
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (12): 1356-1364.DOI: 10.15541/jim20240535
• Topical Section: Key Materials for High-temperature Fuel Cells (Guest Editor: LING Yihan) • Previous Articles Next Articles
JIANG Yuehong( ), SONG Yunfeng(
), SONG Yunfeng( ), ZHANG Leilei(
), ZHANG Leilei( ), MA Ji, SONG Zhaoyuan, LONG Wen
), MA Ji, SONG Zhaoyuan, LONG Wen
Received:2024-12-24
Revised:2025-03-18
Published:2025-12-20
Online:2025-04-09
Contact:
SONG Yunfeng, lecturer. E-mail: yunfs@lnpu.edu.cn;About author:JIANG Yuehong (1994-), female, Master candidate. E-mail: jyh_940315@163.com
Supported by:CLC Number:
JIANG Yuehong, SONG Yunfeng, ZHANG Leilei, MA Ji, SONG Zhaoyuan, LONG Wen. Fluorination of BaZr0.1Ce0.7Y0.1Yb0.1O3 as Electrolyte Material for Proton-conducting Solid Oxide Fuel Cell[J]. Journal of Inorganic Materials, 2025, 40(12): 1356-1364.
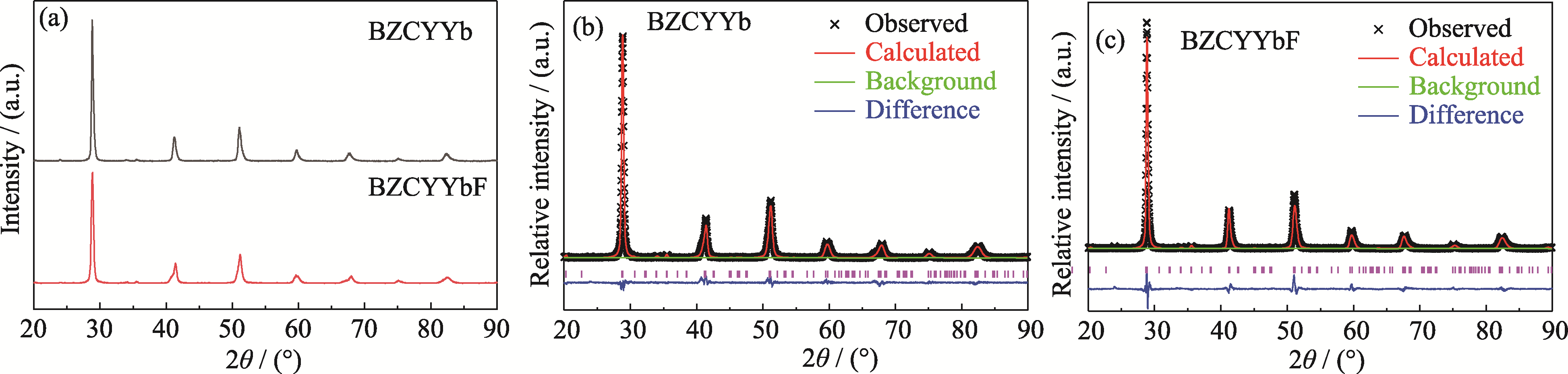
Fig. 1 XRD patterns and Rietveld refinements for BZCYYb and BZCYYbF samples (a) XRD patterns; Rietveld refinements for (b) BZCYYb and (c) BZCYYbF. Colorful figures are available on website

Fig. 4 (a) Electrical conductivities of BZCYYb and BZCYYbF in air; (b, c) Surface SEM images of (b) BZCYYb and (c) BZCYYbF; (d) Electrical conductivities of BZCYYb and BZCYYbF in dry H2

Fig. 5 (a, c, e, g) EIS spectra and (b, d, f, h) DRT curves for BZCYYb and BZCYYbF symmetric cells in (a-d) air and (e-h) wet H2 at (a, b, e, f) 600 and (c, d, g, h) 700 ℃

Fig. 8 (a) I-V/I-P curves for anode-supported single cell at 600 ℃ and (b) cross-sectional SEM image for anode-supported single cell with BZCYYbF electrolyte after cell stability test
| Sample | BZCYYb | BZCYYbF | ||
|---|---|---|---|---|
| Refined | Sigmas | Refined | Sigmas | |
| a/Å | 6.2146 | 0.000851 | 6.210605 | 0.000363 |
| b/Å | 6.2443 | 0.000511 | 6.197102 | 0.000486 |
| c/Å | 8.7578 | 0.00058 | 8.701261 | 0.00077 |
| α/(°) | 90 | - | 90 | - |
| β/(°) | 90 | - | 90 | - |
| γ/(°) | 90 | - | 90 | - |
| V/Å3 | 339.858 | 0.054 | 334.892 | 0.04 |
| χ2 | 1.711 | - | 2.346 | - |
| Rwp/% | 14.83 | - | 14.07 | - |
| Rp/% | 9.99 | - | 11.54 | - |
Table S1 Rietveld refinement results for BZCYYb and BZCYYbF samples based on space group Pbnm
| Sample | BZCYYb | BZCYYbF | ||
|---|---|---|---|---|
| Refined | Sigmas | Refined | Sigmas | |
| a/Å | 6.2146 | 0.000851 | 6.210605 | 0.000363 |
| b/Å | 6.2443 | 0.000511 | 6.197102 | 0.000486 |
| c/Å | 8.7578 | 0.00058 | 8.701261 | 0.00077 |
| α/(°) | 90 | - | 90 | - |
| β/(°) | 90 | - | 90 | - |
| γ/(°) | 90 | - | 90 | - |
| V/Å3 | 339.858 | 0.054 | 334.892 | 0.04 |
| χ2 | 1.711 | - | 2.346 | - |
