
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (12): 1405-1413.DOI: 10.15541/jim20250012
• RESEARCH ARTICLE • Previous Articles Next Articles
YU Zelong1( ), TANG Chun1,2,3(
), TANG Chun1,2,3( ), RAO Jiahao1, GUO Heng1,2,3, ZHOU Ying1,2(
), RAO Jiahao1, GUO Heng1,2,3, ZHOU Ying1,2( )
)
Received:2025-01-08
Revised:2025-04-06
Published:2025-12-20
Online:2025-04-27
Contact:
TANG Chun, associate professor. E-mail: tangchun@swpu.edu.cn;About author:YU Zelong (2000-), male, Master candidate. E-mail: 18535069947@163.com
Supported by:CLC Number:
YU Zelong, TANG Chun, RAO Jiahao, GUO Heng, ZHOU Ying. Preparation and Economic Analysis of High-current-density Electrocatalysts for Alkaline Water Electrolysis[J]. Journal of Inorganic Materials, 2025, 40(12): 1405-1413.
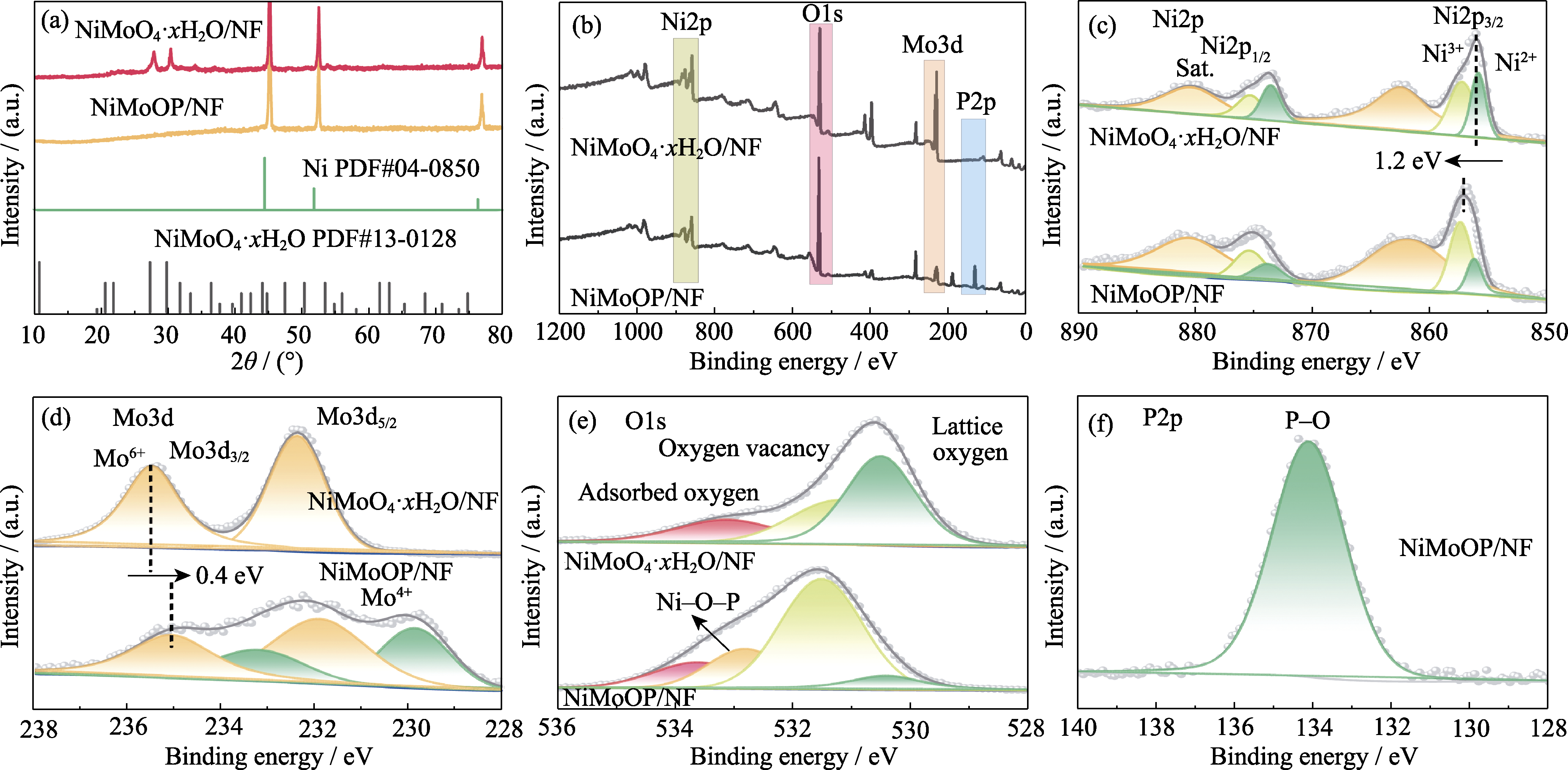
