
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (2): 145-157.DOI: 10.15541/jim20190108
Special Issue: 2020年能源材料论文精选(二):超级电容器; 【虚拟专辑】超级电容器(2020~2021)
• REVIEW • Next Articles
MA Li-Na1,SHI Chuan2,ZHAO Ning2,BI Zhi-Jie2,GUO Xiang-Xin2( ),HUANG Yu-Dong3
),HUANG Yu-Dong3
Received:2019-03-12
Revised:2019-05-17
Published:2020-02-20
Online:2019-06-17
Supported by:CLC Number:
MA Li-Na,SHI Chuan,ZHAO Ning,BI Zhi-Jie,GUO Xiang-Xin,HUANG Yu-Dong. Bacterial Cellulose Based Nano-biomaterials for Energy Storage Applications[J]. Journal of Inorganic Materials, 2020, 35(2): 145-157.
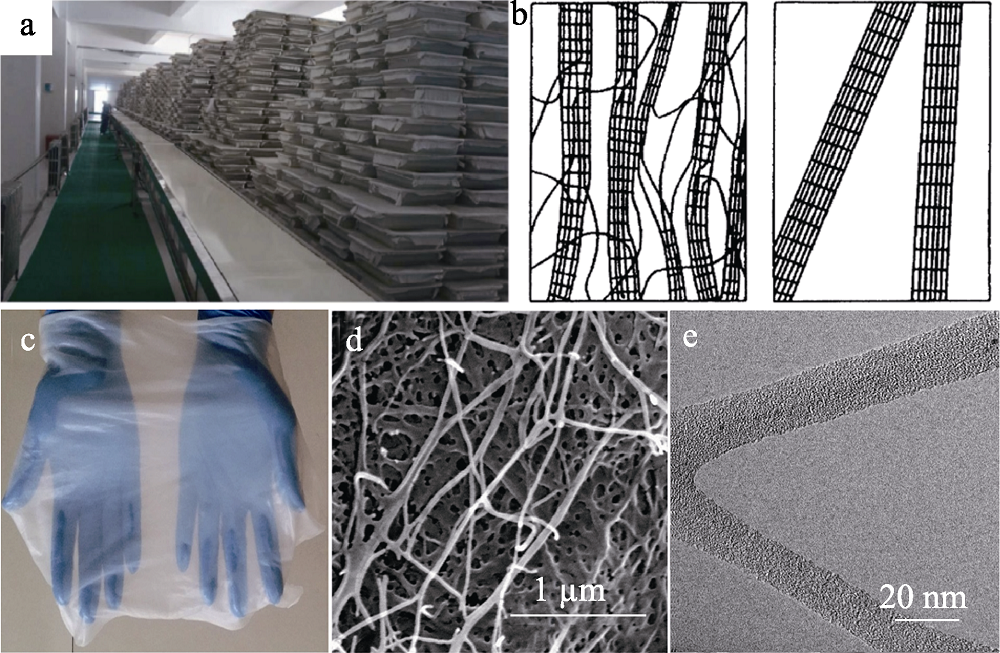
Fig. 1 (a) Photograph of the production line for BC[3]; (b) Schematic model of the plant cellulose fibrils (left) and the BC microfibers (right)[5]; (c) Photograph of BC slice; (d) SEM and (e)TEM images of BC[4]
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CO2 activated CNF | Active material | -0.2-0.2 (vs. Ag/AgCl) | 42 (1 mV∙s-1) (659 mF∙cm-2) | 70% (10 mV∙s-1) | — | — | — | [14] |
| CNF | Active material | -1-0 (vs. Ag/AgCl) | 108 (2 A∙g-1) | — | — | — | — | [15] |
| PCN/CNF | Active material | -1-0 (vs. Hg/HgO) | 261 (2 mV∙s-1) | 76.6 (500 mV∙s-1) | 97.6% (10000) | — | — | [18] |
| PCN/CNF// PCN/CNF | Active material | 0-1.8 | — | — | 94.8% (10000) | 20.4 | 17.8 | [18] |
| N,P-CNF// N,P-CNF | Active material | 0-1 | 204.9 (1 A∙g-1) | — | 100% (4000) | 7.76 | 26.1 | [2] |
| N-S-CNF-700 | Active material | 0-1 (vs. Ag/AgCl) | 171.2 (0.5 A∙g-1) | 105.2 (10 A∙g-1) | >90% (1000) | — | — | [20] |
| KOH activated N-CNF | Active material | 0.9-0.1 (vs. SHE) | 296 (2 mV∙s-1) | 75% (500 mV∙s-1) | 99% (10000) | — | — | [12] |
| N-CNF | Active material | -1-0 (vs. Ag/AgCl) | 120 (1 A∙g-1) | — | 98.2% (5000) | — | — | [13] |
| N,P-CNWs | Active material | -1-0 (vs. Hg/HgO) | 258 (1 A∙g-1) | 208 (10 A∙g-1) | 98% (30000) | — | — | [26] |
| N,P-CNWs// N,P-CNWs | Active material | 0-1 | 74 (0.5 A∙g-1) | — | 87% (6000) | 5.4 | 0.2 | [26] |
| CNF aerogels | Active material | -1-0 (vs. Ag/AgCl) | 194.7 (0.5 A∙g-1) | 108.7(10 A∙g-1) | 94% (5000) | — | — | [19] |
Table 1 BC-based carbon material electrodes for supercapacitor
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CO2 activated CNF | Active material | -0.2-0.2 (vs. Ag/AgCl) | 42 (1 mV∙s-1) (659 mF∙cm-2) | 70% (10 mV∙s-1) | — | — | — | [14] |
| CNF | Active material | -1-0 (vs. Ag/AgCl) | 108 (2 A∙g-1) | — | — | — | — | [15] |
| PCN/CNF | Active material | -1-0 (vs. Hg/HgO) | 261 (2 mV∙s-1) | 76.6 (500 mV∙s-1) | 97.6% (10000) | — | — | [18] |
| PCN/CNF// PCN/CNF | Active material | 0-1.8 | — | — | 94.8% (10000) | 20.4 | 17.8 | [18] |
| N,P-CNF// N,P-CNF | Active material | 0-1 | 204.9 (1 A∙g-1) | — | 100% (4000) | 7.76 | 26.1 | [2] |
