
Journal of Inorganic Materials ›› 2021, Vol. 36 ›› Issue (7): 685-694.DOI: 10.15541/jim20200440
• REVIEW • Previous Articles Next Articles
XIAO Peng1( ), ZHU Yulin2, WANG Song1(
), ZHU Yulin2, WANG Song1( ), YU Yiping1, LI Hao1
), YU Yiping1, LI Hao1
Received:2020-08-10
Revised:2020-10-10
Published:2021-07-20
Online:2020-10-30
Contact:
WANG Song, professor. E-mail:wangs_0731@163.com
About author:XIAO Peng(1991-)male, PhD candidate. E-mail:xspi3@126.com
CLC Number:
XIAO Peng, ZHU Yulin, WANG Song, YU Yiping, LI Hao. Research Progress on the Preparation and Characterization of Ultra Refractory TaxHf1-xC Solid Solution Ceramics[J]. Journal of Inorganic Materials, 2021, 36(7): 685-694.
| Solid solution between metal carbides | Carbonization reaction of metals | Carbothermal reduction of metal oxides | |
|---|---|---|---|
| Advantages | Easy to operate; products have high purity and could achieve densification simultaneously | High temperature is not necessary; the whole process lasts only several seconds, not time consuming | Easy to operate; raw materials are cheap; have potential to synthesize single-phase products with fine grain size at relatively low temperature |
| Disadvantages | Needs high temperature and long time; products are not single-phase solid solution with elements uneven distribution | The reaction process are unable to control; products are usually not pure | The phase and microstructure of products are closely related to the distribution and binding state of oxides and carbon |
Table 1 Advantages and disadvantages of different techniques for preparation of TaxHf1-xC powder
| Solid solution between metal carbides | Carbonization reaction of metals | Carbothermal reduction of metal oxides | |
|---|---|---|---|
| Advantages | Easy to operate; products have high purity and could achieve densification simultaneously | High temperature is not necessary; the whole process lasts only several seconds, not time consuming | Easy to operate; raw materials are cheap; have potential to synthesize single-phase products with fine grain size at relatively low temperature |
