
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (4): 355-364.DOI: 10.15541/jim20200366
Previous Articles Next Articles
WANG Haoxuan1( ), LIU Qiaomu2, WANG Yiguang3(
), LIU Qiaomu2, WANG Yiguang3( )
)
Received:2020-07-02
Revised:2020-09-27
Published:2021-04-20
Online:2020-09-20
Contact:
WANG Yiguang, professor. E-mail: wangyiguang@bit.edu.cn
About author:WANG Haoxuan(1994-), male, PhD candidate. E-mail: wanghaoxuan@mail.nwpu.edu.cn
Supported by:CLC Number:
WANG Haoxuan, LIU Qiaomu, WANG Yiguang. Research Progress of High Entropy Transition Metal Carbide Ceramics[J]. Journal of Inorganic Materials, 2021, 36(4): 355-364.
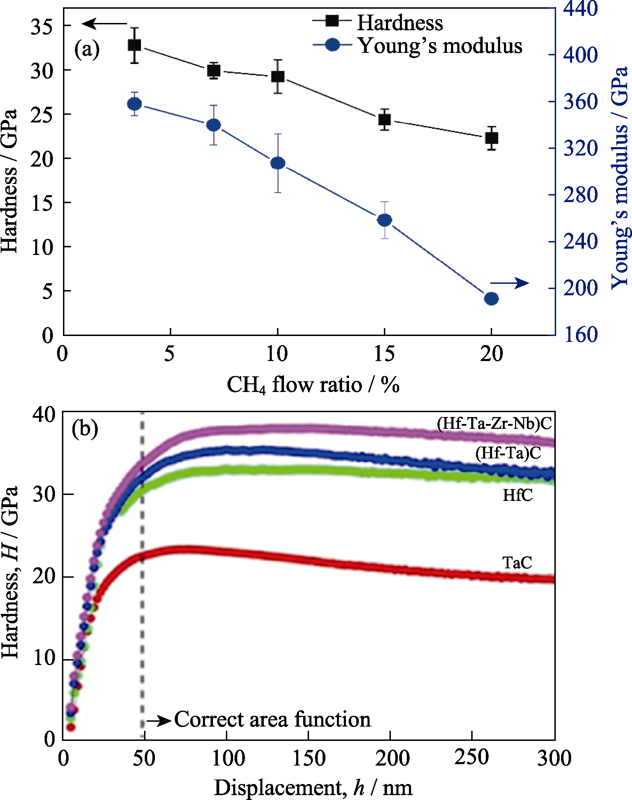
Fig. 2 (a) Hardness of the CrNbSiTiZrCx with different carbon contents[84], and (b) hardness depth-profles of the individual, binary and high-entropy carbide[87]
| HEC | Hardness/GPa | Average/GPa |
|---|---|---|
| HfC[ | 25 | — |
| TaC[ | 14 | — |
| ZrC[ | 24 | — |
| TiC[ | 31 | — |
| NbC[ | 17 | — |
| WC[ | 14 | — |
| VC[ | 29 | — |
| Mo2C[ | 27 | — |
| (ZrNbTiV)C[ | 30 | 25 |
| (HfTaZrNb)C[ | 36 | 20 |
| (TiVNbTaW)C[ | 28 | 21 |
| (TiHfTaWZr)C[ | 33 | 22 |
| (TiHfNbTaMo)C[ | 27 | 23 |
| (TiZrNbTaMo)C[ | 32 | 23 |
| (VNbTaMoW)C[ | 27 | 20 |
| (HfTaZrTiNb)C[ | 32 | 28 |
| (TiZrHfTaW)C[ | 24 | 22 |
| (TiHfNbTaW)C[ | 31 | 20 |
| (TiHfVNbTa)C[ | 29 | 23 |
Table 1 Hardness of some carbide ceramics[31,74,88-90]
| HEC | Hardness/GPa | Average/GPa |
|---|---|---|
| HfC[ | 25 | — |
| TaC[ | 14 | — |
| ZrC[ | 24 | — |
| TiC[ | 31 | — |
| NbC[ | 17 | — |
| WC[ | 14 | — |
| VC[ | 29 | — |
| Mo2C[ | 27 | — |
| (ZrNbTiV)C[ | 30 | 25 |
| (HfTaZrNb)C[ | 36 | 20 |
| (TiVNbTaW)C[ | 28 | 21 |