Fig. 2 XRD patterns and XPS spectra of NiMoO4·xH2O/NF and NiMoOP/NF (a) XRD patterns; (b) Full XPS spectra; (c) Ni2p XPS spectra; (d) Mo3d XPS spectra; (e) O1s XPS spectra; (f) P2p XPS spectrum
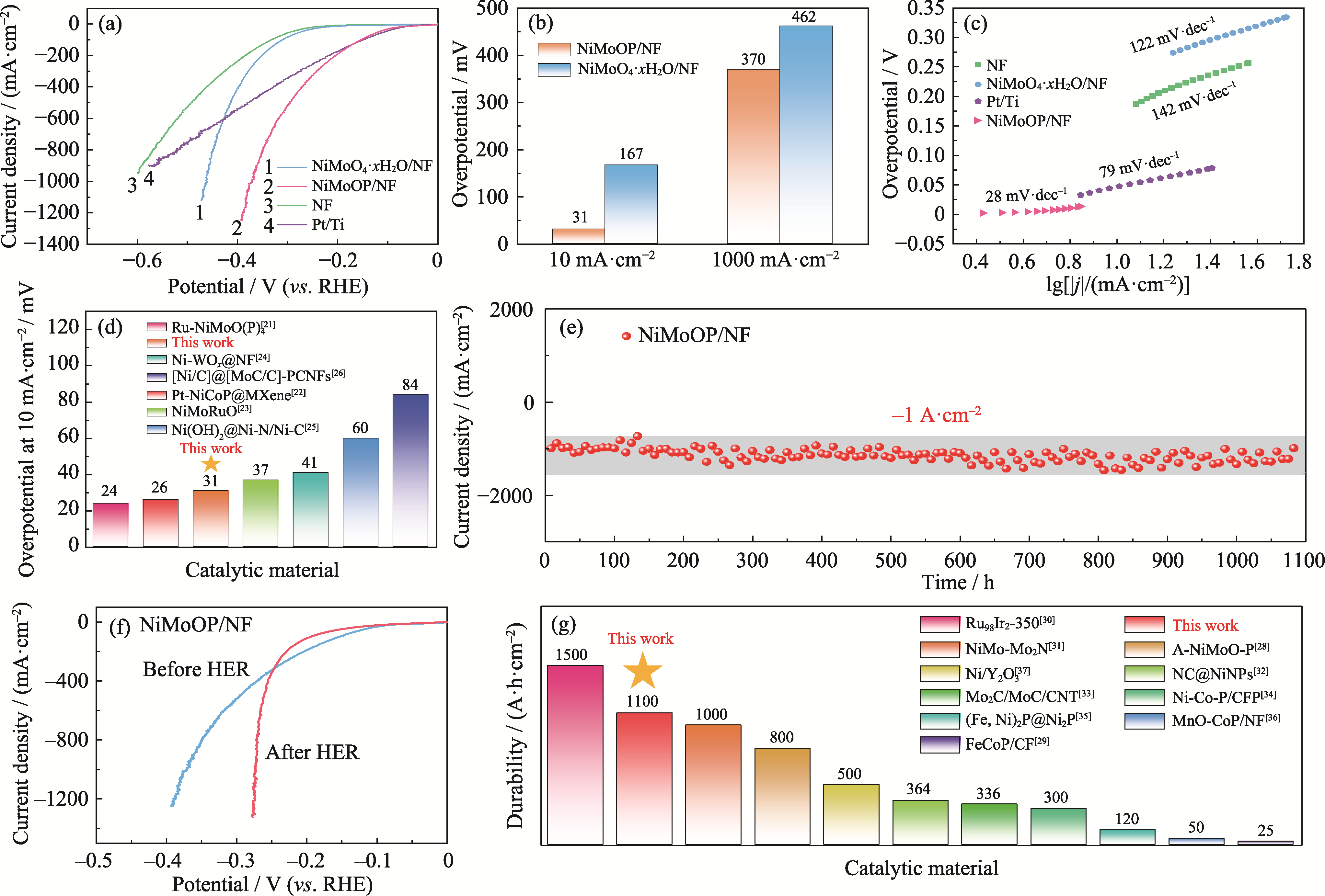
Fig. 3 Electrochemical performance tests of NiMoOP/NF and comparison with other catalytic materials (a) LSV curves; (b) Overpotentials; (c) Tafel plots; (d) Comparison of overpotentials for Ni-based catalysts; (e) I-t curve at -0.9 V (vs. RHE) without IR correction; (f) LSV curves of the catalyst before and after the 1100 h stability test; (g) Comparison of catalysts durability. Colorful figures are available on website
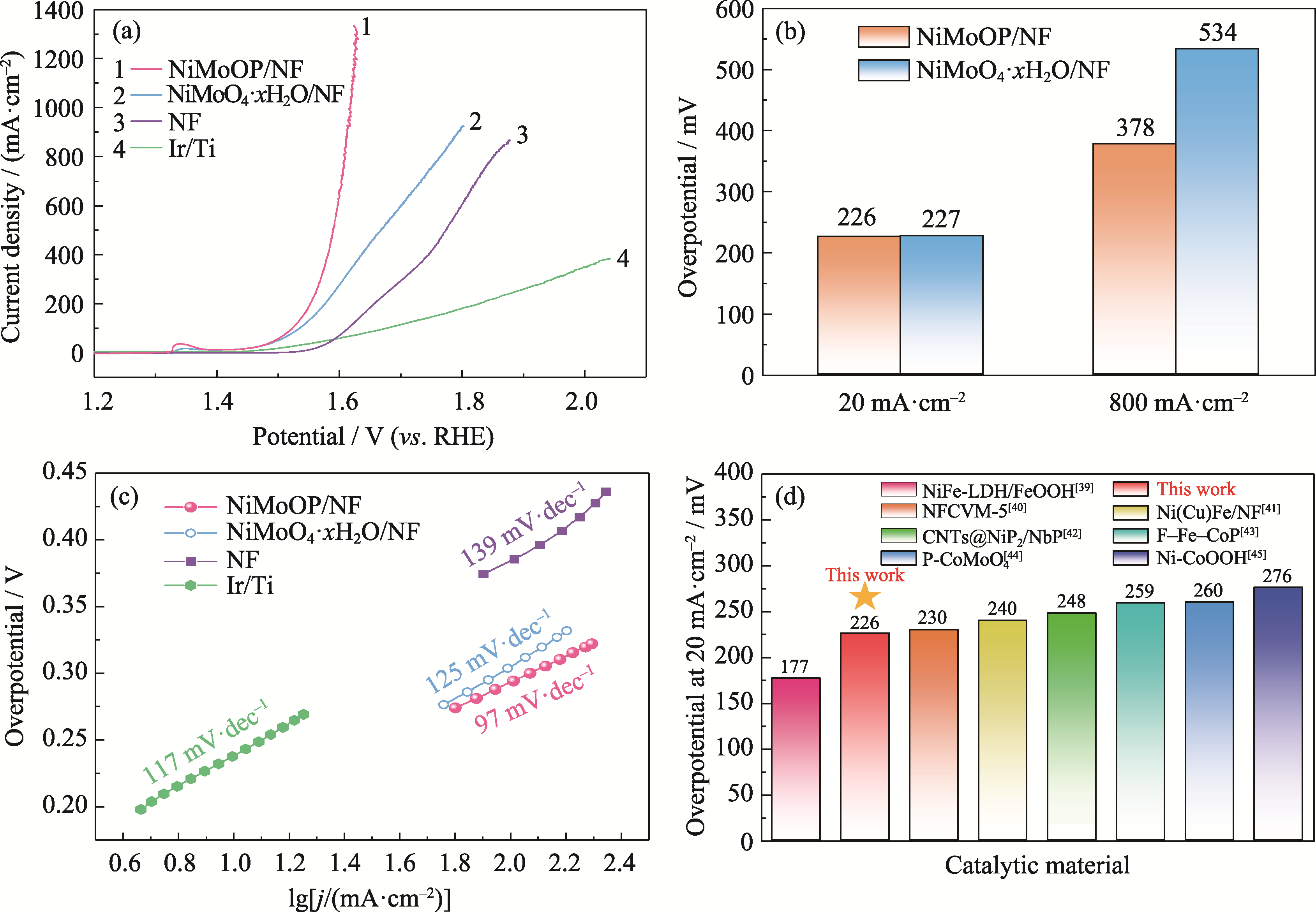
Fig. 4 OER performance of NiMoOP/NF and other catalytic materials (a) LSV curves; (b) Overpotentials; (c) Tafel plots; (d) Comparison of overpotentials with different catalysts. Colorful figures are available on website