| Disadvantages | Needs high temperature and long time; products are not single-phase solid solution with elements uneven distribution | The reaction process are unable to control; products are usually not pure | The phase and microstructure of products are closely related to the distribution and binding state of oxides and carbon |
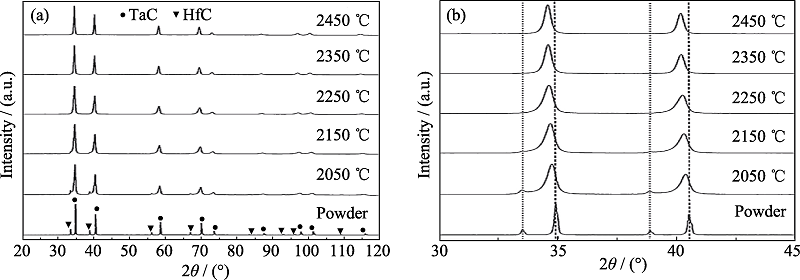
Fig. 1 (a) XRD patterns of Ta0.8Hf0.2C solid solution ceramic before and after SPS at different temperatures and (b) more detailed diffraction patterns between 30° and 45°[32]
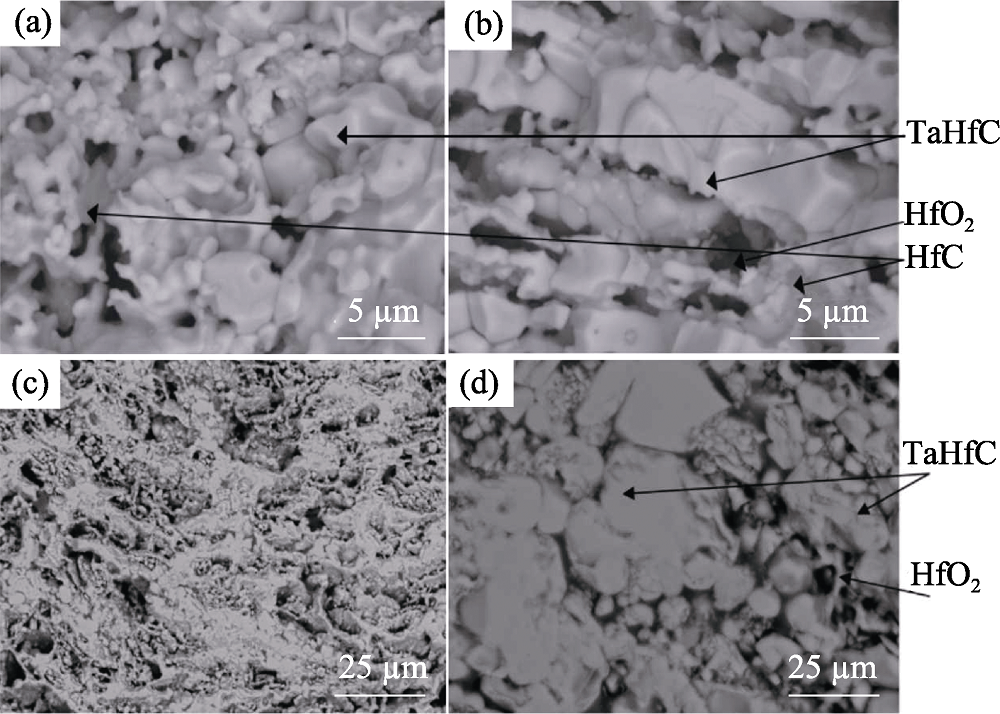
Fig. 2 Morphology of Ta0.8Hf0.2C solid solution ceramics synthesised by different SHS methods[43] (a,b) Mixed Ta, Hf and carbon black simultaneously; (c,d) Mixed Hf and carbon black first
| Raw powder | Sintering method | Relative density/% | Hardness/GPa | Elastic modulus/GPa | KIC/(MPa·m1/2) | Ref. | |
|---|---|---|---|---|---|---|---|
| Ta0.9Hf0.1C-12vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 100.0 | (15.0±0.2) | — | (3.2±0.3) | [26] |
| Ta0.9Hf0.1C-12vol% TaSi2 | TaC, HfC, TaSi2 | SPS | 100.0 | (15.9±0.3) | — | (3.3±0.2) | [26] |
| Ta0.87Hf0.13C | TaC, HfC | HIP | >98.0 | 24.1 | 575.4 | — | [3] |
| Ta0.8Hf0.2C | Ta0.8Hf0.2C | HP | 99.6 | (30.3±1.6) | (462.5±3.1) | (2.2±0.4) | [30] |
| Ta0.8Hf0.2C | TaxHf1-xC | HP | 94.4 | 27.2 | 491.7 | 3.0 | [21] |
| Ta0.8Hf0.2C | TaC, HfC | SPS | 97.8 | (16.7±0.9) | (443.2±23.7) | (4.6±1.1) | [40] |
| Ta0.8Hf0.2C | TaC, HfC | SPS | (97.7±0.1) | (19.3±1.3) | (459.0±5.8) | (2.9±0.9) | [32] |
| Ta0.8Hf0.2C-10vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 99.8 | (18.5±0.5) | (482.0±2.0) | (4.2±0.2) | [29] |
| Ta0.8Hf0.2C-12vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 100.0 | (15.2±0.5) | — | (3.9±0.2) | [26] |
| Ta0.8Hf0.2C-12vol% TaSi2 | TaC, HfC, TaSi2 | SPS | 100.0 | (17.7±0.4) | — | (3.2±0.1) | [26] |
| Ta0.75Hf0.25C | TaC, HfC | HIP | >98.0 | 28.6 | 567.7 | — | [3] |
| Ta0.7Hf0.3C-12vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 97.8 | (15.9±0.6) | — | (3.9±0.1) | [26] |
| Ta0.7Hf0.3C-12vol% TaSi2 | TaC, HfC, TaSi2 | SPS | 98.9 | (18.2±0.7) | — | (2.8±0.1) | [26] |
| Ta0.67Hf0.33C | Ta0.67Hf0.33C | HP | 95.3 | 29.7 | 483.0 | 2.5 | [21] |
