
Journal of Inorganic Materials ›› 2025, Vol. 40 ›› Issue (8): 849-859.DOI: 10.15541/jim20250001
• REVIEW • Previous Articles Next Articles
ZHANG Hongjian1( ), ZHAO Ziyi1,2, WU Chengtie1,2(
), ZHAO Ziyi1,2, WU Chengtie1,2( )
)
Received:2025-01-02
Revised:2025-02-07
Published:2025-08-20
Online:2025-02-19
Contact:
WU Chengtie, professor. E-mail: chengtiewu@mail.sic.ac.cnAbout author:ZHANG Hongjian (1996-), male, PhD. E-mail: zhanghongjian@mail.sic.ac.cn
Supported by:CLC Number:
ZHANG Hongjian, ZHAO Ziyi, WU Chengtie. Inorganic Biomaterials on Regulating Neural Cell Function and Innervated Tissue Regeneration: A Review[J]. Journal of Inorganic Materials, 2025, 40(8): 849-859.
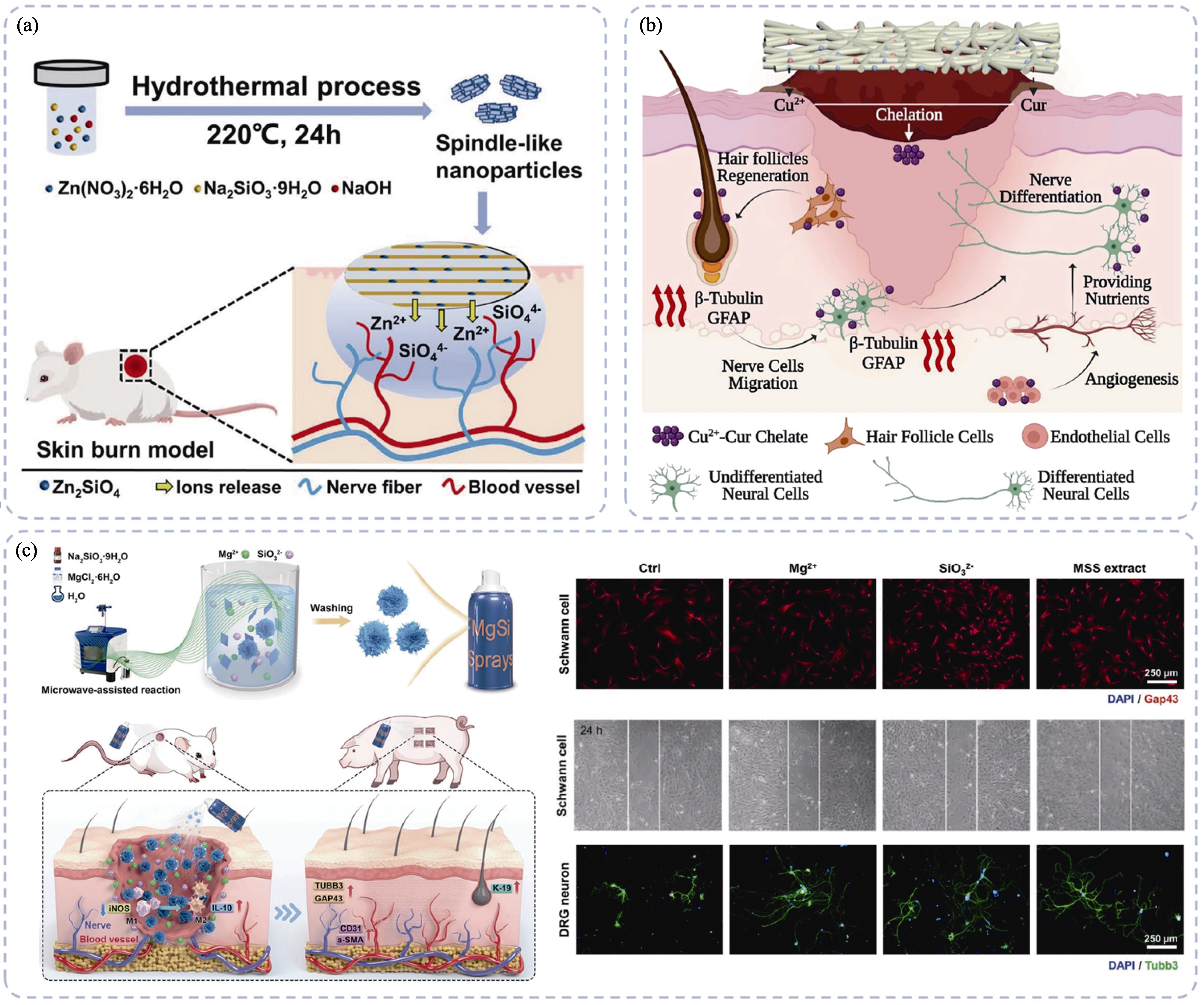
Fig. 1 Inorganic biomaterials promoting innervated skin regeneration through enhancing cellular functions[57-58,60] (a) Spindle zinc silicate