
Journal of Inorganic Materials ›› 2015, Vol. 30 ›› Issue (2): 122-128.DOI: 10.15541/jim20140231
• Orginal Article • Previous Articles Next Articles
MA Ping-Ping1, 2, LIU Zhi-Jian1, XIA Jian-Hua1, LU Zhi-Chao1
Received:2014-05-04
Revised:2014-06-27
Published:2015-02-20
Online:2015-01-27
Supported by:CLC Number:
MA Ping-Ping, LIU Zhi-Jian, XIA Jian-Hua, LU Zhi-Chao. Electrochemical Performance of 0.7LiFePO4ּ0.3Li3V2(PO4)3/C Cathode Materials Using Polyethylene Glycol as Carbon Source[J]. Journal of Inorganic Materials, 2015, 30(2): 122-128.
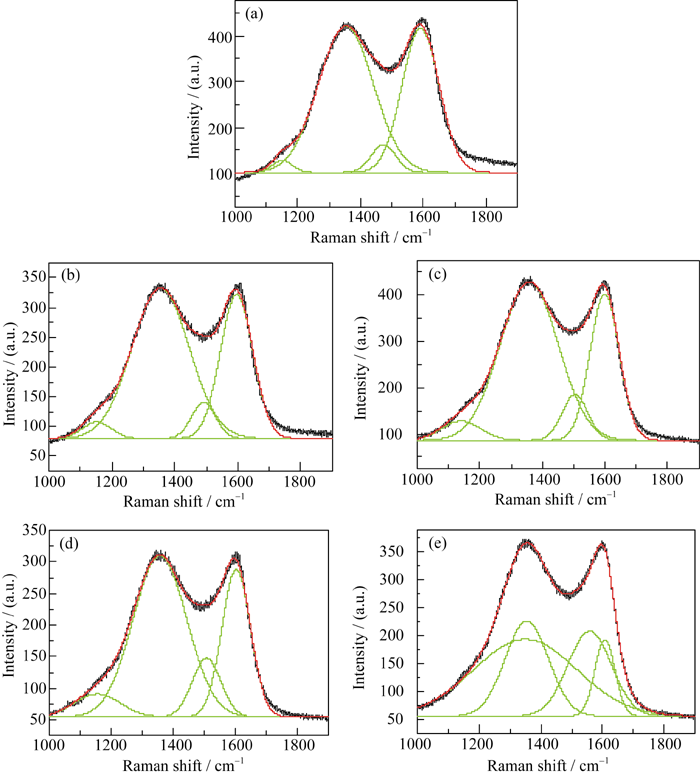
Fig. 3 Raman spectra and its fitted result of the products with different carbon sources (a) PEG-200; (b) PEG-600; (c) PEG-1000; (d) PEG-2000; (e) PEG-6000
| Carbon source | Wavenumber/cm-1 | Area/(a.u.) | AD/AG | Asp3/Asp2 | Carbon content | Electrical conductivity/(S?cm-1) | |
|---|---|---|---|---|---|---|---|
| PEG-200 | sp2 | 1352 | 70022 | 1.50 | 0.07 | 1.73% | 1.89×10-3 |
| 1590 | 46753 | ||||||
| sp3 | 1150 | 2035 | |||||
| 1472 | 5886 | ||||||
| PEG-600 | sp2 | 1354 | 57547 | 1.84 | 0.10 | 1.59% | 1.14×10-4 |
| 1596 | 31336 | ||||||
| sp3 | 1151 | 3165 | |||||
| 1491 | 5744 | ||||||
| PEG-1000 | sp2 | 1357 | 78754 | 2.07 | 0.14 | 1.81% | 2.22×10-5 |
| 1599 | 38080 | ||||||
| sp3 | 1139 | 6453 | |||||
| 1504 | 10158 | ||||||
| PEG-2000 | sp2 | 1358 | 56532 | 2.17 | 0.21 | 1.47% | 8.66×10-5 |
| 1604 | 26020 | ||||||
| sp3 | 1156 | 6771 | |||||
| 1508 | 10759 | ||||||
| PEG-6000 | sp2 | 1354 | 30012 | 2.68 | 2.01 | 1.68% | 1.17×10-5 |
| 1607 | 11208 | ||||||
| sp3 | 1349 | 57748 | |||||
| 1558 | 27004 | ||||||
Table 1 Results of ID/IG ratio, carbon content and electronic conductivity of LFVP/C compounds prepared with different carbon sources
| Carbon source | Wavenumber/cm-1 | Area/(a.u.) | AD/AG | Asp3/Asp2 | Carbon content | Electrical conductivity/(S?cm-1) | |
|---|---|---|---|---|---|---|---|
| PEG-200 | sp2 | 1352 | 70022 | 1.50 | 0.07 | 1.73% | 1.89×10-3 |
| 1590 | 46753 | ||||||
| sp3 | 1150 | 2035 | |||||
| 1472 | 5886 | ||||||
| PEG-600 | sp2 | 1354 | 57547 | 1.84 | 0.10 | 1.59% | 1.14×10-4 |
| 1596 | 31336 | ||||||
| sp3 | 1151 | 3165 | |||||
| 1491 | 5744 | ||||||
| PEG-1000 | sp2 | 1357 | 78754 | 2.07 | 0.14 | 1.81% | 2.22×10-5 |
| 1599 | 38080 | ||||||
| sp3 | 1139 | 6453 | |||||
| 1504 | 10158 | ||||||
| PEG-2000 | sp2 | 1358 | 56532 | 2.17 | 0.21 | 1.47% | 8.66×10-5 |
| 1604 | 26020 | ||||||
| sp3 | 1156 | 6771 | |||||
| 1508 | 10759 | ||||||
| PEG-6000 | sp2 | 1354 | 30012 | 2.68 | 2.01 | 1.68% | 1.17×10-5 |
| 1607 | 11208 | ||||||
| sp3 | 1349 | 57748 | |||||
| 1558 | 27004 | ||||||
| Carbon source | 0.2C | 1C | 5C | 10C |
|---|---|---|---|---|
| PEG-200 | 134 | 134 | 126 | 120 |
| PEG-600 | 142 | 138 | 130 | 112 |
| PEG-1000 | 145 | 139 | 126 | 79 |