| Rwp/% | 14.83 | - | 14.07 | - |
| Rp/% | 9.99 | - | 11.54 | - |

Fig. S2 (a, b) Electrical conductivity curves for (a) BZCYYb and (b) BZCYYbF in air measured by different models; (c) Schematic diagram for three different conductivity testing models; (d, e) Histograms of conductivities for (d) BZCYYb and (e) BZCYYbF measured by different models; (f) Comparison of conductivities for BZCYYb and BZCYYbF

Fig. S3 (a, b) Electrical conductivity curves for (a) BZCYYb and (b) BZCYYbF in H2 measured by different models; (c) Schematic diagram for two different conductivity testing models; (d) Histogram for BZCYYbF measured by different models; (e) Comparison of conductivities of BZCYYb and BZCYYbF
| [1] |
TSVETKOV N, KIM D, JEONG I, et al. Advances in materials and interface understanding in protonic ceramic fuel cells. Advanced Materials Technologies, 2023, 8(20): 2201075.
DOI URL |
| [2] | GAO Y, LIU K C, LI Q, et al. The approaches to conducting in-situ heterostructure electrodes for SOCs: a mini review. Sustainable Materials and Technologies, 2024, 41: e01107. |
| [3] |
LUO Y, ZHANG D, LIU T, et al. In situ exsolution of quaternary alloy nanoparticles for CO2-CO mutual conversion using reversible solid oxide cells. Advanced Functional Materials, 2024, 34(40): 2403922.
DOI URL |
| [4] |
ZHOU M Y, LIU Z J, CHEN M L, et al. Electrochemical performance and chemical stability of proton-conducting BaZr0.8-xCexY0.2O3-δ electrolytes. Journal of the American Ceramic Society, 2022, 105(9): 5711.
DOI URL |
| [5] |
YANG L, WANG S, BLINN K, et al. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2-xYbxO3-δ. Science, 2009, 326(5949): 126.
DOI URL |
| [6] |
GUO Y, LIN Y, RAN R, et al. Zirconium doping effect on the performance of proton-conducting BaZryCe0.8-yY0.2O3-δ (0.0≤y≤0.8) for fuel cell applications. Journal of Power Sources, 2009, 193(2): 400.
DOI URL |
| [7] |
ZHONG Z. Stability and conductivity study of the BaCe0.9-xZrxY0.1O2.95 systems. Solid State Ionics, 2007, 178(3/4): 213.
DOI URL |
| [8] |
CHOI S, KUCHARCZYK C J, LIANG Y, et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nature Energy, 2018, 3(3): 202.
DOI |
| [9] |
CHOI S Y, TIMOTH C, SOSSINA M H. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: exceptional reversibility, stability, and demonstrated Faradaic efficiency. Energy and Environmental Science, 2019, 12(1): 206.
DOI URL |
| [10] |
REN R, YU X, WANG Z, et al. Fluorination inductive effect enables rapid bulk proton diffusion in BaCo0.4Fe0.4Zr0.1Y0.1O3-δ perovskite oxide for high-activity protonic ceramic fuel cell cathode. Applied Catalysis B: Environmental, 2022, 317(1): 121759.