| (TiHfTaWZr)C[ | 33 | 22 |
| (TiHfNbTaMo)C[ | 27 | 23 |
| (TiZrNbTaMo)C[ | 32 | 23 |
| (VNbTaMoW)C[ | 27 | 20 |
| (HfTaZrTiNb)C[ | 32 | 28 |
| (TiZrHfTaW)C[ | 24 | 22 |
| (TiHfNbTaW)C[ | 31 | 20 |
| (TiHfVNbTa)C[ | 29 | 23 |
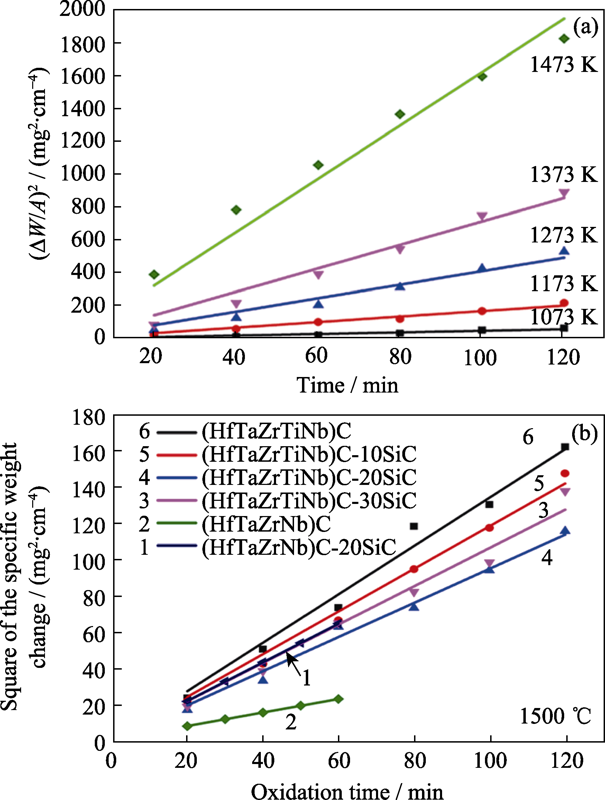
Fig. 4 (a) Square of the specific weight change as a function of oxidation time at 800-1200 ℃ for (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C[95], (b) square of the specific weight change as a function of oxidation time at 1500 ℃ for high-entropy carbide in different systems[33,97,99]
| [1] | MIRACLE D B, SENKOV O N. A critical review of high entropy alloys and related concepts. Acta Mater., 2017,122:448-511. |
| [2] | YEH J W, CHEN S K, LIN S J, et al. Nanostructured high- entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater., 2004,6(5):299-303. |
| [3] |
KUCZA W, DABROWA J, CIESLAK G, et al. Studies of “sluggish diffusion” effect in Co-Cr-Fe-Mn-Ni, Co-Cr-Fe-Ni and Co-Fe-Mn-Ni high entropy alloys; determination of tracer diffusivities by combinatorial approach. J. Alloys Compd., 2018,731:920-928.
DOI URL |
| [4] | UTT D, STUKOWSKI A, ALBE K. Grain boundary structure and mobility in high-entropy alloys: a comparative molecular dynamics study on a 11 symmetrical tilt grain boundary in face-centered cubic CuNiCoFe. Acta Mater., 2020,186:11-19. |
| [5] |
GLUDOVATZ B, HOHENWARTER A, CATOOR D, et al. A fracture-resistant high-entropy alloy for cryogenic applications. Science, 2014,345(6201):1153-1158.
URL PMID |
| [6] |
LI Z M, PRADEEP K G, DENG Y, et al. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature, 2016,534(7606):227-230.
