
Journal of Inorganic Materials ›› 2019, Vol. 34 ›› Issue (7): 694-702.DOI: 10.15541/jim20180512
Special Issue: 离子电池材料
Previous Articles Next Articles
LI Dong1,2,LEI Chao1,2,LAI Hua3,LIU Xiao-Lin1,2,YAO Wen-Li1,2,LIANG Tong-Xiang1,ZHONG Sheng-Wen1,2
Received:2018-10-31
Revised:2019-01-15
Published:2019-07-20
Online:2019-06-26
Supported by:CLC Number:
LI Dong, LEI Chao, LAI Hua, LIU Xiao-Lin, YAO Wen-Li, LIANG Tong-Xiang, ZHONG Sheng-Wen. Recent Advancements in Interface between Cathode and Garnet Solid Electrolyte for All Solid State Li-ion Batteries[J]. Journal of Inorganic Materials, 2019, 34(7): 694-702.
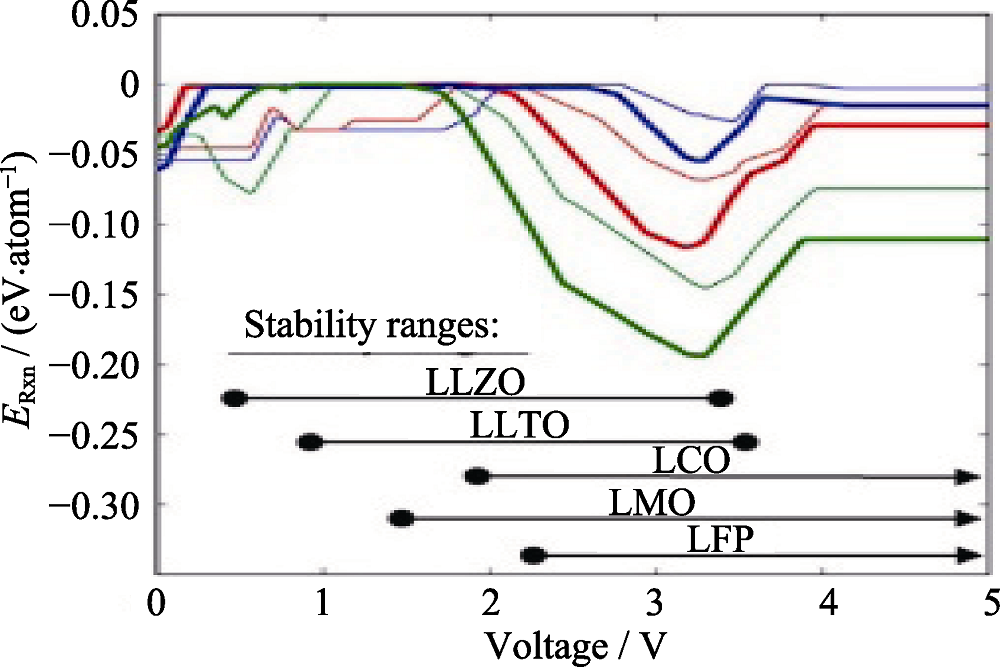
Fig. 2 Driving force for interphase formation between electrolyte, and cathode, with varying voltage from 0 to 5 V vs lithium metal [Legend: blue, LCO; red, LMO; green, LFP; thick line, LLZO; thin line, LLTO]. The calculated intrinsic stability windows are marked along the bottom for reference[50]
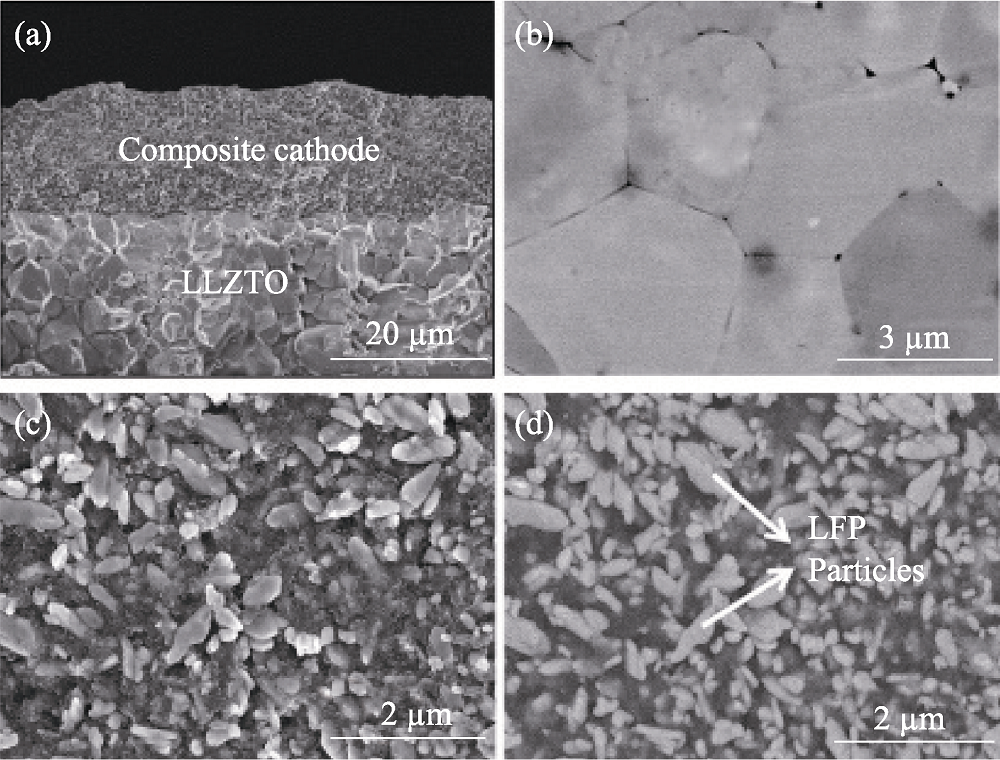
Fig. 3 (a) Typical scanning electron microscope (SEM) image of the interface between composite cathodes and LLZTO electrolyte; (b) SEM image for the surface of LLZTO ceramic; SEM images of the composite cathodes which were measured in (c) the second-electron and (d) the back-scatter-electron mode[53]
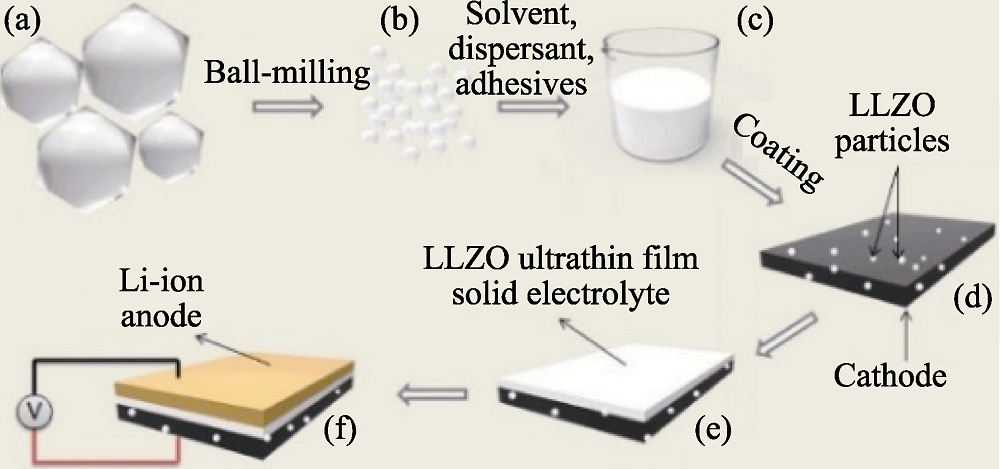
Fig. 4 Schematic illustration of the synthesis procedure[57] (a) Microscale LLZO particles; (b) Nanoscale LLZO particles; (c) Nanoscale LLZO slurry; (d) Cathode layer of LFP; (e) LLZO film; (f) All-solid-state battery of Li/LLZO/LFP
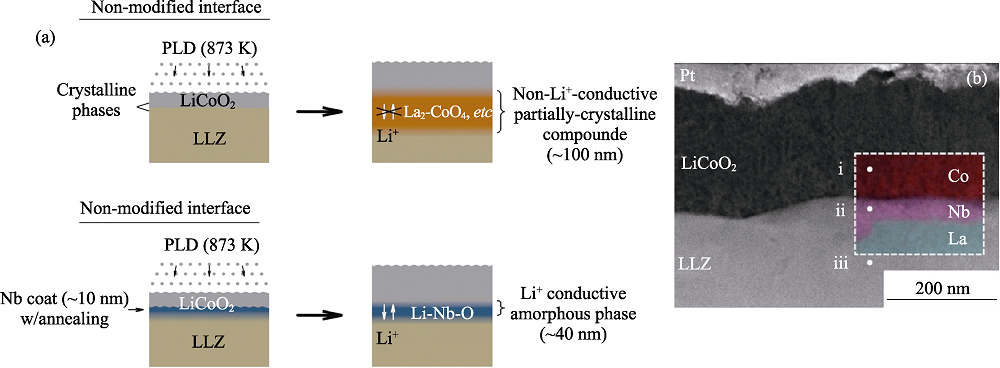