nanoparticles promoting neuro-vascularized wound healing via releasing bioactive Zn and Si ions[57]; (b) Cu-CS containing wound dressing promoting cutaneous neural networks reconstructions and wound healing[58]; (c) Sprayable magnesium silicate particles containing hydrogel promoting vascularized and innervated skin regeneration[60]. Colorful figures are available on website
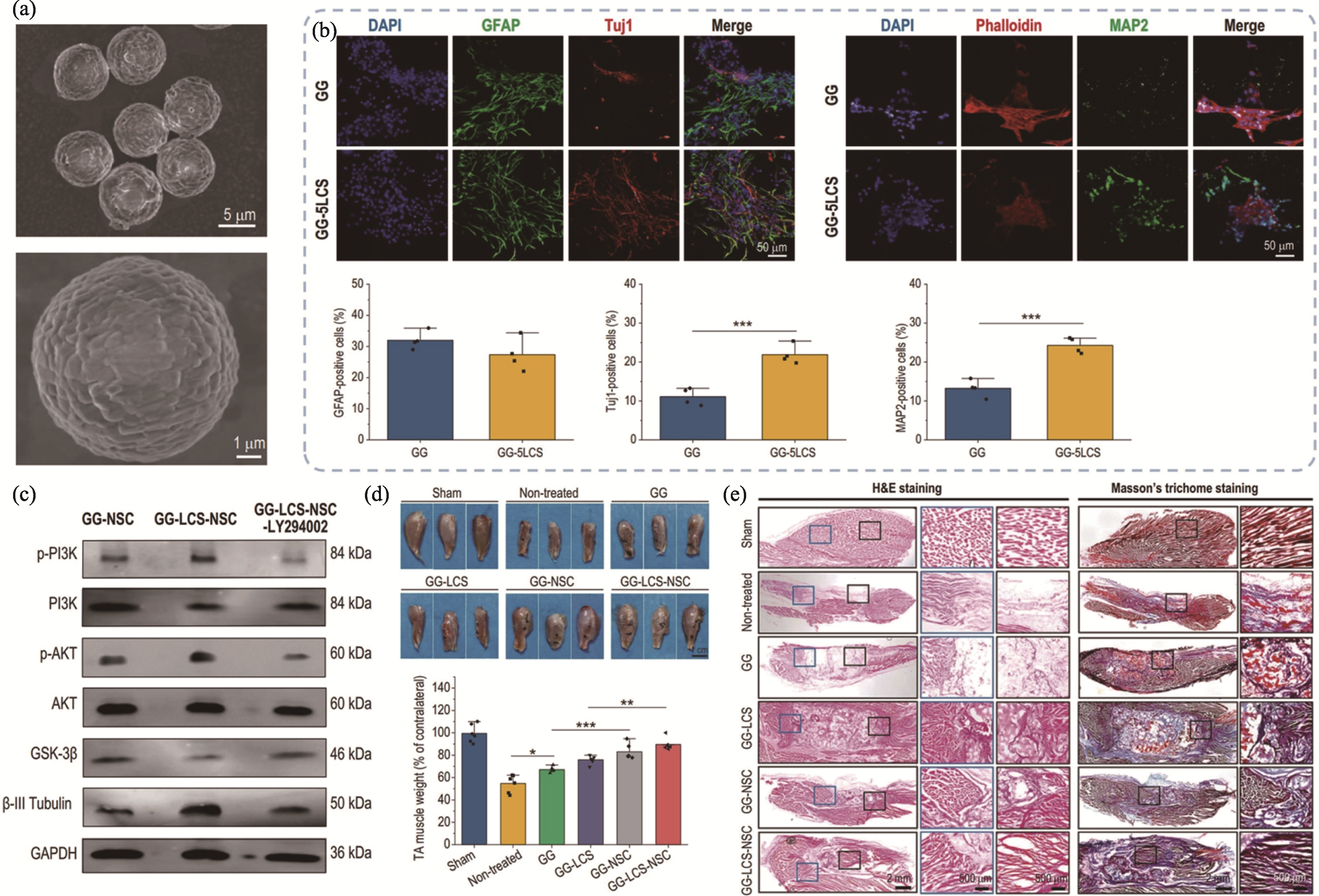
Fig. 2 3D bioprinted Li-Ca-Si bioceramics containing neural constructs for innervated tissue regeneration[62] (a) SEM images of the Li-Ca-Si bioceramic microspheres; (b) Immunofluorescence images and semi-quantitative analysis of Li-Ca-Si bioceramic microspheres inducing the neuronal differentiation and maturation of neural stem cells (NSCs); (c) Li-Ca-Si bioceramic microspheres inducing the neuronal differentiation and maturation of NSCs through