| PEG-2000 | 142 | 141 | 132 | 95 |
| PEG-6000 | 140 | 141 | 129 | 72 |
Table 2 Comparison of discharge capability (mAh/g) of LFVP/C compounds prepared with different carbon sources
| Carbon source | 0.2C | 1C | 5C | 10C |
|---|---|---|---|---|
| PEG-200 | 134 | 134 | 126 | 120 |
| PEG-600 | 142 | 138 | 130 | 112 |
| PEG-1000 | 145 | 139 | 126 | 79 |
| PEG-2000 | 142 | 141 | 132 | 95 |
| PEG-6000 | 140 | 141 | 129 | 72 |
| [1] | RUI X, DING N, LIU J, et al.Analysis of the chemical diffusion coefficient of lithium ions in Li3V2(PO4)3 cathode material.Electrochim. Acta, 2010, 55: 2384-2390. |
| [2] | ZHOU F, COCOCCIONI M, KANG K, et al.The Li intercalation potential of LiMPO4 and LiMSiO4 olivines with M = Fe, Mn, Co, Ni.Electrochem Commun., 2004, 6: 1144-1148. |
| [3] | LEE J, TEJA A S.Synthesis of LiFePO4 micro and nanoparticles in supercritical water.Materials Letters, 2006, 60: 2105-2109. |
| [4] | ZHENG J C, LI X H, WANG Z X, et al.LiFePO4 with enhanced performance synthesized by a novel synthetic route.J. Power Sources, 2008, 184: 574-577. |
| [5] | YUAN L X, WANG Z H, ZHANG W X, et al.Development and challenges of LiFePO4 cathode material for lithium-ion batteries.Energy & Environmental Science, 2011, 4: 269-284. |
| [6] | CHUNG S Y, BLOKING J T, CHIANG Y M.Electronically conductive phospho-olivines as lithium storage electrodes.Nature Materials, 2002, 1: 123-128. |
| [7] | RAVET N, CHOUINARD Y, MAGNAN J F, et al. Electroactivity of natural and synthetic triphylite. J. Power Sources, 2001, 97-98: 503-507. |
| [8] | OH S W, MYUNG S T, OH S M, et al.Double carbon coating of LiFePO4 as high rate electrode for rechargeable lithium batteries .Adv. Mater. , 2010, 22: 4842-4845. |
| [9] | ZHENG J, LI X, WANG Z, et al.Novel synthesis of LiFePO4-Li3V2(PO4)3 composite cathode material by aqueous precipitation and lithiation.J. Power Sources, 2010, 195(9): 2935-2938. |
| [10] | GUO Y, HUANG Y, JIA D, et al.Preparation and electrochemical properties of high-capacity LiFePO4-Li3V2(PO4)3/C composite for lithium-ion batteries.J. Power Sources, 2014, 246: 912-917. |
| [11] | HONG J, WANG C S, CHEN X, et al.Vanadium modified LiFePO4 cathode for Li-ion batteries.Electrochem. Solid-State Lett., 2009, 12: A33-A38. |
| [12] | XIANG J Y, TU J P, ZHANG L, et al.Improved electrochemical performances of 9LiFePO4ּLi3V2(PO4)3/C composite prepared by a simple solid-state method.J. Power Sources, 2010, 195: 8331-8335. |
| [13] | ZHANG B, ZHENG J C, YANG Z H.Structural properties of composite cathode material LiFePO4-Li3V2(PO4)3.Ionics, 2011, 17: 859-862. |
| [14] | LI X L, KANG F Y.A novel network composite cathode of LiFePO4/multiwalled carbon nanotubes with high rate capability for lithium ion batteries.Electrochemistry Communications, 2007, 9: 663-666. |