| Ta0.5Hf0.5C | Ta0.5Hf0.5C | HP | 99.2 | (36.7±1.2) | (559.3±6.5) | (2.9±0.4) | [30] |
| Ta0.5Hf0.5C | Ta0.5Hf0.5C | HP | 97.9 | 37.9 | 591.0 | 2.5 | [21] |
| Ta0.5Hf0.5C | TaC, HfC | SPS | 98.2 | (17.1±1.1) | (523.8±7.0) | (6.0±0.7) | [40] |
| Ta0.5Hf0.5C | TaC, HfC | SPS | (95.7±0.3) | (22.1±1.8) | (549.0±11.2) | (2.9±0.7) | [32] |
| Ta0.5Hf0.5C | TaC, HfC | HIP | >97.0 | 23.5 | 469.9 | — | [3] |
| Ta0.3Hf0.7C | HfO2, Ta2O5, graphite | SPS | 98.7 | (20.0±0.9) | — | (5.2±0.2) | [48] |
| Ta0.25Hf0.75C | Ta0.25Hf0.75C | HP | 96.5 | (29.9±2.2) | (436.4±13.8) | (2.2±0.2) | [30] |
| Ta0.25Hf0.75C | TaC, HfC | HIP | >98.0 | 29.1 | 593.5 | — | [3] |
| Ta0.2Hf0.8C | Ta0.2Hf0.8C | HP | 95.9 | (35.1±1.1) | (554.7±8.8) | (2.3±0.5) | [21] |
| Ta0.2Hf0.8C | TaC, HfC | SPS | (87.0±0.2) | (16.7±3.0) | (438.0±17.8) | (3.4±0.6) | [32] |
| Ta0.2Hf0.8C | TaC, HfC | SPS | 98.8 | (19.1±0.3) | (577.3±6.0) | (5.5±0.6) | [40] |
| Ta0.2Hf0.8C | HfO2, Ta2O5, graphite | SPS | 100.0 | (19.7±0.7) | — | 5.1 | [48] |
| Ta0.17Hf0.83C | TaC, HfC | HIP | >98.0 | 26.6 | 534.2 | — | [3] |
| Ta0.1Hf0.9C | HfO2, Ta2O5, graphite | SPS | 99.3 | (19.7±0.8) | — | — | [48] |
Table 2 Mechanical properties of TaxHf1-xC solid solution ceramics at room temperature
| Raw powder | Sintering method | Relative density/% | Hardness/GPa | Elastic modulus/GPa | KIC/(MPa·m1/2) | Ref. | |
|---|---|---|---|---|---|---|---|
| Ta0.9Hf0.1C-12vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 100.0 | (15.0±0.2) | — | (3.2±0.3) | [26] |
| Ta0.9Hf0.1C-12vol% TaSi2 | TaC, HfC, TaSi2 | SPS | 100.0 | (15.9±0.3) | — | (3.3±0.2) | [26] |
| Ta0.87Hf0.13C | TaC, HfC | HIP | >98.0 | 24.1 | 575.4 | — | [3] |
| Ta0.8Hf0.2C | Ta0.8Hf0.2C | HP | 99.6 | (30.3±1.6) | (462.5±3.1) | (2.2±0.4) | [30] |
| Ta0.8Hf0.2C | TaxHf1-xC | HP | 94.4 | 27.2 | 491.7 | 3.0 | [21] |
| Ta0.8Hf0.2C | TaC, HfC | SPS | 97.8 | (16.7±0.9) | (443.2±23.7) | (4.6±1.1) | [40] |
| Ta0.8Hf0.2C | TaC, HfC | SPS | (97.7±0.1) | (19.3±1.3) | (459.0±5.8) | (2.9±0.9) | [32] |
| Ta0.8Hf0.2C-10vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 99.8 | (18.5±0.5) | (482.0±2.0) | (4.2±0.2) | [29] |
| Ta0.8Hf0.2C-12vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 100.0 | (15.2±0.5) | — | (3.9±0.2) | [26] |