Fig. 5 (a) Schematic illustrations of non-modified and Nb-modified LLZ/LiCoO2 interfaces. The mutual diffusion between LLZO and LiCoO2 produces non-Li+-conductive phases such as a crystalline La2CoO4 phase. Nb-modified LLZ/LiCoO2 interface suppresses the mutual diffusion and produce Li+-conductive amorphous phase; (b) Cross-sectional-HAADF-STEM image of a Nb-modified interface between LLZO and LiCoO2[65]. EDX elemental mappings in (b) for Co (red), Nb (purple), and La (green) are overlaid in the dashed-line-enclosed region. The top Pt is a protective layer for FIB processes
| Electrolyte | Ion-conductivity/ (mS·cm-1) | Cathode materials | Interface Engineering | Test condition | Discharge capacity /(mAh·g-1) | Ref. |
|---|---|---|---|---|---|---|
| Li6.20Ga0.30La2.95Rb0.05Zr2O12 | 1.62 | LiFePO4 | Coating | 60 ℃, 5 μA·cm-2 2.8-4.0 V | 152(1st), 110(20th) | [24] |
| Li6.4La3Zr1.4Ta0.6O12 | 1.60 | LiFePO4 | Coating | 60 ℃, 0.05C 2.76-4.00 V | 150(1st), 140(100th) | [53] |
| Li7La3Zr2O12 | 2.40 | LiFePO4 | Coating | 25 ℃ 0.1C | 160.4 (1st), 136.8 (100th) | [57] |
| Li6.75La3Zr1.75Nb0.25O12 | 1.67 | LiCoO2 | PLD | 25 ℃, 3.5 μA·cm-2 2.5-4.2 V | 129 (1st), 127(100th) | [61] |
| Li6.8(La2.95Ca0.05)(Zr1.75Nb0.25)O12 | 0.36 | LiCoO2 | Co-sintering | 1 μA·g-1, 3.0-4.2 V | 78(1st) | [47] |
| Li7La3Zr2O12 (1.7wt% Al, 0.1wt% Si) | 0.68 | LiCoO2 | PLD | 1 mA·cm-2, 3.2-4.2 V | 80 (1st) | [65] |
| Li6.75La3Zr1.75Nb0.25O12 | 1.23 | LiCoO2 | Screen-printing | 25 ℃, 10 μA·cm-2 3.00-4.05 V | 85(1st) | [64] |
| Li6.75La3Zr1.75Ta0.25O12 | ~1.00 | LiCoO2 | Coating+ co-sintering | 5 μA·cm-2 | 101.3(1st) | [54] |