| [15] | SARAVANAN K, REDDY M V, BALAYA P, et al.Storage performance of LiFePO4 nanoplates.J. Mater. Chem., 2009, 19: 605-610. |
| [16] | BARKER J, SAÏDI M Y, SWOYER J L. A carbothermal reduction method for the preparation of electroactive materials for lithium ion applications.J. Electrochem. Soc., 2003, 150: A684-A688. |
| [17] | NIEN Y H, CAREY J R, CHEN J S.Physical and electrochemical properties of LiFePO4/C composite cathode prepared from various polymer-containing precursors.J. Power Sources, 2009, 193: 822-827. |
| [18] | DOBRYSZYCKI J, BIALLOZOR S.On some organic inhibitors of zinc corrosion in alkaline media.Corros. Sci., 2001, 43: 1309-1319. |
| [19] | HUANG C W, LI Y Y.In situ synthesis of platelet graphite nanofibers from thermal decomposition of poly(ethylene glycol).J. Phys. Chem. B, 2006, 110: 23242-23246. |
| [20] | WANG L N, ZHAN X C, ZHANG Z G, et al.A soft chemistry synthesis routine for LiFePO4-C using a novel carbon source.J. Alloys Compd. , 2008, 456: 461-465. |
| [21] | KIM D K, PARK H M, JUNG S J, et al.Effect of synthesis conditions on the properties of LiFePO4 for secondary lithium batteries.J. Power Sources, 2006, 159: 237-240. |
| [22] | WANG L N, ZHANG Z G, ZHANG K L.A simple, cheap soft synthesis routine for LiFePO4 using iron(III) raw material.J. Power Sources, 2007, 167: 200-205. |
| [23] | DOEFF M M, HU Y, MCLARNON F, et al.Effect of surface carbon structure on the electrochemical performance of LiFePO4.Electrochem. Solid-State Lett., 2003, 6: A207-A209. |
| [24] | HU Y, DOEFF M M, KOSTECKI R, et al.Electrochemical performance of Sol-Gel synthesized LiFePO4 in lithium batteries.J. Electrochem. Soc., 2004, 151: A1279-A1285. |
| [25] | KOSTECKI R, SCHNYDER B, ALLIATA D, et al.Surface studies of carbon films from pyrolyzed photoresist.Thin Solid Films, 2001, 396: 36-43. |
| [26] | SALAH A A, MAUGER A, ZAGHIB K, et al.Reduction Fe3+ of impurities in LiFePO4 from pyrolysis of organic precursor used for carbon deposition.J. Electrochem. Soc., 2006, 153: A1692-A1701. |
| [1] | TAN Bowen, GENG Shuanglong, ZHANG Kai, ZHENG Bailin. Composition-gradient Design of Silicon Electrodes to Mitigate Mechanochemical Coupling Degradation [J]. Journal of Inorganic Materials, 2025, 40(7): 772-780. |
| [2] | LIU Pengdong, WANG Zhen, LIU Yongfeng, WEN Guangwu. Research Progress on the Application of Silicon Slurry in Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2024, 39(9): 992-1004. |
| [3] | YANG Zhuo, LU Yong, ZHAO Qing, CHEN Jun. X-ray Diffraction Rietveld Refinement and Its Application in Cathode Materials for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2023, 38(6): 589-605. |
| [4] | SU Nana, HAN Jingru, GUO Yinhao, WANG Chenyu, SHI Wenhua, WU Liang, HU Zhiyi, LIU Jing, LI Yu, SU Baolian. ZIF-8-derived Three-dimensional Silicon-carbon Network Composite for High-performance Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(9): 1016-1022. |