| Ta0.8Hf0.2C-12vol% TaSi2 | TaC, HfC, TaSi2 | SPS | 100.0 | (17.7±0.4) | — | (3.2±0.1) | [26] |
| Ta0.75Hf0.25C | TaC, HfC | HIP | >98.0 | 28.6 | 567.7 | — | [3] |
| Ta0.7Hf0.3C-12vol% MoSi2 | TaC, HfC, MoSi2 | SPS | 97.8 | (15.9±0.6) | — | (3.9±0.1) | [26] |
| Ta0.7Hf0.3C-12vol% TaSi2 | TaC, HfC, TaSi2 | SPS | 98.9 | (18.2±0.7) | — | (2.8±0.1) | [26] |
| Ta0.67Hf0.33C | Ta0.67Hf0.33C | HP | 95.3 | 29.7 | 483.0 | 2.5 | [21] |
| Ta0.5Hf0.5C | Ta0.5Hf0.5C | HP | 99.2 | (36.7±1.2) | (559.3±6.5) | (2.9±0.4) | [30] |
| Ta0.5Hf0.5C | Ta0.5Hf0.5C | HP | 97.9 | 37.9 | 591.0 | 2.5 | [21] |
| Ta0.5Hf0.5C | TaC, HfC | SPS | 98.2 | (17.1±1.1) | (523.8±7.0) | (6.0±0.7) | [40] |
| Ta0.5Hf0.5C | TaC, HfC | SPS | (95.7±0.3) | (22.1±1.8) | (549.0±11.2) | (2.9±0.7) | [32] |
| Ta0.5Hf0.5C | TaC, HfC | HIP | >97.0 | 23.5 | 469.9 | — | [3] |
| Ta0.3Hf0.7C | HfO2, Ta2O5, graphite | SPS | 98.7 | (20.0±0.9) | — | (5.2±0.2) | [48] |
| Ta0.25Hf0.75C | Ta0.25Hf0.75C | HP | 96.5 | (29.9±2.2) | (436.4±13.8) | (2.2±0.2) | [30] |
| Ta0.25Hf0.75C | TaC, HfC | HIP | >98.0 | 29.1 | 593.5 | — | [3] |
| Ta0.2Hf0.8C | Ta0.2Hf0.8C | HP | 95.9 | (35.1±1.1) | (554.7±8.8) | (2.3±0.5) | [21] |
| Ta0.2Hf0.8C | TaC, HfC | SPS | (87.0±0.2) | (16.7±3.0) | (438.0±17.8) | (3.4±0.6) | [32] |
| Ta0.2Hf0.8C | TaC, HfC | SPS | 98.8 | (19.1±0.3) | (577.3±6.0) | (5.5±0.6) | [40] |
| Ta0.2Hf0.8C | HfO2, Ta2O5, graphite | SPS | 100.0 | (19.7±0.7) | — | 5.1 | [48] |
| Ta0.17Hf0.83C | TaC, HfC | HIP | >98.0 | 26.6 | 534.2 | — | [3] |
| Ta0.1Hf0.9C | HfO2, Ta2O5, graphite | SPS | 99.3 | (19.7±0.8) | — | — | [48] |
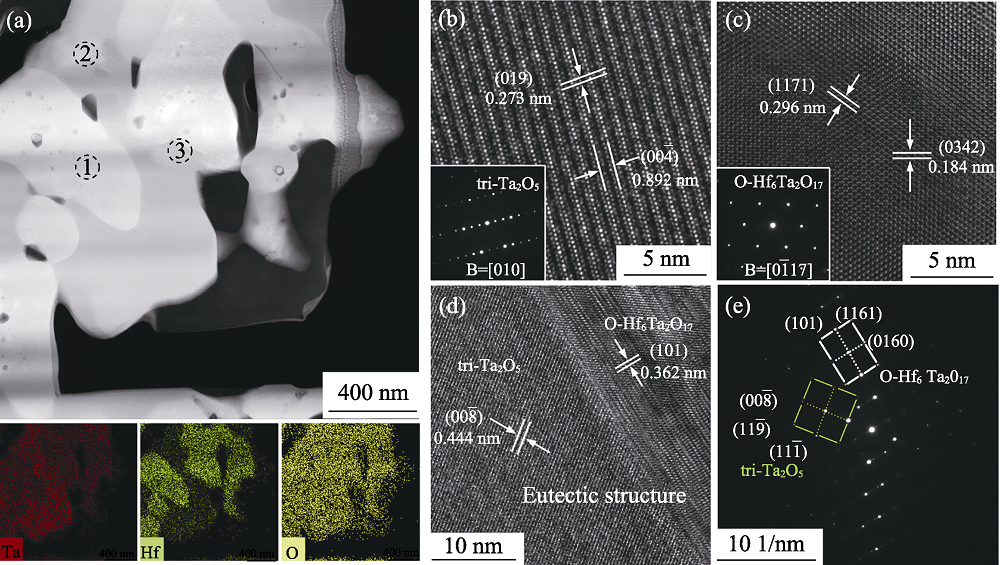
Fig. 6 Morphologies of Hf6Ta2O17-Ta2O5 eutectic structure[6] (a) STEM imagies and associated EDS element mapping; (b) HRTEM image and corresponding SAED pattern of area 2; (c) HRTEM image and corresponding SAED pattern of area 1; (d) HRTEM image of area 3; (e) SAED pattern of area 3
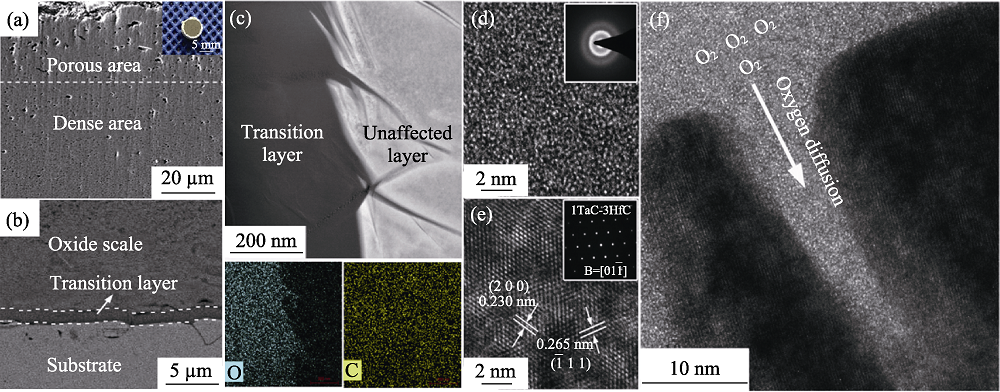
Fig. 7 Morphologies of Ta0.25Hf0.75C ceramic after oxidation[6] (a,b) SEM image of oxide scale; (c) STEM image of transition layer; (d) STEM and SAED results for transition layer; (e) STEM and SAED results for unaffected layer; (f) STEM result for border area of transition layer and unaffected layer
