy technology due to their advantages of efficient medium-to-low temperature power generation (400-600 ℃), excellent fuel compatibility, and high energy conversion efficiency. This review analyzes the development prospects of hydrogen-containing fuel P-SOFCs. Addressing key technological bottlenecks, this review focuses on three core dimensions including material design, reaction mechanisms, and characterization techniques to summarize research progress and technical challenges in hydrocarbon-fueled and ammonia-fueled P-SOFC systems. For hydrocarbon-fueled P-SOFCs, the carbon deposition issue is thoroughly examined. Their formation mechanisms, characterization methods and influencing factors on carbon deposition are discussed in depth. Advanced improvement strategies are highlighted, including modification of reforming catalysts, optimization of proton-conducting electrolytes, and novel design of electrodes. Regarding direct ammonia fuel cells (DAFCs), challenges related to insufficient anode durability are addressed. Critical influencing factors are identified as catalyst activity, support types, nitridation corrosion mechanisms, hydrogen partial pressure, ammonia flow rate, and anode microstructure. Based on cutting-edge research, novel improvement strategies, such as anode modification, optimization of anode catalytic layers and innovative cell structure designs, are summarized. This review outlines future development directions to advance the commercialization of hydrogen-containing fuel P-SOFCs.
|
|
|
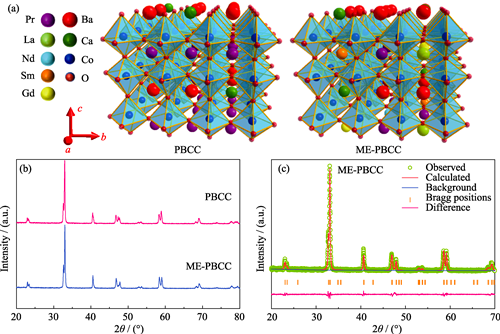
Enhancing Cr-tolerance Ability of Double Perovskite Cathodes through Configuration Entropy Engineering
WANG Zhe, HAO Hongru, WU Zonghui, XU Lingling, LÜ Zhe, WEI Bo
2025 Vol. 40 (12): 1341–1348
 Abstract
Abstract(
401 )
 HTML
HTML(
11)
 PDF
PDF(8091KB)(
144
)
Solid oxide fuel cells (SOFCs) are a kind of highly efficient and clean power generation devices whose cathode performance is critically important for the commercial application of the entire cell. Cation segregation on the surface of cathodes significantly affects the performance and operational stability. The double perovskite oxide PrBa0.8Ca0.2Co2O5+δ (PBCC), a highly active cathode, still suffers from serious surface segregation and insufficient Cr-tolerance ability. In order to improve the stability of cathode, an A-site medium-entropy double perovskite oxide Pr0.6La0.1Nd0.1Sm0.1Gd0.1Ba0.8Ca0.2Co2O5+δ (ME-PBCC) derived from PBCC was prepared, and its segregation behavior in Cr-containing atmosphere was systematically investigated. Compared with traditional PBCC cathode, segregation of BaCrO4 and Co3O4 on the surface of ME-PBCC is significantly suppressed, which is attributed to its higher configurational entropy. Electrical conductivity relaxation (ECR) and electrochemical impedance spectroscopy (EIS) results indicate that electrochemical stability of the ME-PBCC cathode has been significantly improved. Among the improvements, after Cr deposition for 48 h, the oxygen surface exchange coefficient kchem of the medium-entropy cathode decreases from 4.4×10-4 cm·s-1 to 1.8×10-4 cm·s-1, with the reduction of kchem significantly lower than that of PBCC (which decreases from 7.3×10-4 cm·s-1 to 1.2×10-4 cm·s-1). Furthermore, the EIS results after treatment in Cr-containing air at 700 ℃ for 48 h show that the polarization resistance (Rp) of ME-PBCC is only 0.07 Ω·cm2, which is lower than 0.11 Ω·cm2 of PBCC, confirming that the medium-entropy cathode has significantly improved operational stability and Cr resistance. This study demonstrates that ME-PBCC is a promising cathode material for SOFCs.