URL PMID |
| [7] | ZHANG R Z, REECE M J. Review of high entropy ceramics: design, synthesis, structure and properties. J. Mater. Chem. A, 2019,7(39):22148-22162. |
| [8] | MURTY B S, YEH J W, RANGANATHAN S, et al. High-entropy Alloys. United Kingdom: Butterworth-Heinemann, 2019: 165-176. |
| [9] | OSES C, TOHER C, CURTAROLO S. High-entropy ceramics. Nat. Rev. Mater., 2020,5(4):295-309. |
| [10] | LAL M S, SUNDARA R. High entropy oxides-a cost-effective catalyst for the growth of high yield carbon nanotubes and their energy applications. ACS Appl. Mater. Inter., 2019,11(34):30846-30857. |
| [11] |
SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage. Nat. Commun., 2018,9(1):3400.
URL PMID |
| [12] | BERARDAN D, FRANGER S, DRAGOE D, et al. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi-R, 2016,10(4):328-333. |
| [13] | BIESUZ M, SPIRIDIGLIOZZI L, DELLAGLI G, et al. Synthesis and sintering of (Mg,Co,Ni,Cu,Zn)O entropy-stabilized oxides obtained by wet chemical methods. J. Mater. Sci., 2018,53(11):8074-8085. |
| [14] |
OKEJIRI F, ZHANG Z H, LIU J X, et al. Room-temperature synthesis of high-entropy perovskite oxide nanoparticle catalysts via ultrasonication-based method. ChemSusChem, 2020,13(1):111-115.
DOI URL PMID |
| [15] | DEMIRSKYI D, BORODIANSKA H, SUZUKI T S, et al. High- temperature flexural strength performance of ternary high-entropy carbide consolidated via spark plasma sintering of TaC, ZrC and NbC. Scripta Mater., 2019,164:12-16. |
| [16] | LI F, BAO W C, SUN S K, et al. Synthesis of single-phase metal oxycarbonitride ceramics. Scripta Mater., 2020,176:17-22. |
| [17] | KUMAR A, GUPTA M. An insight into evolution of light weight high entropy alloys: a review. Metals Basel, 2016,6(9):199. |
| [18] | ROST C M, SACHET E, BORMAN T, et al. Entropy-stabilized oxides. Nat. Commun., 2015,6(1):8485. |
| [19] | WEI X F, QIN Y, LIU J X, et al. Gradient microstructure development and grain growth inhibition in high-entropy carbide ceramics prepared by reactive spark plasma sintering . J. Eur. Ceram. Soc., 2020,40(4):935-941. |
| [20] | LI F, LU Y, WANG X G, et al. Liquid precursor-derived high- entropy carbide nanopowders. Ceram. Int., 2019,45(7):22437-22441. |
| [21] | WEI X F, LIU J X, LI F, et al. High entropy carbide ceramics from different starting materials. J. Eur. Ceram. Soc., 2019,39(10):2989-2994. |
| [22] | DUSZA J, SVEC P, GIRMAN V, et al. Microstructure of (Hf-Ta-Zr-Nb)C high-entropy carbide at micro and nano/atomic level. J. Eur. Ceram. Soc., 2018,38(12):4303-4307. |
| [23] | ZHOU J Y, ZHANG J Y, ZHANG F, et al. High-entropy carbide: a novel class of multicomponent ceramics. Ceram. Int., 2018,44(17):22014-22018. |
| [24] | JIANG S C, HU T, GILD J, et al. A new class of high-entropy perovskite oxides. Scripta Mater., 2018,142:116-120. |
| [25] | ZHAO Z F, CHEN H, XIANG H M, et al. (Y0.25Yb0.25Er0.25Lu0.25)2(Zr0.5Hf0.5)2O7: a defective fluorite structured high entropy ceramic with low thermal conductivity and close thermal expansion coefficient to Al2O3. J. Mater. Sci. Technol., 2020,39:167-172. |
| [26] | DABROWA J, STYGAR M, MIKULA A, et al. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett., 2018,216:32-36. |
| [27] | ZHAO Z F, CHEN H, XIANG H M, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: a high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3. J. Mater. Sci. Technol., 2020,38:80-85. |
| [28] | GILD J, KAUFMANN K, Vecchio K, et al. Reactive flash spark plasma sintering of high-entropy ultrahigh temperature ceramics. Scripta. Mater., 2019,170:106-110. |
| [29] | YAN J L, LIU F S, MA G H, et al. Suppression of the lattice thermal conductivity in NbFeSb-based half-Heusler thermoelectric materials through high entropy effects. Scripta Mater., 2018,157:129-134. |
| [30] | GILD J, BRAUN J L, KAUFMANN K, et al. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater., 2019,5(3):337-343. |
| [31] |
SARKER P, HARRINGTON T, TOHER C, et al. High-entropy high-hardness metal carbides discovered by entropy descriptors. Nat. Commun., 2018,9(1):4980.