| [1] | BARRAZA O C. Process development and characterisation of (Ta, Hf)C ultra-high temperature ceramics. London: Imperial College London, PhD Thesis, 2015. |
| [2] |
SAVVATIMSKIY A I, ONUFRIEV S V, MUBOYADZHYAN S A. Thermophysical properties of the most refractory carbide Ta0.8Hf0.2C under high temperatures (2000-5000 K). Journal of the European Ceramic Society, 2019,39:907-914.
DOI URL |
| [3] |
SMITH C J, YU X X, GUO Q Y, et al. Phase, hardness, and deformation slip behavior in mixed HfxTa1-xC. Acta Materialia, 2018,145:142-153.
DOI URL |
| [4] |
IVASHCHENKO V I, TURCHI P E A, MEDUKH N R, et al. A first-principles study of the stability and mechanical properties of ternary transition metal carbide alloys. Journal of Applied Physics, 2019,125:235101.
DOI URL |
| [5] |
KIM J, KWON H, KIM B, et al. Finite temperature thermal expansion and elastic properties of (Hf1-xTax)C ultrahigh temperature ceramics. Ceramics International, 2019,45(8):10805-10809.
DOI URL |
| [6] |
ZHANG J, WANG S, LI W, et al. Understanding the oxidation behavior of Ta-Hf-C ternary ceramics at high temperature. Corrosion Science, 2020,164:108348.
DOI URL |
| [7] |
ZHANG C, BOESL B, AGARWAL A. Oxidation resistance of tantalum carbide-hafnium carbide solid solutions under the extreme conditions of a plasma jet. Ceramics International, 2017,43(17):14798-14806.
DOI URL |
| [8] |
PATTERSON M C L, HE S, FEHRENBACHER L L, et al. Advanced HfC-TaC oxidation resistant composite rocket thruster. Materials and Manufacturing Processes, 1996,11(3):367-379.
DOI URL |
| [9] | PATTERSON M C L, FULCHER M, HILMAS G, et al. Advanced tactical and boost nozzle materials. 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, 2005: 4497-4509. |
| [10] | DEADMORE D L. Vaporization of tantalum carbide-hafnium carbide solid solutions at 2500 to 3000 K, NASA TECHNICAL NOTE, TN D-2512, Washington, D. C, 1964: 1-17. |
| [11] |
DEADMORE D L. Normal spectral emittance (0.65) of TaC-HfC solid solutions and tungsten above 1600 ℃. Journal of the American Ceramic Society, 1964,47(12):649-650.