|
|
|
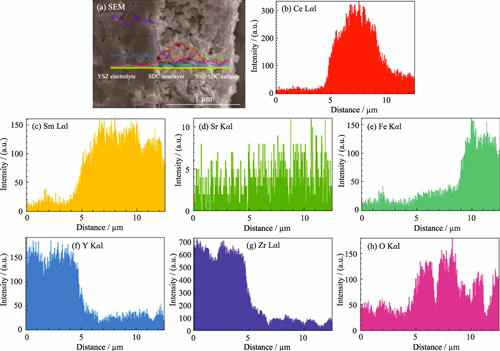
Preparation of Cobalt-free Composite Cathode for Efficient High-temperature Hydrogen Fuel Cell via One-pot Synthesis Method
LIU Tong, HUANG Su, ZHU Shiyue, ZHA Fanglin, HU Xuelei, WANG Yao
2025 Vol. 40 (12): 1349–1355
 Abstract
Abstract(
253 )
 HTML
HTML(
5)
 PDF
PDF(2588KB)(
238
)
High-temperature hydrogen fuel cell, commonly referred to as solid oxide fuel cell (SOFC), is regarded as an effective energy conversion device for achieving the "dual carbon" goals due to its advantages such as high energy conversion efficiency, strong fuel flexibility, environmental friendliness, and all-solid-state structure. To address the issues of high cost, volatility, high thermal expansion coefficient, and easy reaction with electrolyte of traditional cobalt-based cathodes, a cobalt-free composite cathode (O-SSF-SDC), consisting of 75% (in mass) perovskite oxide Sm0.6Sr0.4FeO3-δ (SSF) and 25% (in mass) fluorite structure Ce0.8Sm0.2O1.9 (SDC), was prepared by one-pot synthesis method for SOFC application. The morphological results and element mapping images demonstrated that the O-SSF-SDC composite cathode exhibited uniform particle size, which provided more active reaction sites for the oxygen reduction reaction in the cathode. The symmetrical cell with O-SSF-SDC cathode showed a relatively low electrode polarization resistance of 0.175 Ω·cm2 at 700 ℃ in air, while the maximum output power density of the single cell equipped with O-SSF-SDC cathode reached 609.6 mW·cm-2 when exposing the anode and cathode to 3% H2O wet H2 and ambient air, respectively, demonstrating comparable electrochemical performance to traditional cobalt-based cathodes. This work can provide reference for the development of efficient high-temperature hydrogen fuel cells.
|
|
|
Fluorination of BaZr0.1Ce0.7Y0.1Yb0.1O3 as Electrolyte Material for Proton-conducting Solid Oxide Fuel Cell
JIANG Yuehong, SONG Yunfeng, ZHANG Leilei, MA Ji, SONG Zhaoyuan, LONG Wen
2025 Vol. 40 (12): 1356–1364
 Abstract
Abstract(
367 )
 HTML
HTML(
9)
 PDF
PDF(5138KB)(
184
)
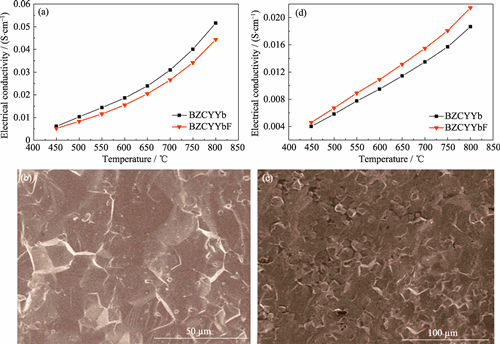
Proton-conducting solid oxide fuel cells (H+-SOFC) have gained great attention due to weak temperature dependence and high energy conversion efficiency. However, how to improve their proton conductivity still remains an open problem. In this work, a fluorination strategy based on the BaZr0.1Ce0.7Y0.1Yb0.1O3 (BZCYYb) electrolyte was proposed to improve the proton conductivity. It is found that the conductivity (σ) of the fluorinated BaZr0.1Ce0.7Y0.1Yb0.1O2.9F0.1 (BZCYYbF) is 4.59×10-3-2.14×10-2 S/cm at 450-800 ℃ in dry H2, higher than that of the primitive BZCYYb electrolyte (σ=3.99×10-3-1.86×10-2 S/cm). The electrolyte fluorination significantly reduces anode polarization resistance for hydrogen oxidation reaction from 2.50 Ω·cm2 to 1.94 Ω·cm2, and total resistance of the single cell with 300-μm-thick electrolyte supporting from 1.54 Ω·cm2 to 1.47 Ω·cm2. Therefore, the BZCYYbF single cell shows much higher maximum power density (172 mW·cm-2) than the BZCYYb single cell (144 mW·cm-2) at 700 ℃. This result is attributed to the fact that the electrolyte fluorination not only improves the proton conduction capacity but also enhances the rates of H2 diffusion and adsorption/dissociation on the anode sides. In conclusion, the fluorination of BZCYYb electrolyte can significantly improve its proton conductivity and thereby contribute to superior electrochemical performance of H+-SOFC.