| N-S-CNF-700 | Active material | 0-1 (vs. Ag/AgCl) | 171.2 (0.5 A∙g-1) | 105.2 (10 A∙g-1) | >90% (1000) | — | — | [20] |
| KOH activated N-CNF | Active material | 0.9-0.1 (vs. SHE) | 296 (2 mV∙s-1) | 75% (500 mV∙s-1) | 99% (10000) | — | — | [12] |
| N-CNF | Active material | -1-0 (vs. Ag/AgCl) | 120 (1 A∙g-1) | — | 98.2% (5000) | — | — | [13] |
| N,P-CNWs | Active material | -1-0 (vs. Hg/HgO) | 258 (1 A∙g-1) | 208 (10 A∙g-1) | 98% (30000) | — | — | [26] |
| N,P-CNWs// N,P-CNWs | Active material | 0-1 | 74 (0.5 A∙g-1) | — | 87% (6000) | 5.4 | 0.2 | [26] |
| CNF aerogels | Active material | -1-0 (vs. Ag/AgCl) | 194.7 (0.5 A∙g-1) | 108.7(10 A∙g-1) | 94% (5000) | — | — | [19] |
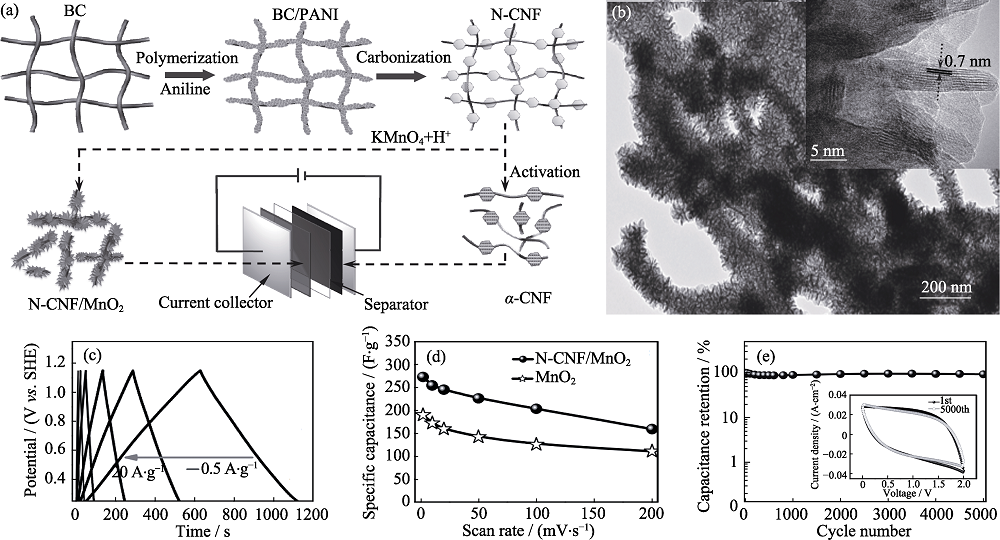
Fig. 2 (a) Schematic diagram for asymmetric supercapacitor device; (b) TEM images, (c) GCD curves, (d) specific capacitance and (e) cycle performance of N-CNF/MnO2[12]
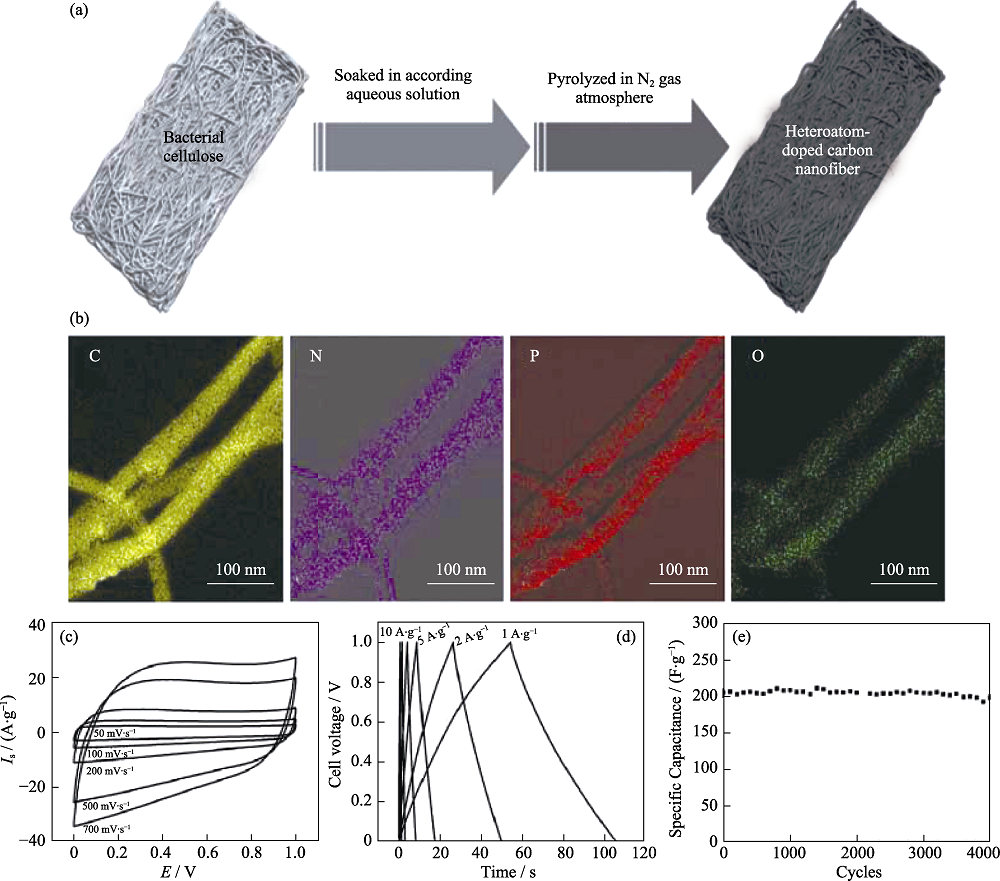
Fig. 3 (a) Fabrication process of heteroatom-doped CNF; (b) Elemental mapping images of C, N, P, and O for N, P-CNF; (c) CV curves, (d) GCD curves and (e) cycling stability test of N,P-CNF supercapacitor[2]
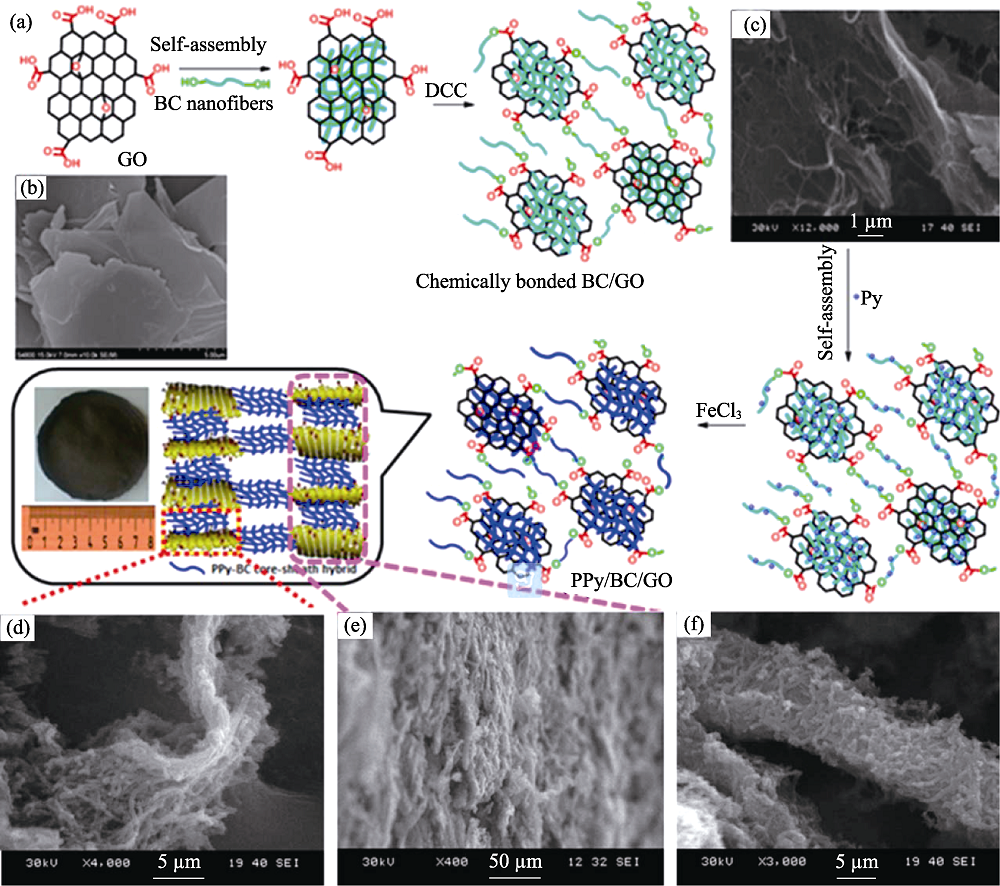
Fig. 4 (a) Synthesis scheme of PPY/BC/GO composites; SEM images of (b) pristine GO, (c) cross-linked BC/GO, (d) a single layer and (e) multilayers of PPY/BC/GO hybrid, and (f) PPY/BC core-sheath hybrid[38]
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CNF@MnO2 | Active material | 0-1 (vs. Ag/AgCl) | 254.64 (1 A∙g-1) | 77.53% (10 A∙g-1) | — | — | — | [24] |
| CNF@MnO2// N-CNF | Active material | 0-2 | — | — | 95.4% (2000) | 32.91 | 284.63 | [24] |
| Ni3S2/CNF | Active material | 0-0.6 (vs. Ag/AgCl) | 957 (1 A∙g-1) | 703 (8 A∙g-1) | 16.5% (1000) | — | — | [15] |
| Ni3S2/CNF//CNF | Active material | 0-1.7 | 56.6 (1 A∙g-1) | 35.4 (10 A∙g-1) | 97% (2500) | 25.8 | 0.425 | [15] |
| CNF/MnO2 | Active material | 0.15-1.15 (vs. SCE) | 273 (2 mV∙s-1) | 75% (100 mV∙s-1) | — | — | — | [12] |
| CNF//CNF/MnO2 | Active material | 0-2 | 113 (20 mV∙s-1) | 53% (10~200 mV∙s-1) | 92% (5000) | 63 | 8 | [12] |
| N-CNF@LDH | Active material | 0-0.5 (Ag/AgCl) | 1949.5 (1 A∙g-1) | 54.7 (10 A∙g-1) | 74.4% (5000) | — | — | [23] |
| N-CNF@LDH// N-CNF | Active material | 0-1.6 | 101.9 (1 A∙g-1) | 63.8 (10 A∙g-1) | 89.3% (2500) | 36.3 | 8 | [23] |
Table 2 BC-based composites electrodes for supercapacitor
| Material | Function of BC | Potential window/V | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density/ (Wh∙kg-1) | Highest power density/ (kW∙kg-1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CNF@MnO2 | Active material | 0-1 (vs. Ag/AgCl) | 254.64 (1 A∙g-1) | 77.53% (10 A∙g-1) | — | — | — | [24] |