activating PI3K-AKT signal pathway; (d) Optical images and their weight of the regenerated muscles; (e) Representative images of H&E and Masson staining assays. Colorful figures are available on website
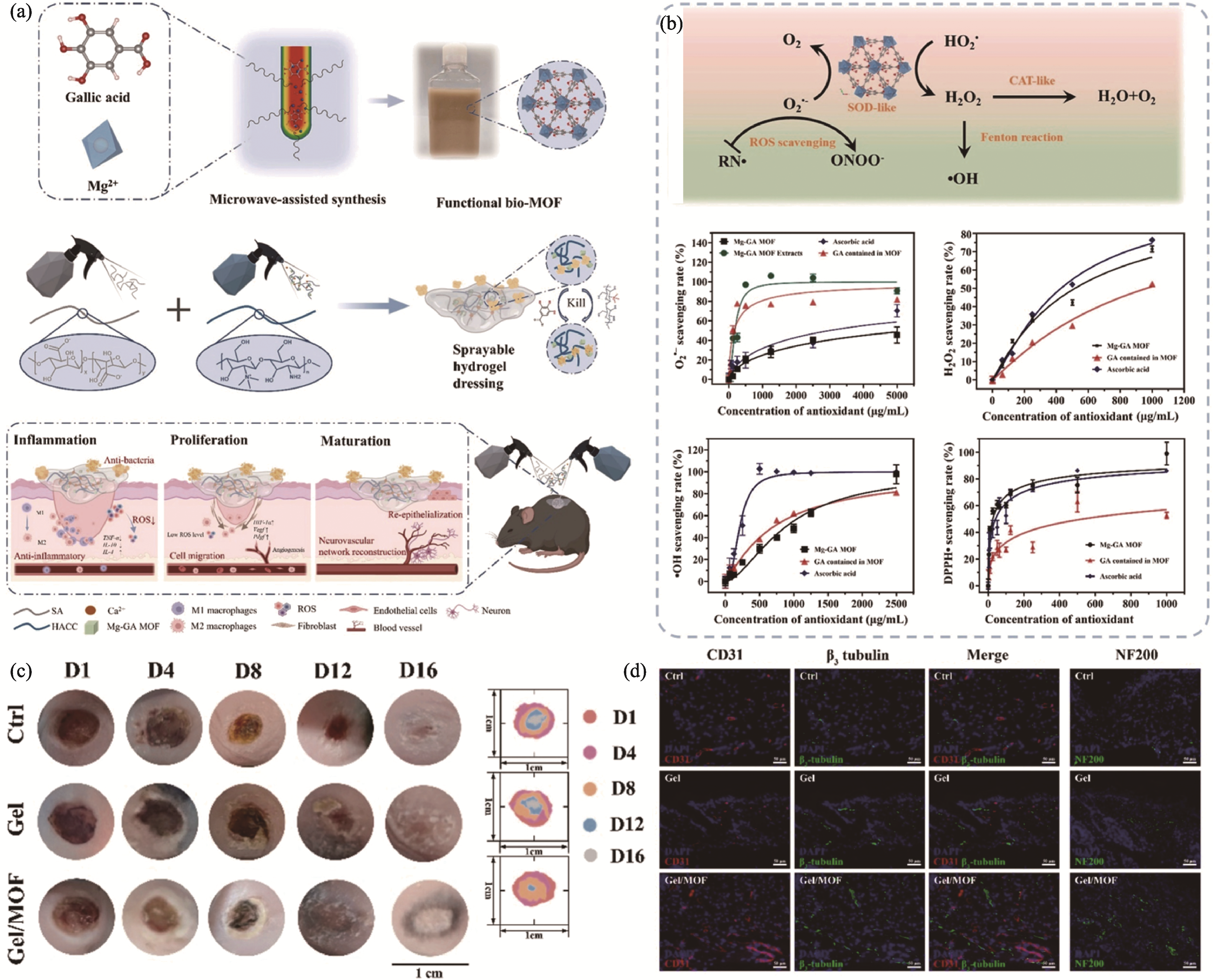
Fig. 3 Sprayable Mg-gallate metal-organic framework-based hydrogel promoting innervated and vascularized wound healing through immune-modulatory approaches[67] (a) Schematic illustrations of synthesis and application of sprayable hydrogel; (b) Enzyme-catalytic behaviors of hydrogel to scavenging ROS; (c) In vivo representative images of the wounds; (d) Representative immunofluorescent images of the regenerated nerves and vessels in the wound area. Colorful figures are available on website
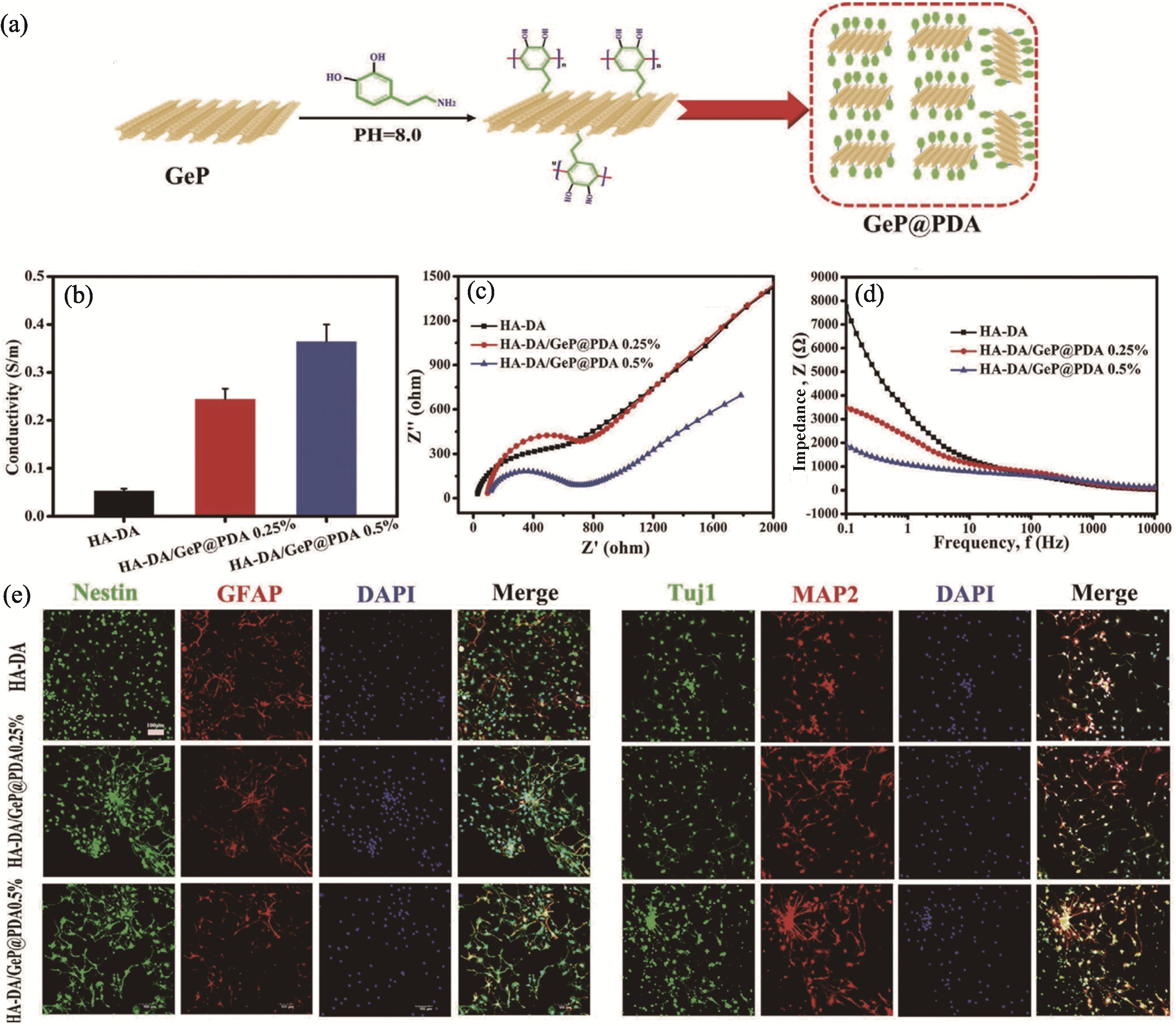