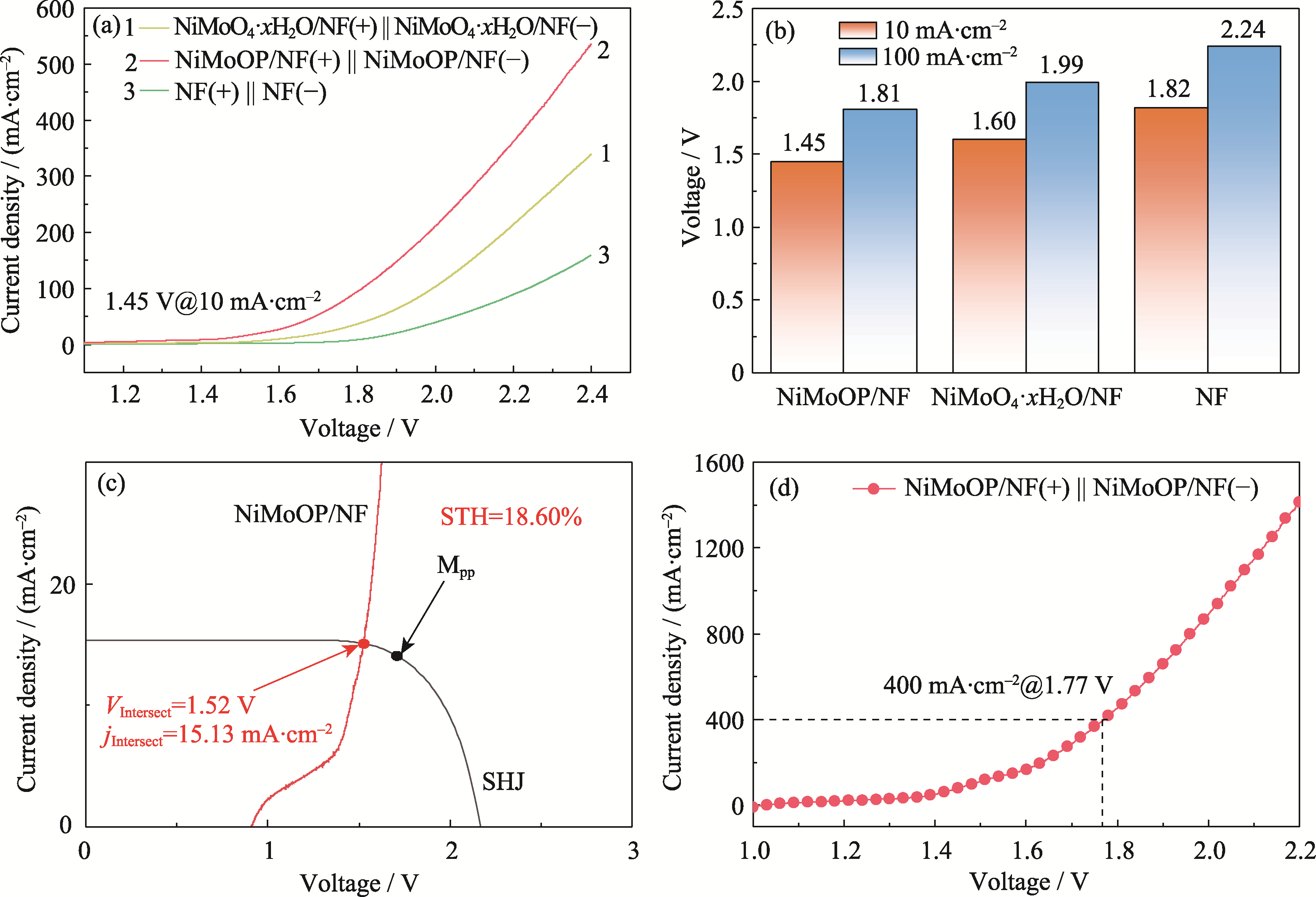
Fig. 5 Performance and STH calculation of overall water splitting for NiMoOP/NF (a) LSV curves for overall water splitting; (b) Comparison of cell voltages at 10 and 100 mA·cm-2; (c) J-V curves of crystalline silicon heterojunction solar cells and polarization curves of the overall water splitting system; (d) Overall water splitting performance test under industrial simulation conditions (30% (in mass) KOH, 60 ℃). Colorful figures are available on website
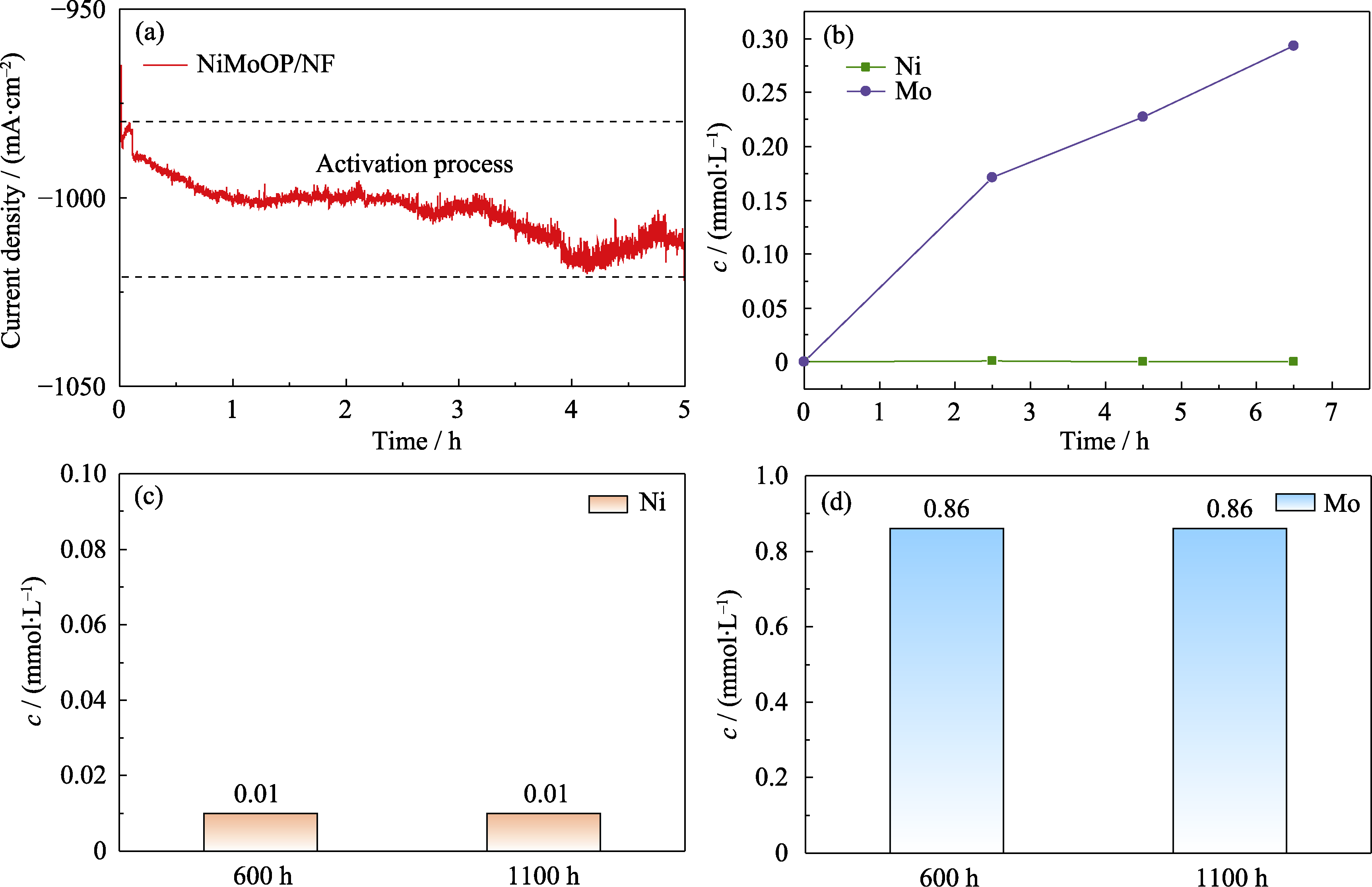
Fig. S5 Electrochemical activation phenomenon and ion dissolution data of NiMoOP/NF (a) I-t curve during initial 5 h of reaction; (b) Ni and Mo elemental concentrations in the electrolyte at initial stage of the reaction; (c) Ni and (d) Mo elemental concentrations in the electrolyte after 600 and 1100 h reaction