|
|
|
Iron-based Perovskite Material La0.25M0.75FeO3-δ (M=Ca, Sr, Ba): Preparation and Performance as Cathode for Solid Oxide Fuel Cells
YANG Hengqiang, ZHANG Xinyue, MA Yichu, ZHOU Qingjun
2025 Vol. 40 (12): 1365–1372
 Abstract
Abstract(
470 )
 HTML
HTML(
15)
 PDF
PDF(2244KB)(
409
)
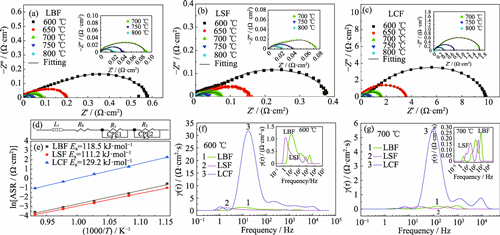
The performance of solid oxide fuel cell (SOFC) is mainly constrained by the cathode's oxygen reduction reaction (ORR), which is crucial for overall cell efficiency. In this study, La0.25M0.75FeO3-δ (M=Ca, Sr, Ba, abbreviated as LCF, LSF, LBF) perovskite cathodes were synthesized based on tolerance factors and structural design, and effects of Ba, Sr, and Ca doping on electrochemical performance was examined. Different alkaline earth elements have a significant effect on the crystal structure. LBF is Pm-3m cubic phase, LSF is R-3c rhombohedral phase, and LCF is a composite phase of P21ma orthogonal and Pcmn rhombohedral. Difference of crystal structure also leads to various thermal expansion coefficient (TEC) and electrical conductivity. LCF possesses the smallest TEC (1.38×10-5 K-1), while LSF possesses the highest conductivity, which reaches 404.4 S·cm-1 at 550 ℃. All three Fe-based cathodes exhibit excellent stability in air and CO2 atmospheres, as well as chemical compatibility with electrolytes. In addition, different alkaline earth elements also affect catalytic activity of the material. LSF and LBF have low area specific resistance (ASR). At 800 ℃, their ASR are only 0.022 and 0.027 Ω·cm2, which are better than the 0.351 Ω·cm2 of LCF. The higher oxygen reduction activity is attributed to its crystal structure, oxygen adsorption and dissociation ability. In view of the advantages and disadvantages of Ba2+, Sr2+ and Ca2+ in cathode performance, medium/high-entropy design can be effectively introduced in A-site in the future to give full play to their respective advantages to obtain SOFC cathode with excellent comprehensive performance.