URL PMID |
| [32] |
SARKAR A, VELASCO L, WANG D, et al. High entropy oxides for reversible energy storage. Nat. Commun., 2018,9(1):3400.
URL PMID |
| [33] |
WANG H X, CAO Y J, LIU W, et al. Oxidation behavior of (Hf0.2Ta0.2Zr0.2Ti0.2Nb0.2)C-xSiC ceramics at high temperature. Ceram. Int., 2020,46(8):11160-11168.
DOI URL |
| [34] | TAN Y Q, CHEN C, Li S G, et al. Oxidation behaviours of high-entropy transition metal carbides in 1200 ℃ water vapor. J. Alloys Compd., 2020,816:152523. |
| [35] | REN K, WANG Q K, SHAO G, et al. Multicomponent high- entropy zirconates with comprehensive properties for advanced thermal barrier coating. Scripta Mater., 2020,178:382-386. |
| [36] | DONG Y, REN K, LU Y H, et al. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc., 2018,39(7):2574-2579. |
| [37] | TENG Z, ZHU L N, TAN Y Q, et al. Synjournal and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc., 2020,40(4):1639-1643. |
| [38] | ZHAO Z F, XIANG H M, DAI F Z, et al. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol., 2019,35(11):2647-2651. |
| [39] | SAVINO R, FUMO M D S, PATERNA D, et al. Aerothermodynamic study of UHTC-based thermal protection systems. Aerosp. Sci. Technol., 2005,9(2):151-160. |
| [40] | OPRKA M M, TALMY I G, ZAYKOSKI J A. Oxidation-based materials selection for 2000 ℃ + hypersonic aerosurfaces: theoretical considerations and historical experience. J. Mater. Sci., 2004,39(19):5887-5904. |
| [41] | KUBOTA Y, YANO M, INOUE R, et al. Oxidation behavior of ZrB2-SiC-ZrC in oxygen-hydrogen torch environment. J. Eur. Ceram. Soc., 2017,38(4):1095-1102. |
| [42] | RAMA RAO G A, VENUGOPAL V. Kinetics and mechanism of the oxidation of ZrC. J. Alloys Compd., 1994,206(2):237-242. |
| [43] | VOITOVICH R F, PUGACH E A. High-temperature oxidation of ZrC and HfC. Powder Metall. Met. C, 1973,12(11):916-921. |
| [44] | CHEN L Y, GU Y L, SHI L, et al. Synjournal and oxidation of nanocrystalline HfB2. J. Alloys Compd., 2004,368(1):353-356. |
| [45] | SHIMADA S. Interfacial reaction on oxidation of carbides with formation of carbon. Solid State Ionics, 2001,141:99-104. |
| [46] | PARTHASARATHY T A, RAPP R A, OPEKA M M, et al. A model for the oxidation of ZrB2, HfB2 and TiB2. Acta Mater., 2007,55(17):5999-6010. |
| [47] | PARTHASARATHY T A, RAPP R A, OPEKA M M, et al. Effect of phase change and oxygen permeability in oxide scales on oxidation kinetics of ZrB2 and HfB2. J. Am. Ceram. Soc., 2009,92(5):1079-1086. |
| [48] | JING Y, YUAN H B, LIAN Z S. Microstructure and mechanical properties of ZrB2-HfC ceramics influenced by HfC addition. Materials, 2018,11(10):2046. |
| [49] | MALLIK M, RAY K K, MITRA R. Oxidation behavior of hot pressed ZrB2-SiC and HfB2-SiC composites. J. Eur. Ceram. Soc., 2011,31(1):199-215. |
| [50] | TRIPP W C, GRAHAM H C. Thermogravimetric study of oxidation of ZrB2 in temperature range of 800 ℃ to 1500 ℃. J. Electrochem. Soc., 1971,118(7):1195-1199. |
| [51] | FAHRENHOLTZ W G. The ZrB2 volatility diagram. J. Am. Ceram. Soc., 2005,88(12):3509-3512. |
| [52] | FAHRENHOLTZ W G. Thermodynamic analysis of ZrB2-SiC oxidation: formation of a SiC-depleted region . J. Am. Ceram. Soc., 2007,90(1):143-148. |