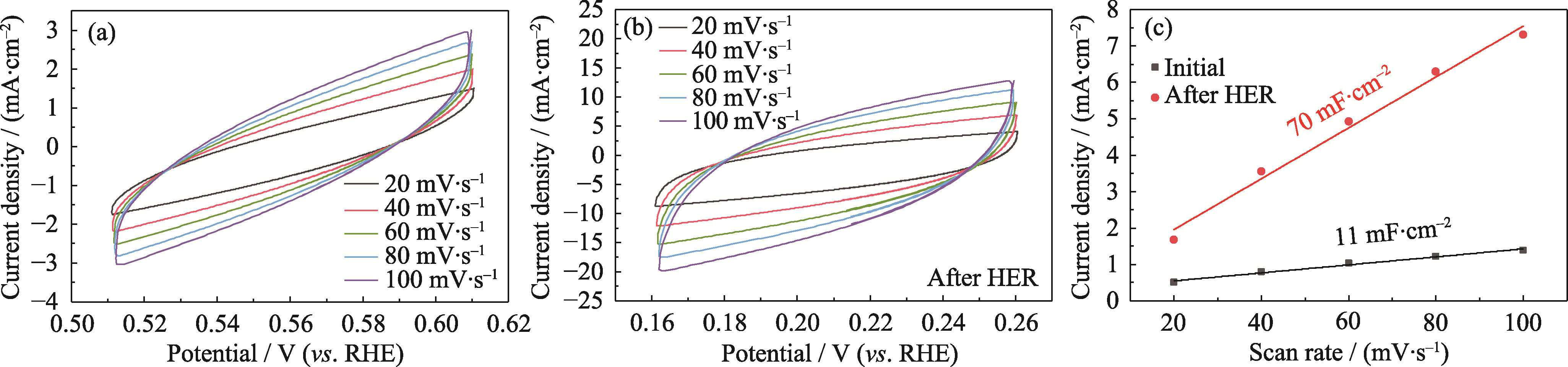
Fig. S7 CV curves and double-layer capacitance of NiMoOP/NF before and after 10 h of HER (a) Initial NiMoOP/NF; (b) NiMoOP/NF after 10 h of HER; (c) Double-layer capacitance before and after HER

Fig. S9 Faraday efficiency testing of NiMoOP/NF (a) Faraday efficiency results; (b) Photo of liquid level before reaction; (c) Photo of liquid level after reaction
| [1] |
DONG B, YU N, WANG Q Y, et al. Double active sites promoting hydrogen evolution activity and stability of CoRuOH/Co2P by rapid hydrolysis. Chinese Chemical Letters, 2024, 35(7): 109221.
DOI URL |
| [2] |
ZHU Y, CHEN B, CHENG T, et al. Amorphous Nd-Ni-B/NF rare earth composites: preparation and HER electrocatalytic performance. Journal of Inorganic Materials, 2021, 36(6): 637.