|
|
|
Micro-spherical Ag2Se: Solvothermal Synthesis and Thermoelectric Properties
MIAO Pengcheng, WANG Lijun, SHEN Ziyi, HUANG Li, YUAN Ningyi, DING Jianning
2025 Vol. 40 (12): 1373–1378
 Abstract
Abstract(
315 )
 HTML
HTML(
4)
 PDF
PDF(3965KB)(
534
)
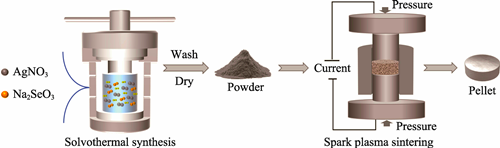
Thermoelectric materials can directly convert thermal energy into electrical energy, offering significant potential for waste heat recovery applications. Ag2Se as a novel low-temperature thermoelectric material has attracted considerable attention due to its unique crystal structure and excellent electronic transport properties. In this study, micro-sized Ag2Se powders were synthesized via solvothermal method, which contributes to precise control over the powders’ structure, composition, and grain size. The results indicate that the synthesized Ag2Se powders exhibit irregular micro-columnar structure with a complete crystal lattice, and the Ag-to-Se ratio is close to the theoretical value (approximately 2 : 1 (in atom)). Furthermore, spark plasma sintering (SPS) was employed to densify the Ag2Se powders at various temperatures, which reveals that the sintering temperature has a significant impact on the microstructure and thermoelectric performance. The fracture surface of the sample sintered at 473 K is relatively dense, whereas samples sintered at 573 and 673 K display the emergence of nanopores. Notably, the nanopores are particularly pronounced in the sample sintered at 673 K, which effectively reduces the material’s lattice thermal conductivity (κ1) to 0.33 W·m-1·K-1 at room temperature. Ultimately, the Ag2Se bulk prepared at a sintering temperature of 573 K exhibits the best thermoelectric performance, achieving a room-temperature thermoelectric figure of merit (ZT) of 0.5. This study not only demonstrates the feasibility of the solvothermal method for fabricating high-performance micro-sized thermoelectric powders but also provides valuable experimental evidence for further optimizing the microstructure and thermoelectric properties of Ag2Se materials.
|
|
|
Non-solvent and Low-temperature Preparation of Porous Silicon-carbon Anodes for Enhanced Lithium Storage
YI Guogang, WU Yaoying, ZU Xihong
2025 Vol. 40 (12): 1379–1386
 Abstract
Abstract(
416 )
 HTML
HTML(
3)
 PDF
PDF(4654KB)(
1412
)
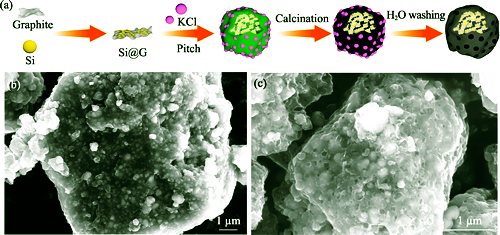
To solve the problems of large volume expansion, poor cycling stability, low electrical conductivity and high energy consumption of silicon-anode materials, a porous silicon-carbon anode material (P-Si@G@C) was prepared by a non-solvent low-temperature method with nano-silicon as active substance, graphite as conductive carrier, asphalt as carbon precursor, and potassium chloride as pore-forming agent. Structure and performance of P-Si@G@C anode were systematically studied by comparing with a series of silicon-carbon anodes. The results showed that insertion of silica nanoparticles in graphite matrix (Si@G) could improve the electrical conductivity of the whole material which is beneficial for electron transport, and the graphite matrix could alleviate the volume expansion of silica nanoparticles. Then the porous carbon shell coated on the surface of Si@G greatly reduced the volume expansion of nano-silicon, and improved the diffusion rate of lithium ion and electron transport rate. Compared with Si@G and unperforated Si@G@C anodes, the P-Si@G@C anode presented the best electrical performance with initial Coulombic efficiency of 85.8%. At the current density of 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0 A·g-1, the specific capacities of P-Si@G@C anode were 1403.6, 1291.7, 1206.1, 1093.6, 868.4, and 609.5 mAh·g-1, respectively. Its recovery rate of specific capacity reached 98.3%, displaying excellent rate performance. The specific capacity still remained 770.7 mAh·g-1 after 200 cycles at 1.0 A·g-1, showing good long-cycle stability.