| CNF@MnO2// N-CNF | Active material | 0-2 | — | — | 95.4% (2000) | 32.91 | 284.63 | [24] |
| Ni3S2/CNF | Active material | 0-0.6 (vs. Ag/AgCl) | 957 (1 A∙g-1) | 703 (8 A∙g-1) | 16.5% (1000) | — | — | [15] |
| Ni3S2/CNF//CNF | Active material | 0-1.7 | 56.6 (1 A∙g-1) | 35.4 (10 A∙g-1) | 97% (2500) | 25.8 | 0.425 | [15] |
| CNF/MnO2 | Active material | 0.15-1.15 (vs. SCE) | 273 (2 mV∙s-1) | 75% (100 mV∙s-1) | — | — | — | [12] |
| CNF//CNF/MnO2 | Active material | 0-2 | 113 (20 mV∙s-1) | 53% (10~200 mV∙s-1) | 92% (5000) | 63 | 8 | [12] |
| N-CNF@LDH | Active material | 0-0.5 (Ag/AgCl) | 1949.5 (1 A∙g-1) | 54.7 (10 A∙g-1) | 74.4% (5000) | — | — | [23] |
| N-CNF@LDH// N-CNF | Active material | 0-1.6 | 101.9 (1 A∙g-1) | 63.8 (10 A∙g-1) | 89.3% (2500) | 36.3 | 8 | [23] |
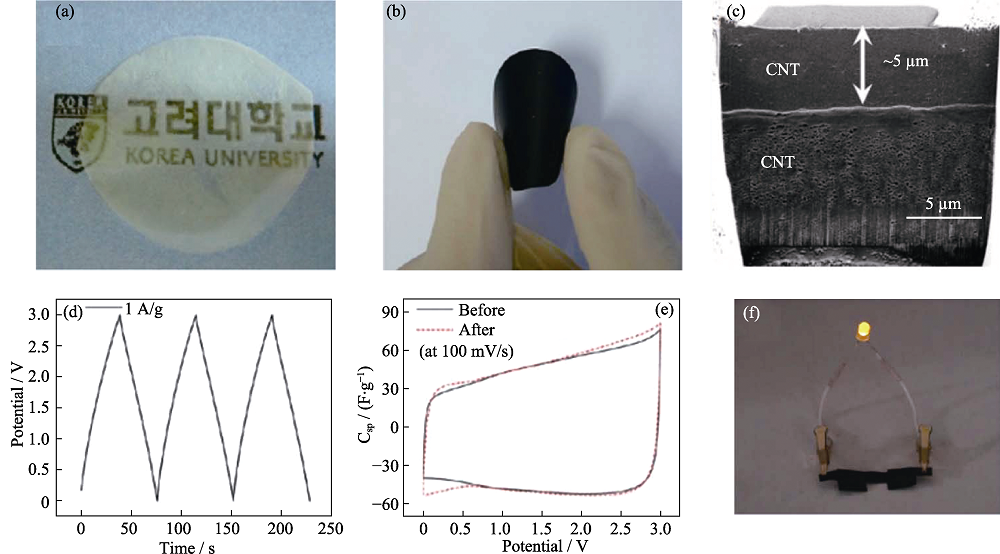
Fig. 5 Photographs of (a) BC paper and (b) flexible CNT/BC paper; (c) Cross-sectional image of CNT/BC paper; (d) GCD curve and (e) CV curves for CNT/BC/ion gel flexible supercapacitors; (f) Photograph of a LED turned on by the flexible supercapacitors[41]
| Material | Function of BC | Potential window/V | Capacitance/ (mF∙cm-2) | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density | Highest power density | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| N-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 2106 (1 mV∙s-1) | 263 | 76% (50 mV∙s-1) | 100% (2×104) | — | — | [45] |
| N-CNF/RGO/BC//N-CNF/RGO/BC | Active material & substrate | 0-1 | 810 (2 mV∙s-1) | — | 755 (50 mV∙s-1) | 99.6% (104) | 0.11 mWh∙cm-2 | 27 mW∙cm-2 | [46] |
| N,P-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 1900 (2 mV∙s-1) | 244.8 | 1554 (50 mV∙s-1) | 100% (2×104) | — | — | [16] |
| N,P-CNF/RGO/ BC//N,P-CNF/ RGO/BC | Active material & substrate | 0-1 | 690 (2 mV∙s-1) | — | 620 (40 mV∙s-1) | 99.6% (1×104) | 0.096 mWh∙cm-2 | 19.98 mW∙cm-2 | [16] |
| BC/GO electrode | Scaffold | -0.2-0.8 (vs. SCE) | — | 160 (0.4 A∙g-1) | 68 (2 A∙g-1) | 90.3% (2×103) | — | — | [44] |
| BC/CNT/ion gel supercapacitors | Substrate | 0-3 | 18.8 (100 mV∙s-1) | 46.9 | 42.0 (500 mV∙s-1) | 99.5% (5×104) | 15.5 Wh∙kg-1 | 1.5 kW∙kg-1 | [41] |
| a-CNF//BC gel// a-CNF supercapacitors | Active material & electrolyte & separator | 0-1 | 289 (0.1 mA∙cm-2) | — | 70% (10 mA∙cm-2) | 66.7% (100) | — | — | [60] |
Table 3 BC-based electrodes for flexible EDLCs
| Material | Function of BC | Potential window/V | Capacitance/ (mF∙cm-2) | Capacitance/ (F∙g-1) | Rate capability | Stability (cycle number) | Highest energy density | Highest power density | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| N-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 2106 (1 mV∙s-1) | 263 | 76% (50 mV∙s-1) | 100% (2×104) | — | — | [45] |