DOI URL |
| [11] |
LI W, LI Y, F L, et al. Enhancing performance of proton ceramic fuel cells through fluorine-doped perovskite oxides. Rare Metals, 2025, 44: 2405.
DOI URL |
| [12] |
JIANG T, LIU Y, WANG Z, et al. An improved direct current sintering technique for proton conductor - BaZr0.1Ce0.7Y0.1Yb0.1O3-δ: the effect of direct current on sintering process. Journal of Power Sources, 2014, 248(8): 70.
DOI URL |
| [13] | ZHANG Y, XIE D, CHI B, et al. Basic properties of proton conductor BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb) material. Asia-Pacific Journal of Chemical Engineering, 2019, 14(4): 58. |
| [14] | CHEN T, JING Y H, ANDERSON L O, et al. Toward durable protonic ceramic cells: hydration-induced chemical expansion correlates with symmetry in the Y-doped BaZrO3-BaCeO3 solid solution. The Journal of Physical Chemistry, 2021, 125(47): 26216. |
| [15] |
LOKEN A, RICOTE S, WACHOWSKI S, et al. Thermal and chemical expansion in proton ceramic electrolytes and compatible electrodes. Crystals, 2018, 8(9): 365.
DOI URL |
| [16] |
STOKES S J, SAIFUL I. Defect chemistry and proton-dopant association in BaZrO3 and BaPrO3. Journal of Materials Chemistry, 2010, 20(6): 6258.
DOI URL |
| [17] |
ZHONG Z Y, SONG T, ZHAO S K, et al. High-performance BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb) protonic ceramic fuel cell electrolytes by the Ba evaporation inhibition strategy. Ceramics International, 2024, 50(2): 3633.
DOI URL |
| [18] |
ZHU H, RICOTE S, COORS W G, et al. Interpreting equilibrium- conductivity and conductivity-relaxation measurements to establish thermodynamic and transport properties for multiple charged defeat conducting ceramics. Faraday Discussions, 2015, 182(3): 49.
DOI URL |
| [19] | ZHU H Y, SANDRINE R, DUAN C C, et al. Defect chemistry and transport within dense BaZr0.1Ce0.7Y0.1Yb0.1O3-δ (BZCYYb) proton- conducting membranes. Journal of the Electrochemical Society, 2018, 10(2): 3633. |
| [20] |
SOMEKAWA T, TACHIKAWA Y, TACHIKAWA Y, et al. Physicochemical properties of proton conductive Ba(Zr0.1Ce0.7Y0.1Yb0.1)O3-δ solid electrolyte in terms of electrochemical performance of solid oxide fuel cells. International Journal of Hydrogen Energy, 2016, 41(39): 17539.
DOI URL |
| [21] |
WANG J, ZHANG D, LIU T, et al. Self-assembled FeRu bimetallic nano catalysts for efficient and durable mutual CO-CO2 conversion in a reversible solid oxide electrochemical cell. Science China Materials, 2024, 67(5): 1471.
DOI |
| [22] |
CHEN H N, ZHANF H C, ZHOU Y J, et al. Structure-conduction correlations in a chlorine-rich superionic lithium-argyrodite solid electrolyte: a DRT analysis. Journal of Power Sources, 2023, 583(1): 233579.
DOI URL |
| [23] |
YANG Q, TIAN D, LIU R, et al. Exploiting rare-earth-abundant layered perovskite cathodes of LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln=La and Nd) for SOFCs. International Journal of Hydrogen Energy, 2021, 46(7): 5630.
DOI URL |
| [24] | XIE D, LING A, YAN D, et al. A comparative study on the composite cathodes with proton conductor and oxygen ion conductor for proton-conducting solid oxide fuel cell. Electrchimica Acta, 2020, 344: 136143. |
| [25] |
SUMI H, SHIMADA H, WAANABE K, et al. External current dependence of polarization resistances for reversible solid oxide and protonic ceramic cells with current leakage. ACS Applied Energy Materials, 2023, 6(3): 1853.
DOI URL |
| [26] |
SHI H G, HU Y, FENG Z X, et al. Solid-state synthesis of BaCe0.16Y0.04Fe0.8O3-δ cathode for protonic ceramic fuel cells. Asia-Pacific Journal of Chemical Engineering, 2022, 17(4): e2789.