| [53] | HU P, GUOLIN W, WANG Z. Oxidation mechanism and resistance of ZrB2-SiC composites. Corros. Sci., 2009,51(11):2724-2732. |
| [54] | JACOBSON N S, MYERS D L. Active oxidation of SiC. Oxid. Met., 2011,75(1):1-25. |
| [55] | JACOBSON N S, HARDER B, MYERS D L, et al. Oxidation transitions for SiC. Part I. Active-to-passive transitions. J. Am. Ceram. Soc., 2013,96(3):838-844. |
| [56] | WANG Y G, LUO L, SUN J, et al. ZrB2-SiC(Al) ceramics with high resistance to oxidation at 1500 ℃. Corros. Sci., 2013,74:154-158. |
| [57] | HE J B, WANG Y G, LUO L, et al. Oxidation behaviour of ZrB2-SiC (Al/Y) ceramics at 1700 ℃. J. Eur. Ceram. Soc., 2016,36(15):3769-3774. |
| [58] | WANG Y G, MA B S, LI L L, et al. Oxidation behavior of ZrB2-SiC-TaC ceramics . J. Am. Ceram. Soc., 2012,95(1):374-378. |
| [59] |
TONG Z W, HE R J, CHENG T B, et al. High temperature oxidation behavior of ZrB2-SiC added MoSi2 ceramics. Ceram. Int., 2018,44(17):21076-21082.
DOI URL |
| [60] | ZAPATASOLVAS E, JAYASEELAN D D, BROWN P, et al. Effect of La2O3 addition on long-term oxidation kinetics of ZrB2-SiC and HfB2-SiC ultra-high temperature ceramics . J. Eur. Ceram. Soc., 2014,34(15):3535-3548. |
| [61] | GILD J, ZHANG Y Y, HARRINGTON T, et al. High-entropy metal diborides: a new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Sci. Rep-UK, 2016,6(1):37946-37946. |
| [62] | YE B L, WEN T Q, HUANG K H, et al. First-principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high- entropy ceramic. J. Am. Ceram. Soc., 2019,102(7):4344-4352. |
| [63] | HOSKING F M. Sodium compatibility of refractory-metal alloy- type 304l stainless-steel joints. Int. J. Refract. Met. H., 1985,64(7):S181-S190. |
| [64] | WERNER E A. Introduction to the thermodynamics of materials. Mat. Sci. Eng., 2008,494(1/2):464. |
| [65] |
CAR R, PARRINELLO M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett., 1985,55(22):2471-2474.
URL PMID |
| [66] | LIU X J, WANG C P, GAO F, et al. Thermodynamic calculation of phase equilibria in the Sn-Ag-Cu-Ni-Au System . J. Electron. Mater., 2007,36(11):1429-1441. |
| [67] | WANG C P, WANG J, GUO S H, et al. Experimental investigation and thermodynamic calculation of the phase equilibria in the Co-Mo-W system. Intermetallics, 2009,17(8):642-650. |
| [68] | FENG R, GAO M C, LEE C, et al. Design of light-weight high- entropy alloys. Entropy Switz., 2016,18(9):333-353. |
| [69] | KIM J. Applicability of special quasi-random structure models in thermodynamic calculations using semi-empirical Debye-Grüneisen theory . J. Alloys Compd., 2015,650:564-571. |
| [70] | VOAS B K, USHER T M, LIU X M, et al. Special quasirandom structures to study the (K0.5Na0.5)NbO3 random alloy. Phys. Rev. B, 2014,90(2):024105-1-6. |
| [71] | SAHARA R, EMURA S, LI S, et al. First-principles study of electronic structures and stability of body-centered cubic Ti-Mo alloys by special quasirandom structures. Sci. Technol. Adv. Mat., 2014,15(3):035014-1-10. |
| [72] |
VITOS L, ABRIKOSOV I A, JOHANSSON B. Anisotropic lattice distortions in random alloys from first-principles theory. Phys. Rev. Lett., 2001,87(15):156401-1-4.