|
|
|
Numerical Simulation of Particle Classification for Spent Hydrogenation Catalyst
ZHAO Lijuan, TAN Zhe, ZHANG Xiaoguang, JIANG Guosai, TAO Ran, PAN De’an
2025 Vol. 40 (12): 1387–1394
 Abstract
Abstract(
211 )
 HTML
HTML(
8)
 PDF
PDF(3377KB)(
442
)
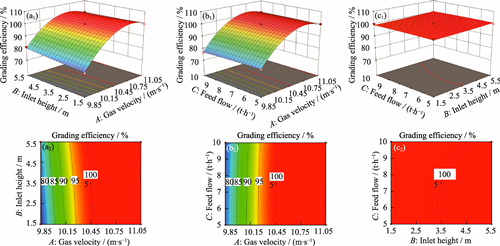
Spent hydrogenation catalysts are an important source of regeneration catalysts due to their large waste volume and high particle integrity. Most of the existing recovery technologies focusing on recovering valuable metals limit studies on the recovery of carrier particles. This study addresses the key challenge of ineffective classification of rod-shaped spent catalyst particles via traditional sieving due to their length-to-diameter ratios exceeding standard specifications. A fluidized bed classification process is innovatively proposed, and a coupled computational fluid dynamics (CFD) and discrete element method (DEM) simulation combined with response surface methodology (RSM) is employed to systematically elucidate the intrinsic mechanisms and optimization principles of fluidized bed classification. The results demonstrate that a fluidized bed enables efficient classification of particles with varying aspect ratios via gas-solid fluidization. Gas velocity is identified as the dominant factor influencing classification efficiency, followed by feed flow, whereas inlet height exhibits a negligible impact. A critical feed flow threshold exists under specific gas velocity and inlet height; exceeding this threshold leads to a decline in classification efficiency. By establishing a Box-Behnken design (BBD) model, optimal conditions are identified as a gas velocity of 10.45 m/s, a feed flow of 7.50 t/h, and an inlet height of 3.50 m, achieving 100% classification efficiency. This study clarifies the multi-physics coupling mechanism in fluidized bed classification and provides theoretical guidance for pre-classification processes of carrier particles during spent hydrogenation catalyst recycling.
|
|
|
Influence of Support Characteristics on Coverage of Ionomer and Oxygen Reduction Performance for Pt/C Catalysts
LI Xueru, MA Zhejie, GUO Yujie, LI Ping
2025 Vol. 40 (12): 1395–1404
 Abstract
Abstract(
306 )
 HTML
HTML(
3)
 PDF
PDF(12580KB)(
109
)
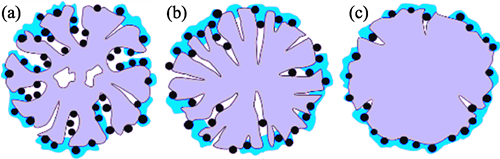
Carbon support is an important component of Pt/C catalyst commonly used in the membrane electrodes of proton exchange membrane fuel cells, and ionomer is one of the key components that make up the catalytic layer. However, the influence of support characteristics on coverage of ionomer and oxygen reduction performance for Pt/C catalysts is still unknown. Here, six different types of representative commercial carbon supports (VC, KB1, KB2, BP, SJR, and AB) were focused. The microstructure and surface chemical properties of the carbon supports and the Pt/C catalysts prepared with and without addition of ionomer were investigated using various characterization methods. The oxygen reduction reaction (ORR) performance of various Pt/C catalysts was tested to explore the electrocatalytic structure-activity relationship of representative carbon supports for Pt catalysts. As revealed, carbon supports with large specific surface areas and rich pore structures, such as KB1, KB2 and BP, contribute to more uniform distribution of Pt particles. Presence of oxygen-containing functional groups on solid carbon supports with strong hydrophilicity, such as VC and SJR, contributes to dispersion of Pt particles. Meanwhile, carbon supports with abundant mesopores in the range of 2-8 nm (KB1 and KB2) are beneficial for improving location of Pt within pores of carbon particles, while those with high specific surface area and full of micropores (BP) and those with medium or low specific surface area (VC, SJR, and AB) have the most Pt nano-particles distributed on the outer surface of the carbon particles. By combining changes in specific surface area and pore structure of various Pt/C samples before and after addition of ionomer, the coverage of ionomer was calculated, and a distribution model of ionomer on different catalyst particles was proposed. On the solid carbon supported catalysts, a certain amount of ionomer basically covers entire outer surface of carbon particles. For the BP supported catalyst which is dominated by micropores, ionomer can block the micropores, resulting in a significant decrease in specific surface area and pore volume. For the mesoporous carbon supported catalysts, the same amount of ionomer is hard to block all micropores and mesopores, leading to lower coverage. Furthermore, ORR activity of Pt/C catalyst mainly depends on the Pt nano-particle size, and the Pt nano-particles located in the pores inside of the carbon particles can be protected from the poisoning of ionomer. Therefore, Pt catalysts supported on the carbon supports of KB series exhibit excellent performance for the ORR kinetics occurring in liquid-phase.