| N-CNF/RGO/BC//N-CNF/RGO/BC | Active material & substrate | 0-1 | 810 (2 mV∙s-1) | — | 755 (50 mV∙s-1) | 99.6% (104) | 0.11 mWh∙cm-2 | 27 mW∙cm-2 | [46] |
| N,P-CNF/RGO/BC | Active material & substrate | -0.8-0.2 (vs. Hg/HgO) | 1900 (2 mV∙s-1) | 244.8 | 1554 (50 mV∙s-1) | 100% (2×104) | — | — | [16] |
| N,P-CNF/RGO/ BC//N,P-CNF/ RGO/BC | Active material & substrate | 0-1 | 690 (2 mV∙s-1) | — | 620 (40 mV∙s-1) | 99.6% (1×104) | 0.096 mWh∙cm-2 | 19.98 mW∙cm-2 | [16] |
| BC/GO electrode | Scaffold | -0.2-0.8 (vs. SCE) | — | 160 (0.4 A∙g-1) | 68 (2 A∙g-1) | 90.3% (2×103) | — | — | [44] |
| BC/CNT/ion gel supercapacitors | Substrate | 0-3 | 18.8 (100 mV∙s-1) | 46.9 | 42.0 (500 mV∙s-1) | 99.5% (5×104) | 15.5 Wh∙kg-1 | 1.5 kW∙kg-1 | [41] |
| a-CNF//BC gel// a-CNF supercapacitors | Active material & electrolyte & separator | 0-1 | 289 (0.1 mA∙cm-2) | — | 70% (10 mA∙cm-2) | 66.7% (100) | — | — | [60] |
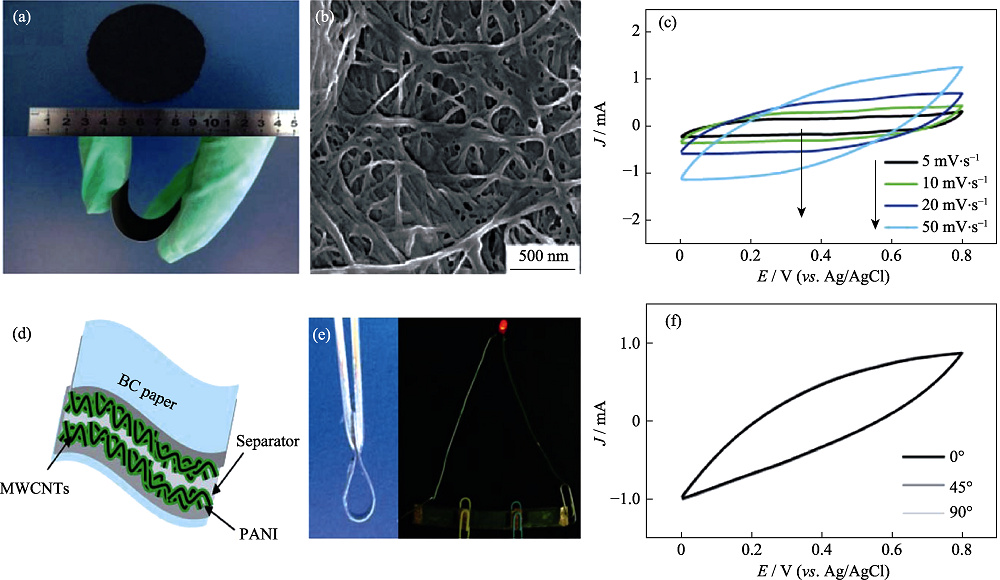
Fig. 6 (a) Photographs of CNT/BC paper and PANI/CNT/BC paper; (b) SEM image and (c) CV curves of PANI/CNT/BC electrode; (d) Schematic structure, (e) digital images and (f) CV curves for flexible supercapacitor[1]
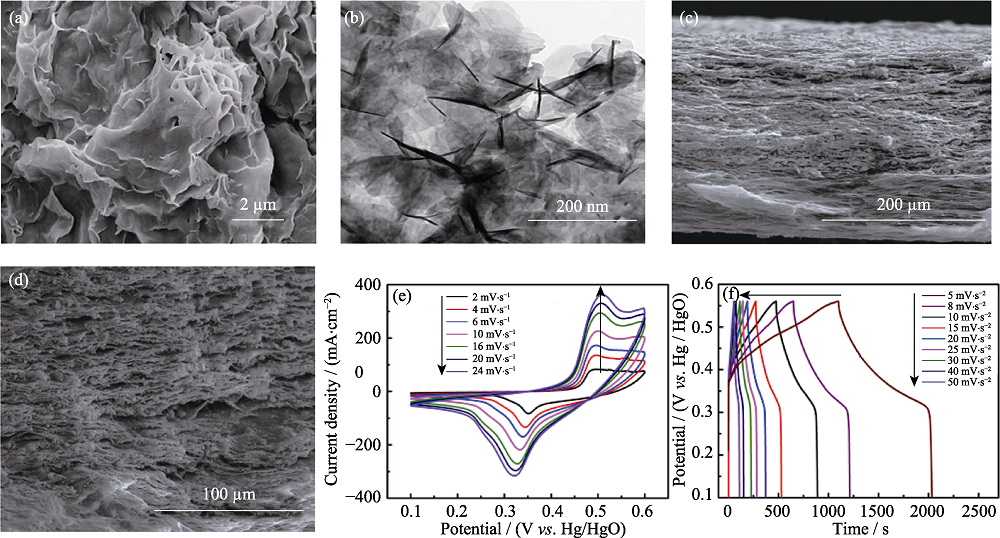
Fig. 7 (a) SEM and (b) TEM images of Ni(OH)2/RGO/BC; (c, d) Cross-sectional SEM micrographs of Ni(OH)2/RGO/BC; (e) CV and (f) GCD curves of Ni(OH)2/RGO/BC electrode[58]
| [1] | LI S H, HUANG D K, ZHANG B Y , et al. Flexible supercapacitors based on bacterial cellulose paper electrodes. Adv. Energy Mater., 2014,4(10):1301655. |
| [2] | CHEN L F, HUANG Z H, LIANG H W , et al. Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv. Funct. Mater., 2014,24(32):5104-5111. |
| [3] | WU Z Y, LIANG H W, CHEN L F , et al. Bacterial cellulose: a robust platform for design of three dimensional carbon-based functional nanomaterials. Acc. Chem. Res., 2016,49(1):96-105. |
| [4] | MA L N, LIU R, NIU H J , et al. Flexible and freestanding electrode based on polypyrrole/graphene/bacterial cellulose paper for supercapacitor. Compos. Sci. Technol., 2016,137(12):87-93. |
| [5] | IGUCHI M, YAMANAKA S, BUDHIOKO A . Bacterial cellulose— a masterpiece of nature’s arts. J. Mater. Sci., 2000,35(2):261-270. |
| [6] | TIAN X D, LI X, YANG T , et al. Recent advances on synthesis and supercapacitor application of binary metal oxide. J. Inorg. Mater., 2017,32(5):459-468. |
| [7] | ZENG Y F, XIN G X, BU L C K , et al. One-step preparation and electrochemical performance of 3D reduced graphene oxide/NiO as supercapacitor electrodes materials. J. Inorg. Mater., 2018,33(10):1070-1076. |
| [8] | QU L, PEI H M, KONG R M , et al. Novel turn-on fluorescent detection of alkaline phosphatase based on green synthesized carbon dots and MnO2 nanosheets. Talanta, 2017,165:136-142. |