DOI URL |
| [1] | WAN Junchi, DU Lulu, ZHANG Yongshang, LI Lin, LIU Jiande, ZHANG Linsen. Structural Evolution and Electrochemical Performance of Na4FexP4O12+x/C Cathode Materials for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(5): 497-503. |
| [2] | XUE Ke, CAI Changkun, XIE Manyi, LI Shuting, AN Shengli. Pr1+xBa1-xFe2O5+δ Cathode Materials for Solid Oxide Fuel Cells: Preparation and Electrochemical Performance [J]. Journal of Inorganic Materials, 2025, 40(4): 363-371. |
| [3] | YANG Hengqiang, ZHANG Xinyue, MA Yichu, ZHOU Qingjun. Iron-based Perovskite Material La0.25M0.75FeO3-δ (M=Ca, Sr, Ba): Preparation and Performance as Cathode for Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2025, 40(12): 1365-1372. |
| [4] | LING Yihan, GUO Sheng, CAO Zhiqiang, TIAN Yunfeng, LIU Fangsheng, JIN Fangjun, GAO Yuan. Research Progress on Preparation Technologies and Performance of Straight-pore Electrode Structures for Solid Oxide Cells [J]. Journal of Inorganic Materials, 2025, 40(12): 1311-1323. |
| [5] | ZHANG Yuting, LI Xiaobin, LIU Zunyi, LI Ning, ZHAO Yu. Composite Yolk-shell NiCo2V2O8@TiO2@NC Material as Anode for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(11): 1221-1228. |
| [6] | ZHANG Haifeng, JIANG Meng, SUN Tingting, WANG Lianjun, JIANG Wan. Preparation of p-type GeMnTe2 Based Thermoelectric Materials with Stable Cubic Phase [J]. Journal of Inorganic Materials, 2025, 40(11): 1245-1251. |
| [7] | ZHANG Jinghui, LU Xiaotong, MAO Haiyan, TIAN Yazhou, ZHANG Shanlin. Effect of Sintering Additives on Sintering Behavior and Conductivity of BaZr0.1Ce0.7Y0.2O3-δ Electrolytes [J]. Journal of Inorganic Materials, 2025, 40(1): 84-90. |
| [8] | WANG Weiming, WANG Weide, SU Yi, MA Qingsong, YAO Dongxu, ZENG Yuping. Research Progress of High Thermal Conductivity Silicon Nitride Ceramics Prepared by Non-oxide Sintering Additives [J]. Journal of Inorganic Materials, 2024, 39(6): 634-646. |
| [9] | JIN Min, MA Yupeng, WEI Tianran, LIN Siqi, BAI Xudong, SHI Xun, LIU Xuechao. Growth and Characterization of Large-size InSe Crystal from Non-stoichiometric Solution via a Zone Melting Method [J]. Journal of Inorganic Materials, 2024, 39(5): 554-560. |
| [10] | CHEN Zhengpeng, JIN Fangjun, LI Mingfei, DONG Jiangbo, XU Renci, XU Hanzhao, XIONG Kai, RAO Muming, CHEN Chuangting, LI Xiaowei, LING Yihan. Double Perovskite Sr2CoFeO5+δ: Preparation and Performance as Cathode Material for Intermediate-temperature Solid Oxide Fuel Cells [J]. Journal of Inorganic Materials, 2024, 39(3): 337-344. |
| [11] | SU Nan, QIU Jieshan, WANG Zhiyu. F-doped Carbon Coated Nano-Si Anode with High Capacity: Preparation by Gaseous Fluorination and Performance for Lithium Storage [J]. Journal of Inorganic Materials, 2023, 38(8): 947-953. |
| [12] | FAN Jiashun, XIA Donglin, LIU Baoshun. Temperature Dependent Transient Photoconductive Response of CsPbBr3 NCs [J]. Journal of Inorganic Materials, 2023, 38(8): 893-900. |
| [13] | WANG Shuling, JIANG Meng, WANG Lianjun, JIANG Wan. n-Type Pb-free AgBiSe2 Based Thermoelectric Materials with Stable Cubic Phase Structure [J]. Journal of Inorganic Materials, 2023, 38(7): 807-814. |
| [14] | CHEN Qiang, BAI Shuxin, YE Yicong. Highly Thermal Conductive Silicon Carbide Ceramics Matrix Composites for Thermal Management: a Review [J]. Journal of Inorganic Materials, 2023, 38(6): 634-646. |
| [15] | ZHANG Shuo, FU Qiangang, ZHANG Pei, FEI Jie, LI Wei. Influence of High Temperature Treatment of C/C Porous Preform on Friction and Wear Behavior of C/C-SiC Composites [J]. Journal of Inorganic Materials, 2023, 38(5): 561-568. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||