DOI |
| [3] |
SUN W, WANG Y, XIANG K, et al. CoP decorated on Ti3C2Tx MXene nanocomposites as robust electrocatalyst for hydrogen evolution reaction. Acta Physico-Chimica Sinica, 2024, 40(8): 2308015.
DOI URL |
| [4] |
SUN Q, CHEN Z, YANG Z, et al. Amorphous vanadium oxide loaded by metallic nickel-copper towards high-efficiency electrocatalyzing hydrogen production. Journal of Inorganic Materials, 2022, 38(6): 647.
DOI URL |
| [5] | XU S, WU Q, LU B A, et al. Recent advances and future prospects on industrial catalysts for green hydrogen production in alkaline media. Acta Physico-Chimica Sinica, 2023, 39(2): 2209001. |
| [6] | 周莹, 饶家豪, 唐春, 等. 光伏电催化硫化氢分解制氢脱硫经济性分析. 天然气工业, 2024, 44(11): 178. |
| [7] |
徐进, 丁显, 宫永立, 等. 电解水制氢厂站经济性分析. 储能科学与技术, 2022, 11(7): 2374.
DOI |
| [8] |
LAGADEC M F, GRIMAUD A. Water electrolysers with closed and open electrochemical systems. Nature Materials, 2020, 19(11): 1140.
DOI PMID |
| [9] | 夏杨红, 胡致远, 韦巍, 等. 可再生能源电解制氢宽范围运行控制策略. 太阳能学报, 2024, 45(8): 34. |
| [10] |
BLEEKER J, VAN KASTEREN C, VAN OMMEN J R, et al. Gas bubble removal from a zero-gap alkaline electrolyser with a pressure swing and why foam electrodes might not be suitable at high current densities. International Journal of Hydrogen Energy, 2024, 57: 1398.
DOI URL |
| [11] |
WANG X, TIAN H, YU X, et al. Advances and insights in amorphous electrocatalyst towards water splitting. Chinese Journal of Catalysis, 2023, 51: 5.
DOI |
| [12] |
SHI Y, ZHOU S, LIU J, et al. An integrated amorphous cobalt phosphoselenide electrocatalyst with high mass activity boosts alkaline overall water splitting. Applied Catalysis B: Environment and Energy, 2023, 341: 123326.