| [9] | ZHAO J, GONG X B, ZHANG R M , et al. Enhanced biosensing platform constructed using urchin-like ZnO-Au@CdS microspheres based on the combination of photoelectrochemical and bioetching strategies. Sens. Actuators B: Chem., 2018,255:1753-1761. |
| [10] | YAO J J, JI P, NAN SHENG N , et al. Hierarchical core-sheath polypyrrole@carbon nanotube/bacterial cellulose macrofibers with high electrochemical performance for allsolid-state supercapacitors. Electrochim. Acta, 2018,283:1578-1588. |
| [11] | LI J, ZHANG G F, CHEN N , et al. Built structure of ordered vertically aligned codoped carbon nanowire arrays for supercapacitors. ACS Appl. Mater. Interfaces, 2017,9:24840-24845. |
| [12] | LONG C L, QI D P, WEI T , et al. Nitrogen-doped carbon networks for high energy density supercapacitors derived from polyaniline coated bacterial cellulose. Adv. Funct. Mater., 2014,24(25):3953-3961. |
| [13] | LEI W, HAN L L, XUAN C J , et al. Nitrogen-doped carbon nanofibers derived from polypyrrole coated bacterial cellulose as high-performance electrode materials for supercapacitors and Li-ion batteries. Electrochim. Acta, 2016,210:130-137. |
| [14] | LEE K Y, QIAN H, TAY F H , et al. Bacterial cellulose as source for activated nanosized carbon for electric double layer capacitors. J. Mater. Sci., 2013,48(1):367-376. |
| [15] | YU W D, LIN W R, SHAO X F , et al. High performance supercapacitor based on Ni3S2/carbon nanofibers and carbon nanofibers electrodes derived from bacterial cellulose. J. Power Sour., 2014,272:137-143. |
| [16] | LIU R, MA L N, MEI J , et al. Large areal mass, mechanically tough and freestanding electrode based on heteroatom-doped carbon nanofibers for flexible supercapacitors. Chem-Eur. J., 2017,23(11):2610-2618. |
| [17] | YUAN D, HUANG X, YAN J , et al. Porous carbon nanofibers derived from bacterial cellulose for sustainable energy storage. Science of Advanced Materials, 2013,5(11):1694-1700. |
| [18] | JIANG Y T, YAN J, WU X L , et al. Facile synthesis of carbon nanofibers-bridged porous carbon nanosheets for high-performance supercapacitors. J. Power Sour., 2016,307:190-198. |
| [19] | LAI F L, MIAO Y E, ZUO L Z , et al. Carbon aerogels derived from bacterial cellulose/polyimide composites as versatile adsorbents and supercapacitor electrodes. ChemNanoMat, 2016,2(3):212-219. |
| [20] | WU Z Y, LIANG H W, LI C , et al. Dyeing bacterial cellulose pellicles for energetic heteroatom doped carbon nanofiber aerogel. Nano Res., 2014,7(12):1861-1872. |
| [21] | LUO H L, DONG J J, ZHANG Y , et al. Constructing 3D bacterial cellulose/graphene/polyaniline nanocomposites by novel layer-by- layer in situ culture toward mechanically robust and highly flexible freestanding electrodes for supercapacitors. Chem. Eng. J., 2018,334:1148-1158. |