DOI URL |
| [12] |
DEADMORE D L. Vaporization of tantalum carbide-hafnium carbide solid solutions. Journal of the American Ceramic Society, 1965,48(7):357-359.
DOI URL |
| [13] | RUDY E. Ternary phase equilibria in transition metal-boron-carbon- silicon systems. Part II. Ternary systems. Vol. I. Ta-Hf-C system. Air Force Materials Laboratory Technical Report, AD-470827/7/XAB, United States, 1965. |
| [14] | RUDY E. Ternary phase equilibra in transition metal-boron-carbon- silicon systems. Part V. Compendium of phase diagram data. Air Force Materials Laboratory Technical Report, AFML-TR-65-2, United States, 1969. |
| [15] |
ANDRIEVSKII R A, STRELNIKOVA N S, POLTORATSKII N I, et al. Melting point in systems ZrC-HfC, TaC-ZrC, TaC-HfC. Soviet Powder Metallurgy and Metal Ceramics, 1967,6(1):65-67.
DOI URL |
| [16] |
BARANTSEVA I G, PADERNO V N, PADERNO Y B. Some physical properties of alloys of the systems ZrC-NbC and TaC-HfC. Soviet Powder Metallurgy and Metal Ceramics, 1967,6:139-141.
DOI URL |
| [17] | BARANTSEVA I G, PADERNO V N. Thermal expansion of solid solutions in the systems ZrC-NbC and HfC-TaC. Refractory Carbides. New York: Springer, 1974: 283-285. |
| [18] |
KIM J, KIM M, ROH K, et al. Bond characteristics, mechanical properties, and high-temperature thermal conductivity of (Hf1-xTax)C composites. Journal of the American Ceramic Society, 2019,102(10):6298-6308.
DOI URL |
| [19] |
PAN Y F, ZHOU P, PENG Y B, et al. A thermodynamic description of the C-Hf-Ta system over the whole composition and temperature ranges. CALPHAD: Computer Coupling of Phase Diagrams and Thermochemistry, 2016,53:1-9.
DOI URL |
| [20] |
JIANG J M, WANG S, LI W. Preparation and characterization of ultrahigh-temperature ternary ceramics Ta4HfC5. Journal of the American Ceramic Society, 2016,99(10):3198-3201.
DOI URL |
| [21] | 蒋进明. Ta-Hf(Zr)-C三元陶瓷的制备及性能研究. 长沙: 国防科技大学博士学位论文, 2017. |
| [22] |
FOROUGHI P, ZHANG CHENG, AGARWAL A, et al. Controlling phase separation of TaxHf1-xC solid solution nanopowders during carbothermal reduction synthesis. Journal of the American Ceramic Society, 2017,100(11):5056-5065.
DOI URL |
| [23] |
SIMONENKO E P, IGNATOV N A, SIMONENKO N P, et al. Synthesis of highly dispersed super-refractory tantalum-zirconium carbide Ta4ZrC5 and tantalum-hafnium carbide Ta4HfC5 via Sol- Gel technology. Russian Journal of Inorganic Chemistry, 2011,56(11):1681-1687.
DOI URL |
| [24] |
ZHANG Z, FU S, AVERSANO F, et al. Arc melting: a novel method to prepare homogeneous solid solutions of transition metal carbides (Zr, Ta, Hf). Ceramics International, 2019,45:9316-9319.
DOI URL |
| [25] |
GABALLA O, COOK B A, RUSSELL A M. Reduced-temperature processing and consolidation of ultra-refractory Ta4HfC5. International Journal of Refractory Metals and Hard Materials, 2013,41:293-299.
DOI URL |
| [26] |
GHAFFARI S A, FAGHIHI-SANI M A, GOLESTANI-FARD F, et al. Spark plasma sintering of TaC-HfC UHTC via disilicides sintering aids. Journal of the European Ceramic Society, 2013,33:1479-1484.
DOI URL |
| [27] |
GHAFFARI S A, FAGHIHI-SANI M A, GOLESTANI-FARD F. Pressureless sintering of Ta0.8Hf0.2C UHTC in the presence of MoSi2. Ceramics International, 2013,39:1985-1989.
DOI URL |
| [28] |
ARIANPOUR F, REZAIE H, GOLESTANI-FARD F, et al. Processing and consolidation of TaC/HfC based composites using MoSi2 and carbon nanotubes. Journal of Nano Research, 2013,21:145-150.