DOI URL |
| [13] |
CHANG Y, KONG L, XU D, et al. Mo migration-induced crystalline to amorphous conversion and formation of RuMo/ NiMoO4 heterogeneous nanoarray for hydrazine-assisted water splitting at large current density. Angewandte Chemie International Edition, 2025, 64(2): e202414234.
DOI URL |
| [14] |
LIU S, WANG Y, ZHAO H, et al. Amorphous W-doped iron phosphide with superhydrophilic surface to boost water-splitting under large current density. Chemical Engineering Journal, 2024, 496: 153956.
DOI URL |
| [15] |
RAJA D S, CHUAH X F, LU S Y. In situ grown bimetallic MOF-based composite as highly efficient bifunctional electrocatalyst for overall water splitting with ultrastability at high current densities. Advanced Energy Materials, 2018, 8(23): 1801065.
DOI URL |
| [16] |
JIN M, ZHANG X, NIU S, et al. Strategies for designing high- performance hydrogen evolution reaction electrocatalysts at large current densities above 1000 mA cm-2. ACS Nano, 2022, 16(8): 11577.
DOI URL |
| [17] |
WANG J, HU J, NIU S, et al. Crystalline-amorphous Ni2P4O12/ NiMoOx nanoarrays for alkaline water electrolysis: enhanced catalytic activity via in situ surface reconstruction. Small, 2022, 18(10): 2105972.
DOI URL |
| [18] |
YU L, ZHU Q, SONG S, et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nature Communications, 2019, 10: 5106.
DOI PMID |
| [19] |
ZHANG Y, LIU J, PAN Y, et al. The evolution of MoS2 properties under oxygen plasma treatment and its application in MoS2 based devices. Journal of Materials Science: Materials in Electronics, 2019, 30(19): 18185.
DOI |
| [20] | HU H, ZHANG Z, ZHANG Y, et al. An ultra-low Pt metal nitride electrocatalyst for sustainable seawater hydrogen production. Energy & Environmental Science, 2023, 16(10): 4584. |
| [21] |
WU S, CHEN D, LI S, et al. Ru cluster incorporated NiMoO(P)4 nanosheet arrays as high-efficient bifunctional catalyst for wind/ solar-to-hydrogen generation systems. Advanced Science, 2023, 10(35): 2304179.
DOI URL |
| [22] | NIU H J, HUANG C, SUN T, et al. Enhancing Ni/Co activity by neighboring Pt atoms in NiCoP/MXene electrocatalyst for alkaline hydrogen evolution. Angewandte Chemie International Edition, 2024, 63(20): e202401819. |
| [23] |
ZHANG Z, WANG H, MA M, et al. Integrating NiMoO wafer as a heterogeneous ‘turbo’ for engineering robust Ru-based electrocatalyst for overall water splitting. Chemical Engineering Journal, 2021, 420: 127686.
DOI URL |
| [24] |
LIANG W, ZHOU M, LIN X, et al. Nickel-doped tungsten oxide promotes stable and efficient hydrogen evolution in seawater. Applied Catalysis B: Environment and Energy, 2023, 325: 122397.
DOI URL |
| [25] |
LIN J, YIN D, HE W, et al. Self-supporting honeycomb coaxial carbon fibers: a new strategy to achieve an efficient hydrogen evolution reaction both in base and acid media. Chemical Engineering Journal, 2024, 488: 151195.
DOI URL |
| [26] |
DASTAFKAN K, SHEN X, HOCKING R K, et al. Monometallic interphasic synergy via nano-hetero-interfacing for hydrogen evolution in alkaline electrolytes. Nature Communications, 2023, 14: 547.
DOI |
| [27] |
LI J, TANG C, ZHANG H, et al. Mesoporous molybdenum carbide for greatly enhanced hydrogen evolution at high current density and its mechanism studies. Materials Reports: Energy, 2023, 3(3): 100215.
DOI URL |
| [28] |
LI Q, CHEN C, LUO W, et al. In situ active site refreshing of electro-catalytic materials for ultra-durable hydrogen evolution at elevated current density. Advanced Energy Materials, 2024, 14(17): 2304099.
DOI URL |
| [29] |
XU Y, ZHAO Y, SUN M, et al. Reconstruction of Fe sacrifice protective layer enables highly effective CoP catalyst for hydrogen evolution reaction at high current density. Chemical Engineering Journal, 2024, 490: 151697.
DOI URL |
| [30] |
YAN S, CHEN X, LI W, et al. Highly active and stable alkaline hydrogen evolution electrocatalyst based on Ir-incorporated partially oxidized Ru aerogel under industrial-level current density. Advanced Science, 2024, 11(7): 2307061.