| [22] | WU H, ZHANG Y Z, YUAN W Y , et al. Highly flexible, foldable and stretchable Ni-Co layered double hydroxide/polyaniline/bacterial cellulose electrodes for high performance all-solid-state supercapacitors. J. Mater. Chem. A, 2018,6(34):16617-16626. |
| [23] | LAI F L, MIAO Y, ZUO L Z , et al. Biomass-derived nitrogen- doped carbon nanofiber network: a facile template for decoration of ultrathin nickel-cobalt layered double hydroxide nanosheets as high-performance asymmetric supercapacitor electrode. Small, 2016,12(24):3235-3244. |
| [24] | CHEN L F, HUANG Z H, LIANG H W , et al. Bacterial-cellulose- derived carbon nanofiber@MnO2 and nitrogen-doped carbon nanofiber electrode materials: an asymmetric supercapacitor with high energy and power density. Adv. Mater., 2013,25(34):4746-4752. |
| [25] | CHEN L F, HUANG Z H, LIANG H W , et al. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen- doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ. Sci., 2013,6(11):3331-3338. |
| [26] | HU Z X, LI S S, CHENG P P , et al. N,P-co-doped carbon nanowires prepared from bacterial cellulose for supercapacitor. J. Mater. Sci., 2016,51(5):2627-2633. |
| [27] | LI S M, YANG S Y, WANG Y S , et al. N-doped structures and surface functional groups of reduced graphene oxide and their effect on the electrochemical performance of supercapacitor with organic electrolyte. J. Power Sources, 2015,278:218-229. |
| [28] | ZHAO L, HU Y S, LI H , et al. Porous Li4Ti5O12 coated with N-doped carbon from ionic liquids for Li-Ion batteries. Advanced Materials, 2011,23(11):1385-1388. |
| [29] | HAO L, LUO B, LI X , et al. Terephthalonitrile-derived nitrogen- rich networks for high performance supercapacitors. Energy & Environmental Science, 2012,5(12):9747-9751. |
| [30] | LIU Y Q, YAN Y, LI K , et al. A high-areal-capacity lithium-sulfur cathode achieved by a boron-doped carbon-sulfur aerogel with consecutive core-shell structures. Chem. Commun., 2019,55(8):1084-1087. |
| [31] | DING L G, YAO B J, LI F , et al. Ionic liquid-decorated COF and its covalent composite aerogel for selective CO2 adsorption and catalytic conversion. J. Mater. Chem. A, 2019, DOI: 10.1039/C8TA12046C. |
| [32] | LIN C, HUANG Q, ZHANG D , et al. Temperature programmed surface reaction test of Co-Ni bimetallic aerogel catalysts for methane reforming. React. Kinet. Mech. Cat., 2019, DOI: 10.1007/s11144-018-01531-3. |
| [33] | GIGOT A, FONTANA M, PIRRI C F , et al. Graphene/ruthenium active species aerogel as electrode for supercapacitor applications. Materials, 2018,11(1):57. |