URL PMID |
| [73] | ABRIKOSOV I A, JOHANSSON B. Applicability of the coherent- potential approximation in the theory of random alloys. Phys. Rev. B, 1998,57(22):14164-14173. |
| [74] | YE B L, WEN T Q, NGUYEN M C, et al. First-principles study, fabrication and characterization of (Zr0.25Nb0.25Ti0.25V0.25)C high- entropy ceramics. Acta Mater., 2019,170:15-23. |
| [75] |
BRAIC V, VLADESCU A, BALACEANU M, et al. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coat. Tech., 2012,211:117-121.
DOI URL |
| [76] |
LIN S Y, CHANG S Y, HUANG Y C, et al. Mechanical performance and nanoindenting deformation of (AlCrTaTiZr)NCy multi-component coatings co-sputtered with bias. Surf. Coat. Tech., 2012,206(24):5096-5102.
DOI URL |
| [77] |
ZHOU J Y, ZHANG J Y, ZHANG F, et al. high-entropy carbide: a novel class of multicomponent ceramics. Ceram. Int., 2018,44(17):22014-22018.
DOI URL |
| [78] |
FENG L, FAHRENHOLTZ W G, HILMAS G E, et al. Synthesis of single-phase high-entropy carbide powders. Scripta Mater., 2019,162(12):90-93.
DOI URL |
| [79] | YE B L, NING S S, LIU D, et al. One-step synthesis of coral-like high-entropy metal carbide powders. J.Am. Ceram. Soc., 102(10):6372-6378. |
| [80] |
DU B, LIU H H, CHU Y H. Fabrication and characterization of polymer-derived high-entropy carbide ceramic powders . J. Am. Ceram. Soc., 2020,103:4063-4068.
DOI URL |
| [81] | NING S S, WEN T Q, YE B L, et al. Low-temperature molten salt synjournal of high-entropy carbide nanopowders . J. Am. Ceram. Soc., 2020,103(3):2244-2251. |
| [82] | JAGADEESH S, VISHNU D S M, KIM H K, et al. Facile electrochemical synthesis of nanoscale (TiNbTaZrHf)C high-entropy carbide powder. Angew. Chem. Int. Ed., 2020 59(29):11830-11835. |
| [83] | BRAIC M, BRAIC V, BALACEANU M, et al. Characteristics of (TiAlCrNbY)C films deposited by reactive magnetron sputtering. Surf. Coat. Tech., 2010,204(12):2010-2014. |
| [84] | JHONG Y S, HUANG C W, LIN S J, et al. Effects of CH4 flow ratio on the structure and properties of reactively sputtered (CrNbSiTiZr)Cx coatings. Mater. Chem. Phys., 2017,210:348-352. |
| [85] | BRAIC M, BALACEANU M, VLADESCU A, et al. Deposition and characterization of multi-principal-element (CuSiTiYZr)C coatings. Appl. Surf. Sci., 2013 284:671-678. |
| [86] | BRAIC V, PARAU A C, PANA I, et al. Effects of substrate temperature and carbon content on the structure and properties of (CrCuNbTiY)C multicomponent coatings. Surf. Coat. Tech., 2014 258:996-1005. |
| [87] | CSANADI T, CASTLE E G, REECE M J, et al. Strength enhancement and slip behaviour of high-entropy carbide grains during micro-compression. Sci. Rep-UK, 2019,9(1):10200. |
| [88] | WANG C, YE Y, GUAN X, et al. An analysis of tribological performance on Cr/GLC film coupling with Si3N4, SiC, WC, Al2O3 and ZrO2 in seawater. Tribol. Int., 2016 96:77-86. |
| [89] | HARRINGTON T J, GILD J, SARKER P, et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater., 2019 166:271-280. |
| [90] | WANG K, CHEN L, XU C G, et al. Microstructure and mechanical properties of (TiZrNbTaMo)C high-entropy ceramic. J. Mater. Sci. Technol., 2020,39:99-105. |
| [91] | HAN X X, VLADIMIR G, RICHARD S, et al. Improved creep resistance of high entropy transition metal carbides . J. Eur. Ceram. Soc., 2020,40(7):2709-2715. |
| [92] | YAN X L, CONSTANTIN L, LU Y F, et al. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics with low thermal conductivity. J. Am. Ceram. Soc., 2018,101(10):4486-4491. |
| [93] | CHEN H, XIANG H M, DAI F Z, et al. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: a novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol., 2019,35(10):2404-2408. |
| [94] | CHEN H, XIANG H M, DAI F Z, et al. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol., 2019,35(8):1700-1705. |
| [95] |
YE B L, WEN T Q, LIU D, et al. Oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics at 1073-1473 K in air. Corros. Sci., 2019,153:327-332.