DOI URL |
| [31] |
JIA H, WANG H, YAN F, et al. Unravelling electrocatalytic concerted diatomic-ensembles over superior hydrogen-evolution array structured by NiMo/Mo2N heteronanojunctions. Applied Catalysis B: Environment and Energy, 2024, 343: 123362.
DOI URL |
| [32] |
TANG Y, LIU F, LIU W, et al. Multifunctional carbon-armored Ni electrocatalyst for hydrogen evolution under high current density in alkaline electrolyte solution. Applied Catalysis B: Environment and Energy, 2023, 321: 122081.
DOI URL |
| [33] |
LI C, WANG Z, LIU M, et al. Ultrafast self-heating synthesis of robust heterogeneous nanocarbides for high current density hydrogen evolution reaction. Nature Communications, 2022, 13: 3338.
DOI PMID |
| [34] |
CHEN X, ZHAO X, WANG Y, et al. Layered Ni-Co-P electrode synthesized by CV electrodeposition for hydrogen evolution at large currents. ChemCatChem, 2021, 13(16): 3619.
DOI URL |
| [35] |
LI Y, YU X, GAO J, et al. Structural and electronic modulation of (Fe, Ni)2P@Ni2P heterostructure for efficient overall water splitting at high current density. Chemical Engineering Journal, 2023, 470: 144373.
DOI URL |
| [36] |
DONG Y, DENG Z, ZHANG H, et al. A highly active and durable hierarchical electrocatalyst for large-current-density water splitting. Nano Letters, 2023, 23(19): 9087.
DOI URL |
| [37] |
SUN H, YAO B, HAN Y, et al. Multi-interface engineering of self- supported nickel/yttrium oxide electrode enables kinetically accelerated and ultra-stable alkaline hydrogen evolution at industrial-level current density. Advanced Energy Materials, 2024, 14(11): 2303563.
DOI URL |
| [38] |
DU W, SHI Y, ZHOU W, et al. Unveiling the in situ dissolution and polymerization of Mo in Ni4Mo alloy for promoting the hydrogen evolution reaction. Angewandte Chemie International Edition, 2021, 60(13): 7051.
DOI URL |
| [39] |
WANG Y H, LI L, SHI J, et al. Oxygen defect engineering promotes synergy between adsorbate evolution and single lattice oxygen mechanisms of OER in transition metal-based (oxy)hydroxide. Advanced Science, 2023, 10(32): 2303321.
DOI URL |
| [40] |
KHATUN S, SHIMIZU K, PAL S, et al. Enthralling anodic protection by molybdate on high-entropy alloy-based electrocatalyst for sustainable seawater oxidation. Small, 2024, 20(43): 2402720.
DOI URL |
| [41] |
WANG H, LIU X, LIU G, et al. Copper doping-induced high- valence nickel-iron-based electrocatalyst toward enhanced and durable oxygen evolution reaction. Chem Catalysis, 2023, 3(3): 100552.
DOI URL |
| [42] |
SINGH S, NGUYEN D C, KIM N H, et al. Interface engineering induced electrocatalytic behavior in core-shelled CNTs@NiP2/NbP heterostructure for highly efficient overall water splitting. Chemical Engineering Journal, 2022, 442: 136120.
DOI URL |
| [43] | XU D, LIU S, ZHANG M, et al. Manipulating the dynamic self- reconstruction of CoP electrocatalyst driven by charge transport and ion leaching. Small, 2023, 19(33): 2300201. |
| [44] |
WANG J, HU J, LIANG C, et al. Surface reconstruction of phosphorus-doped cobalt molybdate microarrays in electrochemical water splitting. Chemical Engineering Journal, 2022, 446: 137094.
DOI URL |
| [45] |
CHEN M, LIU D, FENG J, et al. In-situ generation of Ni-CoOOH through deep reconstruction for durable alkaline water electrolysis. Chemical Engineering Journal, 2022, 443: 136432.
DOI URL |
| [1] | YUE Quanxin, GUO Ruihua, WANG Ruifen, AN Shengli, ZHANG Guofang, GUAN Lili. 3D Core-shell Structured NiMoO4@CoFe-LDH Nanorods: Performance of Efficient Oxygen Evolution Reaction and Overall Water Splitting [J]. Journal of Inorganic Materials, 2024, 39(11): 1254-1264. |
| [2] | ZHANG Wenyu, GUO Ruihua, YUE Quanxin, HUANG Yarong, ZHANG Guofang, GUAN Lili. High-entropy Phosphide Bifunctional Catalyst: Preparation and Performance of Efficient Water Splitting [J]. Journal of Inorganic Materials, 2024, 39(11): 1265-1274. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||