DOI URL |
| [29] |
ARIANPOUR F, GOLESTANI-FARD F, REZAIE H, et al. Processing, phase evaluation and mechanical properties of MoSi2 doped 4TaC-HfC based UHTCs consolidated by spark plasma sintering. International Journal of Refractory Metals and Hard Materials, 2016,56:1-7.
DOI URL |
| [30] |
ZHANG J, WANG S, LI W. Consolidation and characterization of highly dense single phase Ta-Hf-C solid solution ceramics. Journal of the American Ceramic Society, 2019,102(1):58-62.
DOI URL |
| [31] | BARRAZA O C, MANARA D, BOBORIDIS K. Investigating the highest melting temperature materials: a laser melting study of the TaC-HfC system. Scientific Reports, 2016,6:37962. |
| [32] |
BARRAZA O C, GRASSO S, NASIRI N A, et al. Sintering behaviour, solid solution formation and characterisation of TaC, HfC and TaC-HfC fabricated by spark plasma sintering. Journal of the European Ceramic Society, 2016,36:1539-1548.
DOI URL |
| [33] | GUZMÁN P, APERADOR W, YATE L. Enhancement of the pitting corrosion resistance of AISI 316LVM steel with Ta-Hf-C/Au bilayers for biomedical applications. Journal of Nanomaterials, 2017,2017:1-10. |
| [34] |
GUZMÁN P, YATE L, SANDOVAL M, et al. Characterization of the micro-abrasive wear in coatings of TaC-HfC/Au for biomedical implants. Materials, 2017,10(8):842.
DOI URL |
| [35] |
WANG Y L, XIONG X, LI G D, et al. Preparation and ablation properties of Hf(Ta)C co-deposition coating for carbon/carbon composites. Corrosion Science, 2013,66:177-182.
DOI URL |
| [36] |
MONTEYNARD A D, LUO H, CHEHIMI M, et al. The structure, morphology, and mechanical properties of Ta-Hf-C coatings deposited by pulsed direct current reactive magnetron sputtering. Coatings, 2020,10(3):212.
DOI URL |
| [37] | VALENCIA D P, YATE L, APERADOR W, et al. High electrocatalytic response of ultra-refractory ternary alloys of Ta-Hf-C carbide toward hydrogen evolution reaction in acidic media. The Journal of Physical Chemistry C, 2018,122(44):25433-25440. |
| [38] |
YATE L, COY L E, APERADOR W. Robust tribo-mechanical and hot corrosion resistance of ultra-refractory Ta-Hf-C ternary alloy films. Scientific Reports, 2017,7:3080.
DOI URL |
| [39] | GABALLA O. Processing development of 4TaC-HfC and related carbides and borides for extreme environments. Ames: Iowa State University, PhD Thesis, 2012. |
| [40] |
ZHANG C, GUPTA A, SEAL S, et al. Solid solution synthesis of tantalum carbide-hafnium carbide by spark plasma sintering. Journal of the American Ceramic Society, 2017,100(5):1853-1862.
DOI URL |
| [41] |
GHAFFARI S A, FAGHIHI-SANI M A, GOLESTANI-FARD F, et al. Diffusion and solid solution formation between the binary carbides of TaC, HfC and ZrC. International Journal of Refractory Metals and Hard Materials, 2013,41:180-184.
DOI URL |
| [42] |
CASTLE E, CSANADI T, GRASSO S, et al. Processing and properties of high-entropy ultra-high temperature carbides. Scientific Reports, 2018,8:8609.
DOI URL |
| [43] |
KURBATKINA V V, PATSERA E I, LEVASHOV E A, et al. Self- propagating high-temperature synthesis of single-phase binary tantalum-hafnium carbide (Ta, Hf)C and its consolidation by hot pressing and spark plasma sintering. Ceramics International, 2018,44(4):4320-4329.
DOI URL |
| [44] | KURBATKINA V V, PATSERA E I, LEVASHOV E A, et al. SHS processing and consolidation of Ta-Ti-C, Ta-Zr-C, and Ta-Hf-C carbides for ultra-high-temperatures application. Advanced Engineering Materials, 2018,20(8):1701075. |
| [45] |
PATSERA E I, KURBATKINA V V, LEVASHOV E A, et al. Research into the possibility of producing single-phase tantalum- hafnium carbide by SHS. Russian Journal of Non-Ferrous Metals, 2018,59(5):576-582.