Fig. 4 GeP nanosheet-based conductive hydrogel inducing the neuronal differentiation of NSCs[69] (a) Preparation of GeP@PDA nanosheets; (b-d) Conductivity (b), Nyquist curves (c) and impedance spectra (d) of hydrogel; (e) Representative immunostaining images of NSCs under different treatments. Colorful figures are available on website
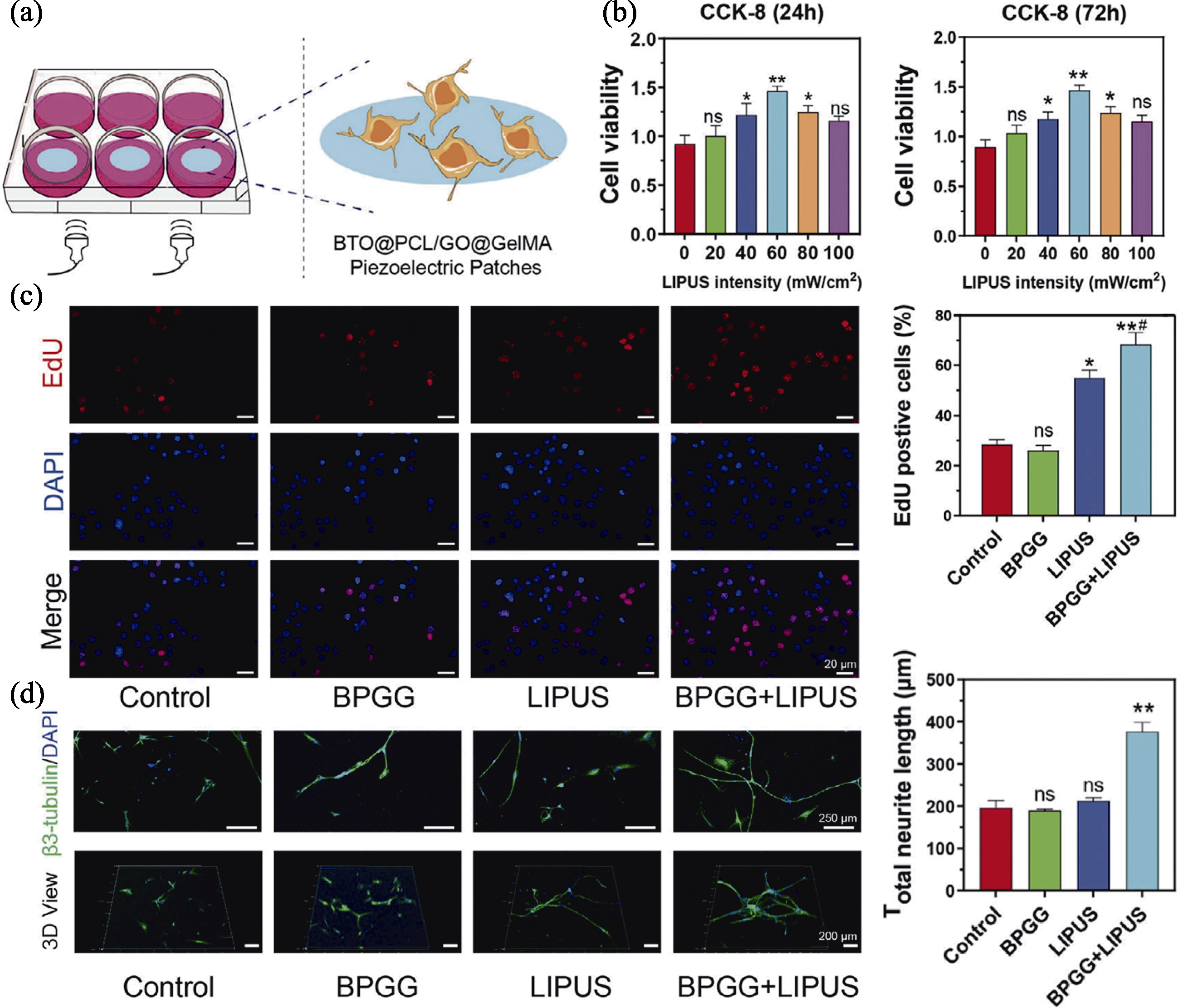
Fig. 5 Ultrasound-triggered wireless electrical stimulation nanopatch promoting neural differentiation[75] (a) Scheme of the nanopatch of stimulating neural cells; (b) Cell viability of nanopatch under different LIPUS stimulation intensities; (c) EdU fluorescence images of cells treated with different groups and corresponding statistical analysis; (d) Representative immunostaining images of β3-tubulin of neurons and corresponding statistical analysis of neurite length. Colorful figures are available on website
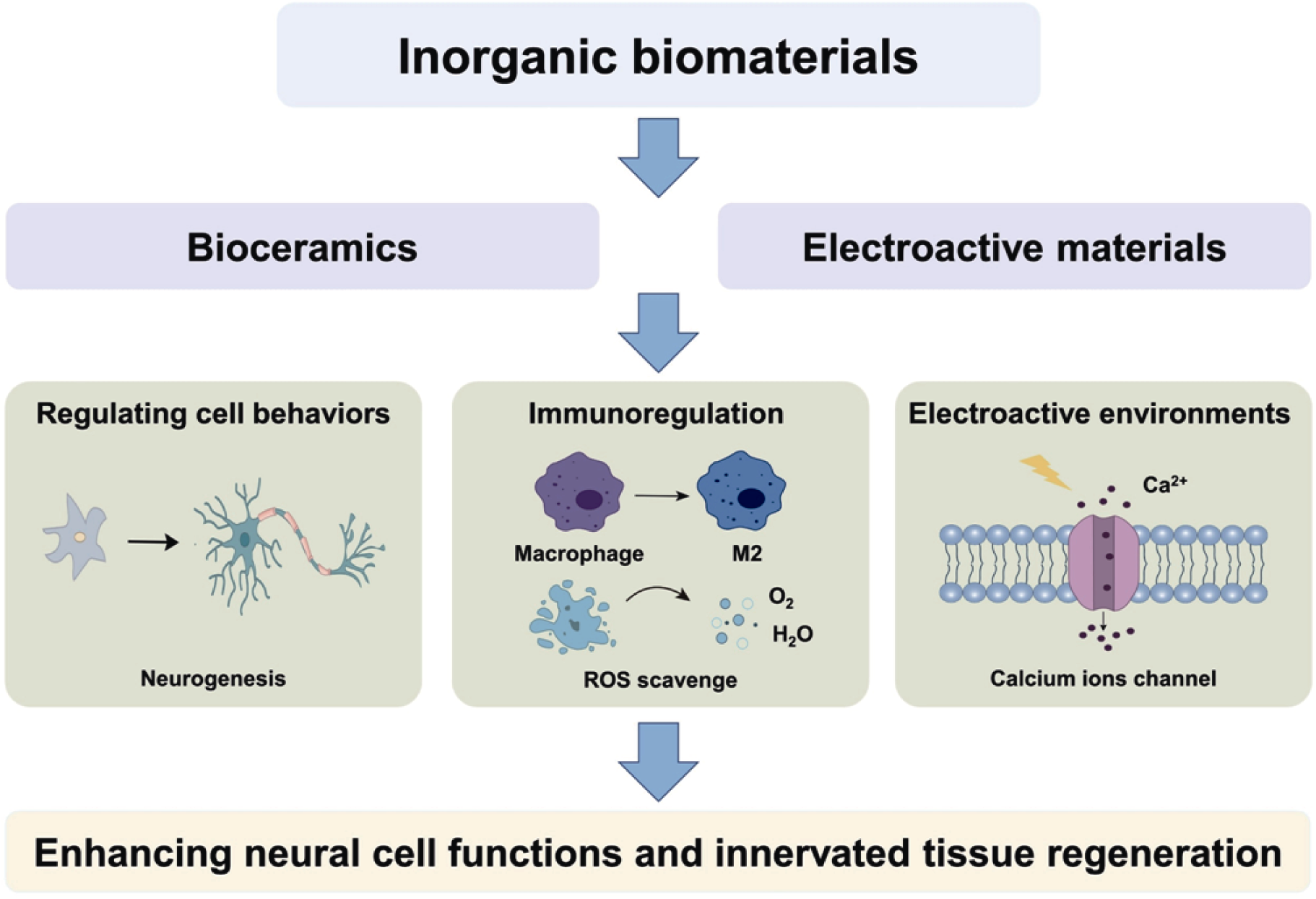
Fig. 6 Inorganic biomaterials enhancing neural cell functions and innervated tissue regeneration through modulating cellular behaviors, regulating immune microenvironment and building electroactive microenvironment
| [1] | WAN Q Q, QIN W P, MA Y X, et al. Crosstalk between bone and nerves within bone. Advanced Science, 2021, 8(7): 2003390. |
| [2] | WANG X D, LI S Y, ZHANG S J, et al. The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach. Theranostics, 2020, 10(11): 4839. |
| [3] |
ELEFTERIOU F. Impact of the autonomic nervous system on the skeleton. Physiological Reviews, 2018, 98(3): 1083.
DOI PMID |
| [4] | BROKESH A M, GAHARWAR A K. Inorganic biomaterials for regenerative medicine. ACS Applied Materials & Interfaces, 2020, 12(5): 5319. |
| [5] |
PEI Z, LEI H, CHENG L. Bioactive inorganic nanomaterials for cancer theranostics. Chemical Society Reviews, 2023, 52(6): 2031.