|
|
|
Preparation and Economic Analysis of High-current-density Electrocatalysts for Alkaline Water Electrolysis
YU Zelong, TANG Chun, RAO Jiahao, GUO Heng, ZHOU Ying
2025 Vol. 40 (12): 1405–1413
 Abstract
Abstract(
834 )
 HTML
HTML(
5)
 PDF
PDF(5642KB)(
727
)
Alkaline water electrolysis (AWE) faces challenges of low efficiency and high costs due to its relatively low current density. It is necessary to develop efficient and stable non-precious metal electrocatalysts under high current densities. In this study, an amorphous NiMoOP/NF electrocatalyst was fabricated by the hydrothermal method combined with phosphorization on a nickel foam (NF) substrate. The amorphous needle-like morphology effectively increases active sites and enhances the stability of hydrogen production through water electrolysis. At current densities of 10 and 1000 mA·cm-2, the hydrogen evolution overpotentials are 31 and 370 mV, respectively, and the catalyst stably runs for 1100 h at a high current density of 1 A·cm-2. The NiMoOP/NF material, when integrated with crystalline silicon heterojunction solar cells for overall water splitting, achieves a theoretical solar-to-hydrogen conversion efficiency of up to 18.60%. Under industrially relevant conditions (60 ℃, 30% (in mass) KOH electrolyte), the electrolysis voltage is 1.77 V, enabling a current density of 400 mA·cm-2, with a hydrogen production energy consumption of 4.19 kWh·Nm-3 (Nm3: Normal cubic meter). Economic analysis of photovoltaic-powered hydrogen production via electrolysis indicates that the minimum hydrogen production cost for an off-grid and non-storage photovoltaic hydrogen production system is ¥28.52 kg-1. The amorphous nanoneedle-like materials developed in this study significantly enhanced both hydrogen evolution activity and stability during water electrolysis, providing valuable insights for design of high-current-density hydrogen evolution catalysts. Furthermore, the combined economic analysis of photovoltaic electrolysis for green hydrogen production supports advancement of green hydrogen industry.
|
|
|
First-principles Study of Novel MAX Phase Zr3InC2 under High Pressure
GUO Jiaxin, CHEN Meijuan, WU Hao, ZHENG Xiaoran, MIN Nan, TIAN Hui, QI Dongli, LI Quanjun, DU Shiyu, SHEN Longhai
2025 Vol. 40 (12): 1414–1424
 Abstract
Abstract(
310 )
 HTML
HTML(
2)
 PDF
PDF(4266KB)(
179
)
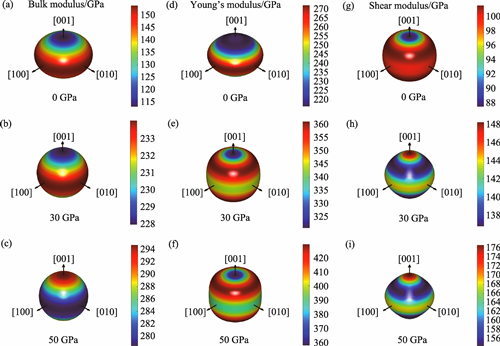
The novel In-based MAX phase Zr3InC2 has recently attracted considerable attention for its excellent physical properties, yet investigations into its behaviour under high pressure remain limited. This study systematically investigates effects of pressure on crystal structure, mechanical properties, electronic structure, and thermodynamic behavior of the novel MAX phase Zr3InC2 using first-principles calculations based on density functional theory (DFT). Comparative analysis with Zr3AlC2 reveals how substituting Al with In at the A-site influences structural and physical properties, as well as responses under high-pressure conditions. Calculated lattice parameters for both Zr3InC2 and Zr3AlC2 show good agreement with previous experimental reports. Results indicate pronounced anisotropic compression, with significantly higher compressibility along the c-axis than the a-axis. Elastic constants and phonon dispersion curves confirm mechanical and dynamic stability of Zr3InC2 up to 50 GPa. Poisson’s ratio analysis suggests brittle behavior at ambient pressure with ductility first appearing at 40 GPa. Discrepancy between the Poisson’s ratio and the Cauchy pressure at 50 GPa suggests that Zr3InC2 may be near the critical region of a brittle-to-ductile transition under high pressure. Compared with Zr3AlC2, Zr3InC2 exhibits greater sensitivity in mechanical properties under high pressure. Electronic structure calculations reveal its metallic nature. Thermodynamic analysis shows a relatively low thermal expansion coefficient at ambient pressure, while increased pressure leads to a significant rise in Debye temperature and minimum thermal conductivity. These findings highlight tunability of Zr3InC2’s thermodynamic properties under pressure, offering theoretical support for potential high-temperature applications.