| [34] | XU X Z, ZHOU J, NAGARAJU D H , et al. Flexible, highly graphitized carbon aerogels based on bacterial cellulose/lignin: catalyst-free synthesis and its application in energy storage devices. Adv. Funct. Mater., 2015,25(21):3193-3202. |
| [35] | MA L N, LIU R, NIU H J , et al. Freestanding conductive film based on polypyrrole/bacterial cellulose/graphene paper for flexible supercapacitor: large areal mass exhibits excellent areal capacitance. Electrochim. Acta, 2016,222(20):429-437. |
| [36] | LIU R, MA L N, HUANG S , et al. Large areal mass, flexible and freestanding polyaniline/bacterial cellulose/graphene film for high- performance supercapacitors. RSC Adv., 2016,6(109):107426-107432. |
| [37] | MULLER D, RECOUVREUX D O S, PORTO L M, , et al. Chemical in situ polymerization of polypyrrole on bacterial cellulose nanofibers. Synthetic Met., 2011,161(1/2):106-111. |
| [38] | LIU Y, ZHOU J, TANG J , et al. Three-dimensional, chemically bonded polypyrrole/bacterial cellulose/graphene composites for high- performance supercapacitors. Chem. Mater., 2015,27(20):7034-7041. |
| [39] | WU H, HUANG Y A, XU F , et al. Energy harvesters for wearable and stretchable electronics: from flexibility to stretchability. Adv. Mater., 2016,28(45):9881-9919. |
| [40] | ZHENG Y, YANG Y B, CHEN S S , et al. Smart, stretchable and wearable supercapacitors: prospects and challenges. CrystEngComm, 2016,18(23):4218-4235. |
| [41] | KANG Y J, CHUN S J, LEE S S , et al. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels. ACS Nano, 2012,6(7):6400-6406. |
| [42] | SUMBOJA A, FOO C Y, WANG X , et al. Large areal mass, flexible and free-standing reduced graphene oxide/manganese dioxide paper for asymmetric supercapacitor device. Adv. Mater., 2013,25(20):2809-2815. |
| [43] | XIONG Z Y, LIAO C L, HAN W H , et al. Mechanically tough large-area hierarchical porous graphene films for high-performance flexible supercapacitor applications. Adv. Mater., 2015,27(30):4469-4475. |
| [44] | LIU Y, ZHOU J, ZHU E W , et al. Facile synthesis of bacterial cellulose fibres covalently intercalated with graphene oxide by one- step cross-linking for robust supercapacitors. J. Mater. Chem. C, 2015,3(5):1011-1017. |
| [45] | MA L N, LIU R, NIU, H J , et al. Flexible and freestanding supercapacitor electrodes based on nitrogen-doped carbon networks/ graphene/bacterial cellulose with ultrahigh areal capacitance. ACS Appl. Mater. Interfaces, 2016,8(49):33608-33618. |
| [46] | XIA C, CHEN W, WANG X B , et al. Highly stable supercapacitors with conducting polymer core-shell electrodes for energy storage applications. Adv. Energy Mater., 2015,5(8):1401805. |
| [47] | YANG C Y, SHEN J L, WANG C Y , et al. All-solid-state asymmetric supercapacitor based on reduced graphene oxide/carbon nanotube and carbon fiber paper/polypyrrole electrodes. J. Mater. Chem. A, 2014,2(5):1458-1464. |