DOI PMID |
| [6] | ZOU Y, HUANG B, CAO L, et al. Tailored mesoporous inorganic biomaterials: assembly, functionalization, and drug delivery engineering. Advanced Materials, 2021, 33(2): 2005215. |
| [7] | QIN C, WU C. Inorganic biomaterials-based bioinks for three-dimensional bioprinting of regenerative scaffolds. VIEW, 2022, 3(4): 20210018. |
| [8] |
ZHAO C, LIU W, ZHU M, et al. Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: a review. Bioactive Materials, 2022, 18: 383.
DOI PMID |
| [9] | ZHOU Y, WU C, CHANG J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Materials Today, 2019, 24: 41. |
| [10] | GOU Y, QI K, WEI Y, et al. Advances of calcium phosphate nanoceramics for the osteoinductive potential and mechanistic pathways in maxillofacial bone defect repair. Nano TransMed, 2024, 3: 100033. |
| [11] | ZHANG Y, XU J, RUAN Y C, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone- fracture healing in rats. Nature Medicine, 2016, 22(10): 1160. |
| [12] | YANG Y, WANG H, YANG H, et al. Magnesium-based whitlockite bone mineral promotes neural and osteogenic activities. ACS Biomaterials Science & Engineering, 2020, 6(10): 5785. |
| [13] |
XU Y, XU C, HE L, et al. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioactive Materials, 2022, 16: 271.
DOI PMID |
| [14] | MA Y X, JIAO K, WAN Q Q, et al. Silicified collagen scaffold induces semaphorin 3A secretion by sensory nerves to improve in-situ bone regeneration. Bioactive Materials, 2022, 9: 475. |
| [15] | MEI P, JIANG S, MAO L, et al. In situ construction of flower-like nanostructured calcium silicate bioceramics for enhancing bone regeneration mediated via FAK/p38 signaling pathway. Journal of Nanobiotechnology, 2022, 20(1): 162. |
| [16] | LI T, ZHAI D, MA B, et al. 3D printing of hot dog-like biomaterials with hierarchical architecture and distinct bioactivity. Advanced Science, 2019, 6(19): 1901146. |
| [17] | MA W P, YANG Z B, LU M X, et al. Hierarchically structured biomaterials for tissue regeneration. Microstructures, 2024, 4(2): 2024014. |
| [18] | YANG Z, XUE J, SHI Z, et al. Naturally derived flexible bioceramics: biomass recycling approach and advanced function. Matter, 2024, 7(3): 1275. |
| [19] | LIU Z, WAN X, WANG Z L, et al. Electroactive biomaterials and systems for cell fate determination and tissue regeneration: design and applications. Advanced Materials, 2021, 33(32): 2007429. |
| [20] | QIAN Y, LIN H, YAN Z, et al. Functional nanomaterials in peripheral nerve regeneration: scaffold design, chemical principles and microenvironmental remodeling. Materials Today, 2021, 51: 165. |
| [21] |
ZHANG M, ZHAI X, MA T, et al. Sequential therapy for bone regeneration by cerium oxide-reinforced 3D-printed bioactive glass scaffolds. ACS Nano, 2023, 17(5): 4433.
DOI PMID |
| [22] | KIM J W, MAHAPATRA C, HONG J Y, et al. Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Advanced Science, 2017, 4(10): 1700034. |
| [23] |
ZHENG Y, WU J, ZHU Y, et al. Inorganic-based biomaterials for rapid hemostasis and wound healing. Chemical Science, 2022, 14(1): 29.
DOI PMID |
| [24] | MA J, WU C. Bioactive inorganic particles-based biomaterials for skin tissue engineering. Exploration, 2022, 2(5): 20210083. |
| [25] | HAN D, LIU X, WU S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chemical Society Reviews, 2022, 51(16): 7138. |
| [26] | 张兴栋, WILLIAMS D. 二十一世纪生物材料定义. 北京: 科学出版社, 2021: 25. |
| [27] | WU C T, CHANG J. Silicate bioceramics for bone tissue regeneration. Journal of Inorganic Materials, 2013, 28(1): 29. |
| [28] | WU H, HUA Y, WU J, et al. The morphology of hydroxyapatite nanoparticles regulates clathrin-mediated endocytosis in melanoma cells and resultant anti-tumor efficiency. Nano Research, 2022, 15(7): 6256. |
| [29] | ZHU D, LU B, YANG Q, et al. Lanthanum-doped mesoporous bioglasses/chitosan composite scaffolds enhance synchronous osteogenesis and angiogenesis for augmented osseous regeneration. Chemical Engineering Journal, 2021, 405: 127077. |
| [30] |
GUPTA B, PAPKE J B, MOHAMMADKHAH A, et al. Effects of chemically doped bioactive borate glass on neuron regrowth and regeneration. Annals of Biomedical Engineering, 2016, 44(12): 3468.
PMID |
| [31] | HUANG J, HUANG J, ZHANG X, et al. A bioactive multifunctional dressing with simultaneous visible monitoring of pH values and H2O2 concentrations for promoting diabetic wound healing. Materials Horizons, 2025, 12: 267. |
| [32] | HUANG J, ZHENG Y, NIU H, et al. A multifunctional hydrogel for simultaneous visible H2O2 monitoring and accelerating diabetic wound healing. Advanced Healthcare Materials, 2024, 13(3): 2302328. |
| [33] | ZHANG Z, KLAUSEN L H, CHEN M, et al. Electroactive scaffolds for neurogenesis and myogenesis: graphene-based nanomaterials. Small, 2018, 14(48): 1801983. |
| [34] | 宁成云, 毛传斌. 电活性生物材料. 北京: 科学出版社, 2017: 1. |
| [35] |
SHIN M, LIM J, PARK Y, et al. Carbon-based nanocomposites for biomedical applications. RSC Advances, 2024, 14(10): 7142.