|
|
|
Boosting the Thermoelectric Performance of Full-Heusler Fe2VAl Alloy via Substituting Al Site with V
ZHENG Yuanshun, YU Jian, YE Xianfeng, LIANG Dong, ZHU Wanting, NIE Xiaolei, WEI Ping, ZHAO Wenyu, ZHANG Qingjie
2025 Vol. 40 (12): 1425–1432
 Abstract
Abstract(
330 )
 HTML
HTML(
6)
 PDF
PDF(3535KB)(
168
)
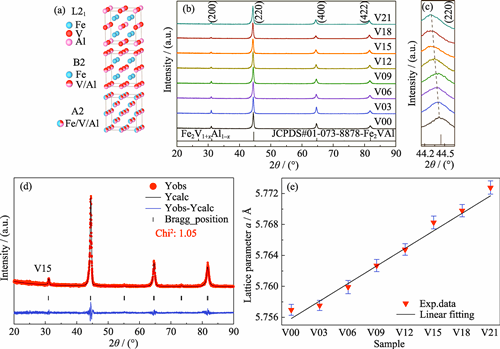
Full-Heusler Fe2VAl alloy has received significant attention for thermoelectric (TE) applications due to its high mechanical strength, favorable electrical transport behavior, and earth-abundant constituent elements. However, its intrinsically high lattice thermal conductivity hinders the enhancement of the figure of merit (zT). In this study, a series of bulk materials with the nominal composition of Fe2V1+xAl1-x (x=0-0.21) were prepared by the arc-melting method. Effects of substituting Al site with V on the phase composition, microstructure, band structure, and TE transport properties were systematically investigated. All materials exhibit a single phase with a partially disordered B2 structure. V-doping shifts the Fermi level into the conduction band, significantly enhancing the carrier concentration, and resulting in a high power factor of 4.5 mW·K-2·m-1. Additionally, the lattice thermal conductivity is substantially reduced due to enhanced phonon scattering induced by the mass and stress fluctuations. Ultimately, a maximum zT of 0.14 is achieved for the material with x=0.15, which is nearly 280 times larger than that of undoped Fe2VAl. This work demonstrates that substituting Al site with V can effectively improve the TE performance of Fe2VAl alloy.