DOI URL |
| [46] |
NAZAROVA S Z, KURMAEV E Z, MEDVEDEVA N I. Physical properties and electronic structure of TaC-HfC solid solutions. Russian Journal of Inorganic Chemistry, 2007,52(2):233-237.
DOI URL |
| [47] |
FENG L, KIM J, LEE S, et al. Synthesis of a fine (Ta0.8, Hf0.2)C powder from carbide or oxide powder mixtures. Journal of the American Ceramic Society, 2016,99(4):1129-1132.
DOI URL |
| [48] |
HA D, KIM J, HAN J, et al. Synthesis and properties of (Hf1-xTax)C solid solution carbides. Ceramics International, 2018,44(16):19247-19253.
DOI URL |
| [49] |
LU Y, SUN Y N, ZHANG T Z, et al. Polymer-derived Ta4HfC5 nanoscale ultrahigh-temperature ceramics: synthesis, microstructure and properties. Journal of the European Ceramic Society, 2019,39:205-211.
DOI URL |
| [50] |
SUN Y N, YANG C M, LU Y, et al. Transformation of metallic polymer precursor into nanosized HfTaC2 ceramics. Ceramics International, 2020,46:6022-6028.
DOI URL |
| [51] |
REZAEI F, KAKROUDI M G, SHAHEDIFAR V, et al. Consolidation and mechanical properties of hot pressed TaC-HfC-VC composites. Ceramics International, 2017,43(17):15537-15543.
DOI URL |
| [52] |
ZHANG B H, YIN J, CHEN J, et al. Pressureless densification, microstructure tailoring and properties of Ta0.8Hf0.2C-based composites. Journal of the European Ceramic Society, 2018,38:1227-1236.
DOI URL |
| [53] |
ZHANG B H, YIN J, ZHENG J Q, et al. High temperature ablation behavior of pressureless sintered Ta0.8Hf0.2C-based ultra-high temperature ceramics. Journal of the European Ceramic Society, 2020,40:1784-1789.
DOI URL |
| [54] |
ZHANG B H, YIN J, HUANG Y H, et al. Harmonized toughening and strengthening in pressurelessly reactive-sintered Ta0.8Hf0.2C-SiC composite. Journal of the European Ceramic Society, 2018,38:5610-5614.
DOI URL |
| [55] | HONG Q J, WALLE A V D. Prediction of the material with highest known melting point from ab initio molecular dynamics calculations. Physical Review B, 2015,92(2):020104. |
| [56] | ZHANG C, LOGANATHAN A, BOESL B. Thermal analysis of tantalum carbide-hafnium carbide solid solutions from room temperature to 1400 ℃. Coatings, 2017,7(8):111. |
| [57] | COURTRIGHT E L, PRATER J T, HOLCOMB G R, et al. Oxidation of hafnium carbide and hafnium carbide with additions of tantalum and praseodymium. Oxidation of Metals, 1991,36(5/6):423-437. |
| [58] | MCCORMACK S J, TSENG K P, WEBER R J K, et al. In-situ determination of the HfO2-Ta2O5-temperature phase diagram up to 3000 ℃. Journal of the American Ceramic Society, 2019,102(8):4848-4861. |
| [59] | MCCORMACK S J, WEBER R J, KRIVEN W M. In-situ investigation of Hf6Ta2O17 anisotropic thermal expansion and topotactic, peritectic transformation. Acta Materialia, 2018,161:127-137. |
| [60] | FENG G H, LI H J, YAO X Y, et al. Ablation resistance of TaC- modified HfC coating prepared by supersonic plasma spraying for SiC-coated carbon/carbon composites. Ceramics International, 2019,45(14):17936-17945. |
| [61] | FENG G H, LI H J, YANG L, et al. Investigation on the ablation performance and mechanism of HfC coating modified with TaC. Corrosion Science, 2020,170:108649. |
| [62] | REN J C, ZHANG Y L, FU Y Q, et al. Effects of the second phase on the microstructure and ablation resistance of HfC coating on C/C composites. Surface & Coatings Technology, 2018,344:250-258. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | ZHOU Fan, TIAN Zhilin, LI Bin. Research Progress on Carbide Ultra-high Temperature Ceramic Anti-ablation Coatings for Thermal Protection System [J]. Journal of Inorganic Materials, 2025, 40(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||