DOI PMID |
| [36] | ZHENG Y, HONG X, WANG J, et al. 2D nanomaterials for tissue engineering and regenerative nanomedicines: recent advances and future challenges. Advanced Healthcare Materials, 2021, 10(7): 2001743. |
| [37] | WANG X, HAN X, LI C, et al. 2D materials for bone therapy. Advanced Drug Delivery Reviews, 2021, 178: 113970. |
| [38] | GU G, CUI Z, DU X, et al. Recent advances in biomacromolecule-reinforced 2D material (2DM) hydrogels: from interactions, synthesis, and functionalization to biomedical applications. Advanced Functional Materials, 2024, 34(48): 2408367. |
| [39] | WANG Z L. Progress in piezotronics and piezo-phototronics. Advanced Materials, 2012, 24(34): 4632. |
| [40] | KAPAT K, SHUBHRA Q T H, ZHOU M, et al. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Advanced Functional Materials, 2020, 30(44): 1909045. |
| [41] | KIM W, LEE H, LEE J, et al. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials, 2020, 230: 119632. |
| [42] | HE J, HAO M, DUAN J, et al. Synergistic effect of endocellular calcium ion release and nanotopograpy of one-dimensional hydroxyapatite nanomaterials for accelerating neural stem cell differentiation. Composites Part B-Engineering, 2021, 219: 108944. |
| [43] | DONG X, LIU S, YANG Y, et al. Aligned microfiber-induced macrophage polarization to guide Schwann-cell-enabled peripheral nerve regeneration. Biomaterials, 2021, 272: 120767. |
| [44] | HAO M, ZHANG Z, LIU C, et al. Hydroxyapatite nanorods function as safe and effective growth factors regulating neural differentiation and neuron development. Advanced Materials, 2021, 33(33): 2100895. |
| [45] | DAI H, FAN Q, WANG C. Recent applications of immunomodulatory biomaterials for disease immunotherapy. Exploration, 2022, 2(6): 20210157. |
| [46] | LI L, XIAO B, MU J, et al. A MnO2 nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS Nano, 2019, 13(12): 14283. |
| [47] |
SUN Y, ZHANG H, ZHANG Y, et al. Li-Mg-Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioactive Materials, 2023, 28: 227.
DOI PMID |
| [48] |
LEVIN M. Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell, 2021, 184(8): 1971.
DOI PMID |
| [49] | MURILLO G, BLANQUER A, VARGAS-ESTEVEZ C, et al. Electromechanical nanogenerator-cell interaction modulates cell activity. Advanced Materials, 2017, 29(24): 1605048. |
| [50] | ZHANG X, WANG T, ZHANG Z, et al. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Materials Today, 2023, 68: 177. |
| [51] | KHARE D, BASU B, DUBEY A K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials, 2020, 258: 120280. |
| [52] | SEBASTIAN A, VOLK S W, HALAI P, et al. Enhanced neurogenic biomarker expression and reinnervation in human acute skin wounds treated by electrical stimulation. Journal of Investigative Dermatology, 2017, 137(3): 737. |
| [53] | FAN L, XIAO C, GUAN P, et al. Extracellular matrix-based conductive interpenetrating network hydrogels with enhanced neurovascular regeneration properties for diabetic wounds repair. Advanced Healthcare Materials, 2022, 11(1): 2101556. |
| [54] | TAN M H, XU X H, YUAN T J, et al. Self-powered smart patch promotes skin nerve regeneration and sensation restoration by delivering biological-electrical signals in program. Biomaterials, 2022, 283: 121413. |
| [55] | GAO J, YU X, WANG X, et al. Biomaterial-related cell microenvironment in tissue engineering and regenerative medicine. Engieering, 2022, 13: 31. |
| [56] |
ZHANG H, WU C. 3D printing of biomaterials for vascularized and innervated tissue regeneration. International Journal of Bioprinting, 2023, 9(3): 706.
DOI PMID |
| [57] | ZHANG H, MA W, MA H, et al. Spindle-like zinc silicate nanoparticles accelerating innervated and vascularized skin burn wound healing. Advanced Healthcare Materials, 2022, 10: 2102359. |
| [58] | ZHANG Z, CHANG D, ZENG Z, et al. CuCS/Cur composite wound dressings promote neuralized skin regeneration by rebuilding the nerve cell “factory” in deep skin burns. Materials Today Bio, 2024, 26: 101075. |
| [59] | KANG Y, LIU K, CHEN Z, et al. Healing with precision: a multi- functional hydrogel-bioactive glass dressing boosts infected wound recovery and enhances neurogenesis in the wound bed. Journal of Controlled Release, 2024, 370: 210. |
| [60] | XU S, ZHANG Y, DAI B, et al. Green-prepared magnesium silicate sprays enhance the repair of burn-skin wound and appendages regeneration in rats and minipigs. Advanced Functional Materials, 2024, 34(9): 2307439. |
| [61] | SAMANDARI M, QUINT J, RODRIGUEZ-DELAROSA A, et al. Bioinks and bioprinting strategies for skeletal muscle tissue engineering. Advanced Materials, 2022, 34(12): 2105883. |
| [62] | ZHANG H, QIN C, SHI Z, et al. Bioprinting of inorganic- biomaterial/neural-stem-cell constructs for multiple tissue regeneration and functional recovery. National Science Review, 2024, 11(4): nwae035. |
| [63] | ZHU Y, ZHANG X, CHANG G, et al. Bioactive glass in tissue regeneration: unveiling recent advances in regenerative strategies and applications. Advanced Materials, 2025, 37(2): 2312964. |
| [64] |
WOLTERINK R G J K, WU G S, CHIU I M, et al. Neuroimmune interactions in peripheral organs. Annual Review of Neuroscience, 2022, 45: 339.