|
|
|
Enhanced Performance of La0.7Sr0.3FeO3-δ Cathode for SOFC via Implementation of B-site High-entropy Strategy
LIU Hongming, ZHANG Jinke, CHEN Zhengpeng, LI Mingfei, QIAN Xiuyang, SUN Chuanqi, XIONG Kai, RAO Mumin, CHEN Chuangting, GAO Yuan, LING Yihan
2025 Vol. 40 (12): 1433–1442
 Abstract
Abstract(
774 )
 HTML
HTML(
2)
 PDF
PDF(7235KB)(
312
)
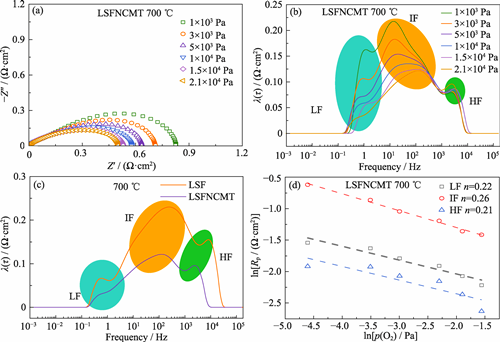
As classical cathode materials of solid oxide fuel cell (SOFC), Fe-based perovskite materials are favored for their affordable price, low thermal expansion coefficient and high stability. In this study, B-site high-entropy perovskite oxide La0.7Sr0.3(FeNiCo)0.8Mo0.1Ti0.1O3-δ (LSFNCMT) was prepared by the citric acid-nitrate combustion method. Due to the faster oxygen surface exchange rate of the high-entropy material, the LSFNCMT cathode shows excellent oxygen reduction reaction (ORR) activity with a polarization impedance (Rp) of 0.11 Ω·cm2 at 800 ℃, which is much lower than that of the La0.7Sr0.3FeO3-δ (LSF) cathode (0.31 Ω·cm2). Furthermore, the high-entropy material exhibits superior stability due to incorporation of highly acidic Ni, Co, and Mo cations as well as Ti cation with more negative average bonding energy (ABE) of metal-oxygen. In the 22 h-stability test of the symmetric cell with LSFNCMT cathode in the Cr-containing atmosphere, Rp only increases from 1.07 Ω·cm2 to 2.98 Ω·cm2, while Rp of the LSF cathode increases from 2.62 Ω·cm2 to 7.90 Ω·cm2 under the same conditions, indicating better Cr-resistance of LSFNCMT due to the high-entropy strategy. The fact that the maximum power density (MPD) of the single cell with LSFNCMT cathode at 800 ℃ is 1105.26 mW·cm-2, significantly higher than that of LSF cathode (830.74 mW·cm-2), and Rp at 800 ℃ is 0.24 Ω·cm2, lower than that of LSF cathode (0.36 Ω·cm2), confirming excellent toxicity resistance of the high-entropy cathode. This study shows that B-position high entropy is an effective way to improve the catalytic activity and chromium resistance of cathode materials.
|
|
|
Large-size Functional Wafer Temporary Bonding and Thinning
ZHU Yixin, YU Zhikui, WAN Qing
2025 Vol. 40 (12): 1443–1444
 Abstract
Abstract(
812 )
 HTML
HTML(
3)
 PDF
PDF(404KB)(
553
)
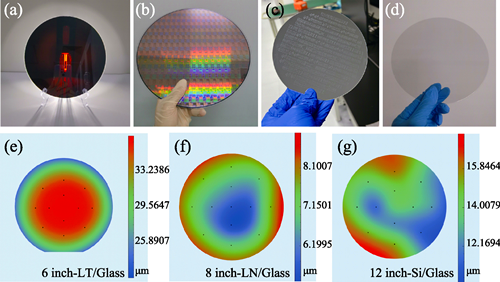
In the post-Moore era, temporary bonding and ultra-thin wafer thinning of large-size functional wafers have emerged as essential technologies underpinning innovation within the semiconductor industry. However, challenges such as wafer warpage and breakage commonly encountered during wafer thinning severely limit device performance and yield. To address these issues, WAN’s group at Yongjiang Laboratory developed a cost-effective, room-temperature ultra-flat temporary bonding technique. This innovative process has significantly reduced the risk of wafer warpage while achieving high flatness and stability in wafer bonding. By integrating this process with domestically developed thinning equipment, the group successfully thinned 8-inch silicon wafers down to 8 µm, 12-inch silicon power chips to 15 µm with a total thickness variation (TTV) ≤2 µm, and 8-inch lithium niobate wafers to 8-10 µm, thereby satisfying diverse piezoelectric micro-electro-mechanical system (MEMS) application demands. Currently, this technology is widely applied in heterogeneous integration of various wafer materials, including silicon, lithium niobate/lithium tantalate, gallium oxide, and indium phosphide, providing crucial support for the localization and development of power chips and high-performance MEMS devices.
|
|