| Li6.75La3Zr1.75Ta0.25O12 | 0.74 | LiNi0.5Co0.2Mn0.3O2 | Tape casting | 80 ℃, 5 μA·cm-2 3.0-4.6 V | 123.3 (1st), 76.6 (5th) | [56] |
| Li6.25Al0.25La3Zr2O12 | 0.50 | Li4Ti5O12 | Coating | 95 ℃, 2-8 μA·g-1 1.0-2.5 V | 15(1st) | [81] |
Table 1 Performances of ASSLBs based on garnet-type Li7La3Zr2O12 solid electrolytes
| Electrolyte | Ion-conductivity/ (mS·cm-1) | Cathode materials | Interface Engineering | Test condition | Discharge capacity /(mAh·g-1) | Ref. |
|---|---|---|---|---|---|---|
| Li6.20Ga0.30La2.95Rb0.05Zr2O12 | 1.62 | LiFePO4 | Coating | 60 ℃, 5 μA·cm-2 2.8-4.0 V | 152(1st), 110(20th) | [24] |
| Li6.4La3Zr1.4Ta0.6O12 | 1.60 | LiFePO4 | Coating | 60 ℃, 0.05C 2.76-4.00 V | 150(1st), 140(100th) | [53] |
| Li7La3Zr2O12 | 2.40 | LiFePO4 | Coating | 25 ℃ 0.1C | 160.4 (1st), 136.8 (100th) | [57] |
| Li6.75La3Zr1.75Nb0.25O12 | 1.67 | LiCoO2 | PLD | 25 ℃, 3.5 μA·cm-2 2.5-4.2 V | 129 (1st), 127(100th) | [61] |
| Li6.8(La2.95Ca0.05)(Zr1.75Nb0.25)O12 | 0.36 | LiCoO2 | Co-sintering | 1 μA·g-1, 3.0-4.2 V | 78(1st) | [47] |
| Li7La3Zr2O12 (1.7wt% Al, 0.1wt% Si) | 0.68 | LiCoO2 | PLD | 1 mA·cm-2, 3.2-4.2 V | 80 (1st) | [65] |
| Li6.75La3Zr1.75Nb0.25O12 | 1.23 | LiCoO2 | Screen-printing | 25 ℃, 10 μA·cm-2 3.00-4.05 V | 85(1st) | [64] |
| Li6.75La3Zr1.75Ta0.25O12 | ~1.00 | LiCoO2 | Coating+ co-sintering | 5 μA·cm-2 | 101.3(1st) | [54] |
| Li6.75La3Zr1.75Ta0.25O12 | 0.74 | LiNi0.5Co0.2Mn0.3O2 | Tape casting | 80 ℃, 5 μA·cm-2 3.0-4.6 V | 123.3 (1st), 76.6 (5th) | [56] |
| Li6.25Al0.25La3Zr2O12 | 0.50 | Li4Ti5O12 | Coating | 95 ℃, 2-8 μA·g-1 1.0-2.5 V | 15(1st) | [81] |