| [48] | ZHAO Y, LIU J, HU Y , et al. Highly compression-tolerant supercapacitor based on polypyrrole-mediated graphene foam electrodes. Adv. Mater., 2013,25(4):591-595. |
| [49] | SONG Y, XU J L, LIU X X . Electrochemical anchoring of dual doping polypyrrole on graphene sheets partially exfoliated from graphite foil for high-performance supercapacitor electrode. J. Power Sour., 2014,249:48-58. |
| [50] | LEE H J, CHUANG T J, KWON H J , et al. Fabrication and evaluation of bacterial cellulose-polyaniline composites by interfacial polymerization. Cellulose, 2012,19:1251-1258. |
| [51] | XU J, ZHU L G, BAI Z K , et al. Conductive polypyrrole-bacterial cellulose nanocomposite membranes as flexible supercapacitor electrode. Org. Electron., 2013,14(12):3331-3338. |
| [52] | PENG S, FAN L L, WEI C Z , et al. Flexible polypyrrole/copper sulfide/bacterial cellulose nanofibrous composite membranes as supercapacitor electrodes. Carbohyd. Polym., 2017,157:344-352. |
| [53] | PENG S, XU Q, FAN L L , et al. Flexible polypyrrole/cobalt sulfide/ bacterial cellulose composite membranes for supercapacitor application. Synthetic Met., 2016,222:285-292. |
| [54] | PENG S, FAN L L, WEI C Z , et al. Polypyrrole/nickel sulfide/ bacterial cellulose nanofibrous composite membranes for flexible supercapacitor electrodes. Cellulose, 2016,23(4):2639-2651. |
| [55] | WANG F, KIM H J, PARK S , et al. Bendable and flexible supercapacitor based on polypyrrole-coated bacterial cellulose core-shell composite network. Compos. Sci. Technol., 2016,128(18):33-40. |
| [56] | YUAN L, YAO B, HU B , et al. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci., 2013,6(2):470-476. |
| [57] | LIU R, MA L N, HUANG S , et al. A flexible polyaniline/ graphene/bacterial cellulose supercapacitor electrode. New J. Chem., 2017,41(2):857-864. |
| [58] | MA L N, LIU R, WANG F , et al. Facile synthesis of Ni(OH)2/graphene/bacterial cellulose paper for large areal mass, mechanically tough and flexible supercapacitor electrodes. J. Power Sour., 2016,335(15):76-83. |
| [59] | LIU R, MA L N, HUANG S , et al. Large areal mass and high scalable and flexible cobalt oxide/graphene/bacterial cellulose electrode for supercapacitors. J. Phys. Chem. C, 2016,120(50):28480-28488. |
| [60] | WANG X, KONG D, ZHANG Y , et al. All-biomaterial supercapacitor derived from bacterial cellulose. Nanoscale, 2016,8(17):9146-9150. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | ZHOU Fan, TIAN Zhilin, LI Bin. Research Progress on Carbide Ultra-high Temperature Ceramic Anti-ablation Coatings for Thermal Protection System [J]. Journal of Inorganic Materials, 2025, 40(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||