DOI URL |
| [96] | YE B L, WEN T Q, CHU Y H. High-temperature oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics in air. J. Am. Ceram. Soc., 2019,103(1):500-507. |
| [97] | WANG H X, HAN X, LIU W, et al. Oxidation behavior of high-entropy carbide (Hf0.2Ta0.2Er0.2Ti0.2Nb0.2)C at 1400-1600 ℃. DOI: 10.1016/j.ceramint.2020.12.201. |
| [98] | BACKMAN L, GILD J, LUO J, et al. Theoretical predictions of preferential oxidation in refractory high entropy materials. Acta Mater., 2020,197:20-27. |
| [99] | WANG H X, WANG S Y, CAO Y J, et al. Oxidation behaviors of (Hf0.25Zr0.25Ta0.25Nb0.25)C and (Hf0.25Zr0.25Ta0.25Nb0.25)C-SiC at 1300-1500 ℃. J. Mater. Sci. Technol., 2021,60:147-155. |
| [100] |
BRAIC V, BALACEANU M, BRAIC M, et al. Characterization of multi-principal-element (TiZrNbHfTa)N and (TiZrNbHfTa)C coatings for biomedical applications. J. Mech. Behav. Biomed., 2019,10:197-205.
DOI URL |
| [101] | WANG F, YAN X L, WANG T Y, et al. Irradiation damage in (Zr0.25Ta0.25Nb0.25Ti0.25)C high-entropy carbide ceramics. Acta Mater., 2020,195:739-749. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | SUN Yuxuan, WANG Zheng, SHI Xue, SHI Ying, DU Wentong, MAN Zhenyong, ZHENG Liaoying, LI Guorong. Defect Dipole Thermal-stability to the Electro-mechanical Properties of Fe Doped PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(5): 545-551. |
| [8] | CHEN Yi, QIU Haipeng, CHEN Mingwei, XU Hao, CUI Heng. SiC/SiC Composite: Matrix Boron Modification and Mechanical Properties [J]. Journal of Inorganic Materials, 2025, 40(5): 504-510. |
| [9] | CUI Ning, ZHANG Yuxin, WANG Lujie, LI Tongyang, YU Yuan, TANG Huaguo, QIAO Zhuhui. Single-phase Formation Process and Carbon Vacancy Regulation of (TiVNbMoW)Cx High-entropy Ceramics [J]. Journal of Inorganic Materials, 2025, 40(5): 511-520. |
| [10] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [11] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [12] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [13] | LI Ziwei, GONG Weilu, CUI Haifeng, YE Li, HAN Weijian, ZHAO Tong. (Zr, Hf, Nb, Ta, W)C-SiC Composite Ceramics: Preparation via Precursor Route and Properties [J]. Journal of Inorganic Materials, 2025, 40(3): 271-280. |
| [14] | GAO Chenguang, SUN Xiaoliang, CHEN Jun, LI Daxin, CHEN Qingqing, JIA Dechang, ZHOU Yu. SiBCN-rGO Ceramic Fibers Based on Wet Spinning Technology: Microstructure, Mechanical and Microwave-absorbing Properties [J]. Journal of Inorganic Materials, 2025, 40(3): 290-296. |
| [15] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||