| [1] | QIU Z P, ZHANG Y J, XIA S B , et al. Research progress on interface properties of inorganic solid state lithium ion batteries. Acta Chim.Sinica, 2015,73(10):992-1001. |
| [2] | XU X X, LI H . A review of solid-state lithium batteries. Energ. Stor. Sci. Technol., 2018,7(1):1-7. |
| [3] |
KIM J G, SON B, MUKHERJEE S , et al. A review of lithium and non-lithium based solid state batteries.[J]. Power Sources, 2015,282:299-322.
DOI URL |
| [4] |
MAUGER A, ARMAND M, JULIEN C M , et al. Challenges and issues facing lithium metal for solid-state rechargeable batteries.[J]. Power Sources, 2017,353:333-342.
DOI URL |
| [5] |
SUN C, LIU J, GONG Y , et al. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy, 2017,33:363-386.
DOI URL |
| [6] |
TAKADA K . Progress and prospective of solid-state lithium batteries. Acta Mater., 2013,61(3):759-770.
DOI URL |
| [7] |
MEESALA Y, JENA A, CHANG H , et al. Recent advancements in Li-ion conductors for all-solid-state Li-ion batteries. ACS Energy Lett., 2017,2(12):2734-2751.
DOI URL |
| [8] |
KERMAN K, LUNTZ A, VISWANATHAN V , et al. Review- practical challenges hindering the development of solid state Li ion batteries.[J]. Electrochem. Soc., 2017,164(7):A1731-A1744.
DOI URL |
| [9] | CHEN L, CHI S S, DONG Y , et al. Research progress of key materials for all-solid-state lithium batteries.[J]. Chin. Ceram. Soc., 2018,46(1):21-34. |
| [10] |
KAZUNORI TAKADA . Progress in solid electrolytes toward realizing solid-state lithium batteries.[J]. Power Sources, 2018,394:74-85.
DOI URL |
| [11] |
CHENG J, LI H, WANG C . Recent progress in solid-state electrolytes for alkali-ion batteries. Sci. Bull., 2017,62(21):1473-1490.