DOI PMID |
| [65] | LU Y Z, NAYER B, SINGH S K, et al. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature, 2024, 628: 604. |
| [66] | CUI X, WANG L, GAO X, et al. Self-assembled silk fibroin injectable hydrogels based on layered double hydroxides for spinal cord injury repair. Matter, 2024, 7(2): 620. |
| [67] |
LIAN C, LIU J, WEI W, et al. Mg-gallate metal-organic framework-based sprayable hydrogel for continuously regulating oxidative stress microenvironment and promoting neurovascular network reconstruction in diabetic wounds. Bioactive Materials, 2024, 38: 181.
DOI PMID |
| [68] | PENG L H, XU X H, HUANG Y F, et al. Self-adaptive all-in-one delivery chip for rapid skin nerves regeneration by endogenous mesenchymal stem cells. Advanced Functional Materials, 2020, 30(40): 2001751. |
| [69] | XU C, CHANG Y, WU P, et al. Two-dimensional-germanium phosphide-reinforced conductive and biodegradable hydrogel scaffolds enhance spinal cord injury repair. Advanced Functional Materials, 2021, 31(41): 2104440. |
| [70] | YU Q, JIN S, WANG S, et al. Injectable, adhesive, self-healing and conductive hydrogels based on MXene nanosheets for spinal cord injury repair. Chemical Engineering Journal, 2023, 452: 139252. |
| [71] |
AN G, GUO F, LIU X, et al. Functional reconstruction of injured corpus cavernosa using 3D-printed hydrogel scaffolds seeded with HIF-1α-expressing stem cells. Nature Communications, 2020, 11: 2687.
DOI PMID |
| [72] | WANG S, WANG Z, YANG W, et al. In situ-sprayed bioinspired adhesive conductive hydrogels for cavernous nerve repair. Advanced Materials, 2024, 36(19): 2311264. |
| [73] | PI W, CHEN H, LIU Y, et al. Flexible sono-piezo patch for functional sweat gland repair through endogenous microenvironmental remodeling. ACS Nano, 2024, 18(31): 20283. |
| [74] | CHEN P, XU C, WU P, et al. Wirelessly powered electrical- stimulation based on biodegradable 3D piezoelectric scaffolds promotes the spinal cord injury. ACS Nano, 2022, 16(10): 16513. |
| [75] | LIU Y, ZHANG Z, ZHAO Z, et al. An easy nanopatch promotes peripheral nerve repair through wireless ultrasound-electrical stimulation in a band-aid-like way. Advanced Functional Materials, 2024, 34(44): 2407411. |
| [76] |
WANG L, DU J, LIU Q, et al. Wrapping stem cells with wireless electrical nanopatches for traumatic brain injury therapy. Nature Communications, 2024, 15: 7223.
DOI PMID |
| [77] |
VARADARAJAN S G, HUNYARA J L, HAMILTON N R, et al. Central nervous system regeneration. Cell, 2022, 185(1): 77.
DOI PMID |
| [1] | YU Shengyang, SU Haijun, JIANG Hao, YU Minghui, YAO Jiatong, YANG Peixin. A Review of Pore Defects in Ultra-high Temperature Oxide Ceramics by Laser Additive Manufacturing: Formation and Suppression [J]. Journal of Inorganic Materials, 2025, 40(9): 944-956. |
| [2] | LIU Jiangping, GUAN Xin, TANG Zhenjie, ZHU Wenjie, LUO Yongming. Research Progress on Catalytic Oxidation of Nitrogen-containing Volatile Organic Compounds [J]. Journal of Inorganic Materials, 2025, 40(9): 933-943. |
| [3] | XIAO Xiaolin, WANG Yuxiang, GU Peiyang, ZHU Zhenrong, SUN Yong. Advances in Regulation of Damaged Skin Regeneration by Two-dimensional Inorganic Materials [J]. Journal of Inorganic Materials, 2025, 40(8): 860-870. |
| [4] | MA Jingge, WU Chengtie. Application of Inorganic Bioceramics in Promoting Hair Follicle Regeneration and Hair Growth [J]. Journal of Inorganic Materials, 2025, 40(8): 901-910. |
| [5] | AI Minhui, LEI Bo. Micro-nanoscale Bioactive Glass: Functionalized Design and Angiogenic Skin Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 921-932. |
| [6] | WANG Yutong, CHANG Jiang, XU He, WU Chengtie. Advances in Silicate Bioceramic/Bioglass for Wound Healing: Effects, Mechanisms and Application Ways [J]. Journal of Inorganic Materials, 2025, 40(8): 911-920. |
| [7] | MA Wenping, HAN Yahui, WU Chengtie, LÜ Hongxu. Application of Inorganic Bioactive Materials in Organoid Research [J]. Journal of Inorganic Materials, 2025, 40(8): 888-900. |
| [8] | LUO Xiaomin, QIAO Zhilong, LIU Ying, YANG Chen, CHANG Jiang. Inorganic Bioactive Materials Regulating Myocardial Regeneration [J]. Journal of Inorganic Materials, 2025, 40(8): 871-887. |
| [9] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [10] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [11] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [12] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [13] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [14] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [15] | CHEN Xi, YUAN Yuan, TAN Yeqiang, LIU Changsheng. Strategic Study on the Development of Inorganic Non-metallic Biomaterials [J]. Journal of Inorganic Materials, 2025, 40(5): 449-456. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||