| [5] | WANG Yang, FAN Guangxin, LIU Pei, YIN Jinpei, LIU Baozhong, ZHU Linjian, LUO Chengguo. Microscopic Mechanism of K+ Doping on Performance of Lithium Manganese Cathode for Li-ion Battery [J]. Journal of Inorganic Materials, 2022, 37(9): 1023-1029. |
| [6] | ZHU Hezhen, WANG Xuanpeng, HAN Kang, YANG Chen, WAN Ruizhe, WU Liming, MAI Liqiang. Enhanced Lithium Storage Stability Mechanism of Ultra-high Nickel LiNi0.91Co0.06Al0.03O2@Ca3(PO4)2 Cathode Materials [J]. Journal of Inorganic Materials, 2022, 37(9): 1030-1036. |
| [7] | FENG Kun, ZHU Yong, ZHANG Kaiqiang, CHEN Zhang, LIU Yu, GAO Yanfeng. Boehmite Nanosheets-coated Separator with Enhanced Performance for Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(9): 1009-1015. |
| [8] | CHEN Ying, LUAN Weiling, CHEN Haofeng, ZHU Xuanchen. Multi-scale Failure Behavior of Cathode in Lithium-ion Batteries Based on Stress Field [J]. Journal of Inorganic Materials, 2022, 37(8): 918-924. |
| [9] | WANG Yutong, ZHANG Feifan, XU Naicai, WANG Chunxia, CUI Lishan, HUANG Guoyong. Research Progress of LiTi2(PO4)3 Anode for Aqueous Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2022, 37(5): 481-492. |
| [10] | LI Kunru, HU Xinghui, ZHANG Zhengfu, GUO Yuzhong, HUANG Ruian. Three-dimensional Porous Biogenic Si/C Composite for High Performance Lithium-ion Battery Anode Derived from Equisetum Fluviatile [J]. Journal of Inorganic Materials, 2021, 36(9): 929-935. |
| [11] | WANG Ying, ZHANG Wenlong, XING Yanfeng, CAO suqun, DAI Xinyi, LI Jingze. Performance of Amorphous Lithium Phosphate Coated Lithium Titanate Electrodes in Extended Working Range of 0.01-3.00 V [J]. Journal of Inorganic Materials, 2021, 36(9): 999-1005. |
| [12] | WANG Yanan, LI Hua, WANG Zhengkun, LI Qingfeng, LIAN Chen, HE Xin. Progress on Failure Mechanism of Lithium Ion Battery Caused by Diffusion Induced Stress [J]. Journal of Inorganic Materials, 2020, 35(10): 1071-1087. |
| [13] | Jian-Huang KE, Kai XIE, Yu HAN, Wei-Wei SUN, Shi-Qiang LUO, Jin-Feng LIU. Morphology Controlling of the High-voltage Cathode Materials with Different Co-solvents [J]. Journal of Inorganic Materials, 2019, 34(6): 618-624. |
| [14] | GUO Rong-Nan, HAN Wei-Qiang. Effects of Structure and Properties of Polar Polymeric Binders on Lithium-ion Batteries [J]. Journal of Inorganic Materials, 2019, 34(10): 1021-1029. |
| [15] | LEE Sai-Xi, WANG Xue-Yin, GU Qing-Wen, XIA Yong-Gao, LIU Zhao-Ping, HE Jie. Tuning Electrochemical Performance through Non-stoichiometric Compositions in High-voltage Spinel Cathode Materials [J]. Journal of Inorganic Materials, 2018, 33(9): 993-1000. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||