DOI URL |
| [12] |
ZHENG F, KOTOBUKI M, SONG S , et al. Review on solid electrolytes for all-solid-state lithium-ion batteries.[J]. Power Sources, 2018,389:198-213.
DOI URL |
| [13] |
ZHU Y, HE X, MO Y . Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces, , 2015,7(42):23685-23693.
DOI URL |
| [14] | ZHONG S W, HUANG B . Effects of excess lithium salt on properties of perovskite-type solid electrolyte Li3/8Sr7/16Ta3/4Hf1/4O3. Nonferr. Metal. Sci. Eng., 2017,8(1):70-74. |
| [15] | XU C, LUO J B, PENG W W , et al. SPS sintering and properties of NASICON type solid electrolyte Li1.1Y0.1Zr1.9(PO4)3. Nonferr. Metal. Sci. Eng., 2018,9(1):66-70. |
| [16] | LUO J B, LI T T, YOU W X , et al. Preparation of Li3/8Sr7/16Ta3/4Hf1/4O3 perovskite solid electrolyte by hot pressing sintering. Nonferr. Metal. Sci. Eng., 2018,9(4):66-69. |
| [17] |
BACHMAN J C, MUY S, GRIMAUD A , et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev., 2016,116(1):140-162.
DOI URL |
| [18] | LIN Z J, HE X M, LI J J , et al. Recent advances of all solid state polymer electrolyte for Li-ion batteries. Prog. Chem., 2006,18(4):459-466. |
| [19] |
RICHARDS W D, MIARA L J, WANG Y , et al. Interface stability in solid-state batteries. Chem. Mater., 2016,28:266-273.
DOI URL |
| [20] |
DUAN H, ZHENG H, ZHOU Y , et al. Stability of garnet-type Li ion conductors: an overview. Solid State Ionics, 2018,318:45-53.
DOI URL |
| [21] |
CHAN C K, YANG T, WELLER J M . Nanostructured garnet-type Li7La3Zr2O12: synthesis, properties, and opportunities as electrolytes for Li-ion batteries. Electrochim. Acta, 2017,253:268-280.
DOI URL |
| [22] |
RAMAKUMAR S, DEVIANNAPOORANI C, DHIVYA L , et al. Lithium garnets: synthesis, structure, Li +, conductivity, Li +, dynamics and applications . Prog. Mater. Sci., 2017,88:325-411.
DOI URL |
| [23] |
LIU Q, GENG Z, HAN C , et al. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries.[J]. Power Sources, 2018,389:120-134.
DOI URL |
| [24] |
WU J F, PANG W K, PETERSON V K , et al. Garnet-type fast Li-ion conductors with high ionic conductivities for all-solid-state batteries. ACS Appl. Mater. Interfaces, 2017,9(14):12461-12468.
DOI URL |
| [25] | HAN X, GONG Y, FU K K , et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater., 2017,16(5):572-579. |
| [26] |
FU K K, GONG Y, LIU B , et al. Toward garnet electrolyte-based Li metal batteries: an ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface. Sci. Adv., 2017,3(4):e1601659.
DOI URL |
| [27] | WANG C, GONG Y, LIU B , et al. Conformal, nanoscale ZnO surface modification of garnet-based solid state electrolyte for lithium metal anodes. Nano Lett., 2016,17(1):565-571. |
| [28] |
TIAN Y, DING F, ZHONG H , et al. Li6.75La3Zr1.75Ta0.25O12 @amorphous Li3OCl composite electrolyte for solid state lithium- metal batteries. Energy Storage Mater., 2018,14:49-57.
DOI URL |
| [29] | GAO Z H, SUN H B, FU L , et al. Promises, challenges,recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv Mater., 2018, 30(17): 1705702-1-27. |
| [30] |
ZHENG B, WANG H, MA J, GONG Z, YANG Y . A review of inorganic solid electrolyte/electrode interface in all-solid-state lithium batteries. Sci. Sin. Chim., 2017,47(5):579-593.
DOI URL |
| [31] |
YU S, SCHMIDT R D, GARCIA-MENDEZ R , et al. Elastic properties of the solid electrolyte Li7La3Zr2O12( LLZO). Chem. Mater., 2016,28(1):197-206.
DOI URL |
| [32] |
ZHAN X, LAI S, GOBET M P , et al. Defect chemistry and electrical properties of garnet-type Li7La3Zr2O12. Phys. Chem. Chem. Phys., 2018,20(3):1447-1459.
DOI URL |
| [33] |
LIU T, ZHANG Y, CHEN R , et al. Non-successive degradation in bulk-type all-solid-state lithium battery with rigid interfacial contact. Electrochem. Commun., 2017,79:1-4.
DOI URL |
| [34] |
YAMADA H, ITO T, HONGAHALLY BASAPPA R , et al. Influence of strain on local structure and lithium ionic conduction in garnet-type solid electrolyte.[J]. Power Sources, 2017,368:97-106.
DOI URL |
| [35] |
BUCCI G, SWAMY T, CHIANG Y M , et al. Modeling of internal mechanical failure of all-solid-state batteries during electrochemical cycling, and implications for battery design. J. Mater. Chem. A, 2017,5(36):19422-19430.
DOI URL |
| [36] |
CHENG L, CRUMLIN E J, CHEN W , et al. The origin of high electrolyte-electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys. Chem. Chem. Phys., 2014,16(34):18294-18300.
DOI URL |
| [37] |
CHENG L, WU C H, JARRY A , et al. Interrelationships among grain size, surface composition, air stability, and interfacial resistance of Al-substituted Li7La3Zr2O12 solid electrolytes. ACS Appl. Mater. Interfaces, 2015,7(32):17649-19655.
DOI URL |
| [38] |
SHARAFI A . Impact of air exposure and surface chemistry on Li-Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A, 2017,5(26):13475-13487.
DOI URL |
| [39] |
AHN C W, CHOI J J, RYU J , et al. Electrochemical properties of Li7La3Zr2O12-based solid state battery.[J]. Power Sources, 2014,272:554-558.
DOI URL |
| [40] |
XIA W, XU B, DUAN H , et al. Reaction mechanisms of lithium garnet pellets in ambient air: the effect of humidity and CO2.[J]. Am. Ceram. Soc., 2017,100:2832-2839.
DOI URL |
| [41] |
KANG S G, SHOLL D S . First-principles study of chemical stability of the lithium oxide garnets Li7La3M2O12 (M=Zr, Sn, or Hf). J. Phys. Chem. C, 2014,118(31):17402-17406.
DOI URL |
| [42] |
HOFSTETTER K, SAMSON A J, NARAYANAN S , et al. Present understanding of the stability of Li-stuffed garnets with moisture, carbon dioxide, and metallic lithium.[J]. Power Sources, 2018,390:297-312.
DOI URL |
| [43] |
JIN Y, MCGINN P J . Li7La3Zr2O12, electrolyte stability in air and fabrication of a Li/Li7La3Zr2O12/Cu0.1V2O5 solid-state battery.[J]. Power Sources, 2013,239(10):326-331.
DOI URL |
| [44] |
WANG Y X, LAI W . Phase transition in lithium garnet oxide ionic conductors Li7La3Zr2O12: the role of Ta substitution and H2O/CO2 exposure.[J]. Power Sources, 2015,275:612-620.
DOI URL |
| [45] |
TIAN Y, SHI T, RICHARDS W D , et al. Compatibility issues between electrodes and electrolytes in solid-state batteries. Energy Environ. Sci., 2017,10:1150-1166.
DOI URL |
| [46] |
KIM K H, IRIYAMA Y, YAMAMOTO K , et al. Characterization of the interface between LiCoO2, and Li7La3Zr2O12, in an all-solid-state rechargeable lithium battery.[J]. Power Sources, 2011,196(2):764-767.
DOI URL |
| [47] |
OHTA S, SEKI J, YAGI Y , et al. Co-sinterable lithium garnet-type oxide electrolyte with cathode for all-solid-state lithium ion battery.[J]. Power Sources, 2014,265:40-44.
DOI URL |
| [48] |
REN Y, LIU T, SHEN Y , et al. Chemical compatibility between garnet-like solid state electrolyte Li6.75La3Zr1.75Ta0.25O12, and major commercial lithium battery cathode materials.[J]. Materiomics, 2016,2(3):256-264.
DOI URL |
| [49] |
MIARA L, WINDMÜLLER A, TSAI C L , et al. About the compatibility between high voltage spinel cathode materials and solid oxide electrolytes as function of temperature. ACS Appl. Mater. Interfaces, 2016,8(40):26842-26850.
DOI URL |
| [50] |
MIARA L J, RICHARDS W D, WANG Y E , et al. First-principles studies on cation dopants and electrolyte|cathode interphases for lithium garnets. Chem. Mater., 2015,27(11):4040-4047.
DOI URL |
| [51] |
HÄNSEL C, AFYON S, RUPP J L . Investigating the all-solid-state batteries based on lithium garnets and a high potential cathode- LiMn1.5Ni0.5O4. Nanoscale, 2016,8(43):18412-18420.
DOI URL |
| [52] |
PARK K, YU B C, JUNG J W , et al. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem. Mater., 2016,28(21):8051-8059.
DOI URL |
| [53] |
DU F, ZHAO N, LI Y , et al. All solid state lithium batteries based on lamellar garnet-type ceramic electrolytes.[J]. Power Sources, 2015,300:24-28.
DOI URL |
| [54] |
LIU T, REN Y, SHEN Y , et al. Achieving high capacity in bulk-type solid-state lithium ion battery based on Li6.75La3Zr1.75Ta0.25O12, electrolyte: interfacial resistance.[J]. Power Sources, 2016,324:349-357.
DOI URL |
| [55] |
HE M, CUI Z, HAN F , et al. Construction of conductive and flexible composite cathodes for room-temperature solid-state lithium batteries.[J]. Alloys Compd., 2018,762:157-162.
DOI URL |
| [56] |
LIU T, ZHANG Y, ZHANG X , et al. Enhanced electrochemical performance of bulk type oxide ceramic lithium battery enabled by interface modification. J. Mater. Chem. A, 2018,6:4649-4657.
DOI URL |
| [57] |
YAN X F, LI Z B, WEN Z Y , et al. Li/Li7La3Zr2O12/LiFePO4 all-solid-state battery with ultrathin nanoscale solid electrolyte. J. Phys. Chem. C, 2017,121(3):1431-1435.
DOI URL |
| [58] |
WAKAYAMA H, YONEKURA H, KAWAI Y . Three-dimensional bicontinuous nanocomposite from a self-assembled block copolymer for a high-capacity all-solid-state lithium battery cathode. Chem. Mater., 2016,28(12):4453-4459.
DOI URL |
| [59] |
WAKAYAMA H, KAWAI Y . Effect of LiCoO2/Li7La3Zr2O12 ratio on the structure and electrochemical properties of nanocomposite cathodes for all-solid-state lithium batteries. J. Mater. Chem. A, 2017,5:18816-18822.
DOI URL |
| [60] | GAI J, ZHAO E, MA F , et al. Improving the Li-ion conductivity and air stability of cubic Li7La3Zr2O12 by the co-doping of Nb, Y on the Zr site.[J]. Eur. Ceram. Soc., 2017,38(4):1673-1678. |
| [61] |
OHTA S, KOBAYASHI T, SEKI J , et al. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte.[J]. Power Sources, 2012,202(1):332-335.
DOI URL |
| [62] |
KOTOBUKI M, MUNAKATA H, KANAMURA K , et al. Compatibility of Li7La3Zr2O12 solid electrolyte to all-solid-state battery using Li metal anode.[J]. Electrochem. Soc., 2010,157(10):A1076-A1079.
DOI URL |
| [63] |
LIU B, FU K, GONG Y , et al. Rapid thermal annealing of cathode- garnet interface toward high temperature solid state batteries. Nano Lett., 2017,17(8):4917-4923.
DOI URL |
| [64] |
OHTA S, KOMAGATA S, SEKI J , et al. All-solid-state lithium ion battery using garnet-type oxide and Li3BO3, solid electrolytes fabricated by screen-printing.[J]. Power Sources, 2013,238(28):53-56.
DOI URL |
| [65] |
KATO T, HAMANAKA T, YAMAMOTO K , et al. In-situ Li7La3Zr2O12/LiCoO2 interface modification for advanced all-solid- state battery.[J]. Power Sources, 2014,260(16):292-298.
DOI URL |
| [66] |
OHTA N, TAKADA K, ZHANG L , et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater., 2006,18(17):2226-2229.
DOI URL |
| [67] |
KITAURA H, HAYASHI A, TADANAGA K , et al. Improvement of electrochemical performance of all-solid-state lithium secondary batteries by surface modification of LiMn2O4, positive electrode. Solid State Ionics, 2011,192(1):304-307.
DOI URL |
| [68] |
SAKUDA A, KITAURA H, HAYASHI A , et al. Improvement of high-rate performance of all-solid-state lithium secondary batteries using LiCoO2 coated with Li2O-SiO2 glasses. Electrochem. Solid-State Lett., 2008,11(1):A1-A3.
DOI URL |
| [69] |
JIN Y, LI N, CHEN C H , et al. Electrochemical characterizations of commercial LiCoO2 powders with surface modified by Li3PO4 nanoparticles. Electrochem. Solid-State Lett., 2006,9(6):A273-A276.
DOI URL |
| [70] |
LIU B, GONG Y, FU K , et al. Garnet solid electrolyte protected Li-metal batteries. ACS Appl. Mater. Interfaces, 2017,9(22):18809-18815.
DOI URL |
| [71] |
ZHANG J, ZHAO N, ZHANG M , et al. Flexible and ion- conducting membrane electrolytes for solid-state lithium batteries: dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy, 2016,28:447-454.
DOI URL |
| [72] |
CHEN R J, ZHANG Y B, LIU T , et al. Addressing the interface issues in all-solid-state bulk-type lithium ion battery via an all composite approach. ACS Appl. Mater. Interfaces, 2017,9(11):9654-9661.
DOI URL |
| [73] |
ZHANG X, LIU T, ZHANG S , et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly (vinylidene fluoride) induces high ionic conductivity, mechanical strength and thermal stability of solid composite electrolytes. J. Am. Chem. Soc., 2017,139(39):13779-13785.
DOI URL |
| [74] |
ZHANG W, NIE J, LI F , et al. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy, 2018,45:413-419.
DOI URL |
| [75] |
YOSHIMA K, HARADA Y, TAKAMI N . Thin hybrid electrolyte based on garnet-type lithium-ion conductor Li7La3Zr2O12, for 12V-class bipolar batteries.[J]. Power Sources, 2016,302:283-290.
DOI URL |
| [76] |
ZHANG J, ZANG X, WEN H , et al. High-voltage and free- standing poly(propylene carbonate)/Li6.75La3Zr1.75Ta0.25O12 composite solid electrolyte for wide temperature range and flexible solid lithium ion battery. J. Mater. Chem. A, 2017,5(10):4940-4948.
DOI URL |
| [77] |
HUO H, SUN J, CHEN C , et al. Flexible interfaces between Si anodes and composite electrolytes consisting of poly(propylene carbonates) and garnets for solid-state batteries.[J]. Power Sources, 2018,383:150-156.
DOI URL |
| [78] |
HUO H, ZHAO N, SUN J , et al. Composite electrolytes of polyethylene oxides/garnets interfacially wetted by ionic liquid for room-temperature solid-state lithium battery.[J]. Power Sources, 2017,372:1-7.
DOI URL |
| [79] |
WANG Z, WANG Z, YANG L , et al. Boosting Interfacial Li +, transport with a MOF-based ionic conductor for solid-state batteries . Nano Energy, 2018,49:580-587.
DOI URL |
| [80] |
XU B, DUAN H, LIU H , et al. Stabilization of garnet/liquid electrolyte interface using superbase additives for hybrid Li batteries. ACS Appl. Mater. Interfaces, 2017,9(25):21077-21082.
DOI URL |
| [81] | JAN V D B, AFYON S, RUPP J L M . Interface-engineered all-solid-state Li-ion batteries based on garnet-type fast Li + conductors. Adv. Energy Mater., 2016, 6(19): 1600736-1-11. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | ZHOU Fan, TIAN Zhilin, LI Bin. Research Progress on Carbide Ultra-high Temperature Ceramic Anti-ablation Coatings for Thermal Protection System [J]. Journal of Inorganic Materials, 2025, 40(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||