无机材料学报 ›› 2026, Vol. 41 ›› Issue (2): 150-158.DOI: 10.15541/jim20250101 CSTR: 32189.14.10.15541/jim20250101
刘占一1,2,3( ), 李勉2,3(
), 李勉2,3( ), 欧阳晓平4, 柴之芳2,3, 黄庆2,3(
), 欧阳晓平4, 柴之芳2,3, 黄庆2,3( )
)
收稿日期:2025-03-08
修回日期:2025-04-16
出版日期:2025-05-09
网络出版日期:2025-05-09
通讯作者:
李 勉, 研究员. E-mail: limian@nimte.ac.cn;作者简介:刘占一(2001-), 男, 硕士研究生. E-mail: liuzhanyi@nimte.ac.cn
基金资助:
LIU Zhanyi1,2,3( ), LI Mian2,3(
), LI Mian2,3( ), OUYANG Xiaoping4, CHAI Zhifang2,3, HUANG Qing2,3(
), OUYANG Xiaoping4, CHAI Zhifang2,3, HUANG Qing2,3( )
)
Received:2025-03-08
Revised:2025-04-16
Published:2025-05-09
Online:2025-05-09
Contact:
LI Mian, professor. E-mail: limian@nimte.ac.cn;About author:LIU Zhanyi (2001-), male, Master candidate. E-mail: liuzhanyi@nimte.ac.cn
Supported by:摘要:
干法后处理技术具有耐辐照、防扩散和简化废物处理等特点, 是未来先进快堆乏燃料后处理的优选技术。其中, 熔盐电解精炼是干法后处理的核心技术, 主要利用铀、钚等锕系元素与其他裂变元素在熔盐体系中的氧化还原电位差来实现锕系元素的分离回收。然而, 在电解精炼过程中, 镧系元素和Sr/Cs等裂变元素在熔盐中不断积累, 改变了熔盐的理化性质, 严重影响电解精炼效率。另外, 90Sr和137Cs等裂变产物属于水溶性长寿命核素, 若处理不当, 将对环境造成巨大危害。因此, 有效净化熔盐中的Sr/Cs等裂变元素不仅是提高熔盐电解干法后处理效率的迫切需求, 也是减少放射性废物排放的重要手段。本文总结了熔盐中的Sr/Cs去除方法的研究现状, 对比分析了电解法、结晶法、减压蒸馏法、沉淀法和离子交换法等不同方法的分离原理和分离效果, 并探讨了未来的发展方向及潜在的可用材料体系。
中图分类号:
刘占一, 李勉, 欧阳晓平, 柴之芳, 黄庆. 干法后处理熔盐中Sr/Cs去除方法的研究进展[J]. 无机材料学报, 2026, 41(2): 150-158.
LIU Zhanyi, LI Mian, OUYANG Xiaoping, CHAI Zhifang, HUANG Qing. Recent Progress on Removal of Sr/Cs from Molten Salt in Dry Reprocessing[J]. Journal of Inorganic Materials, 2026, 41(2): 150-158.
图2 典型轻水反应堆燃料辐照至40000兆瓦天/吨产生的废物[11]
Fig. 2 Wastes arising from typical light water reactor fuel irradiated to 40000 MW·d/t[11] Elements present shadowed in grey. Numbers represent amount in milligram per kilogram of uranium
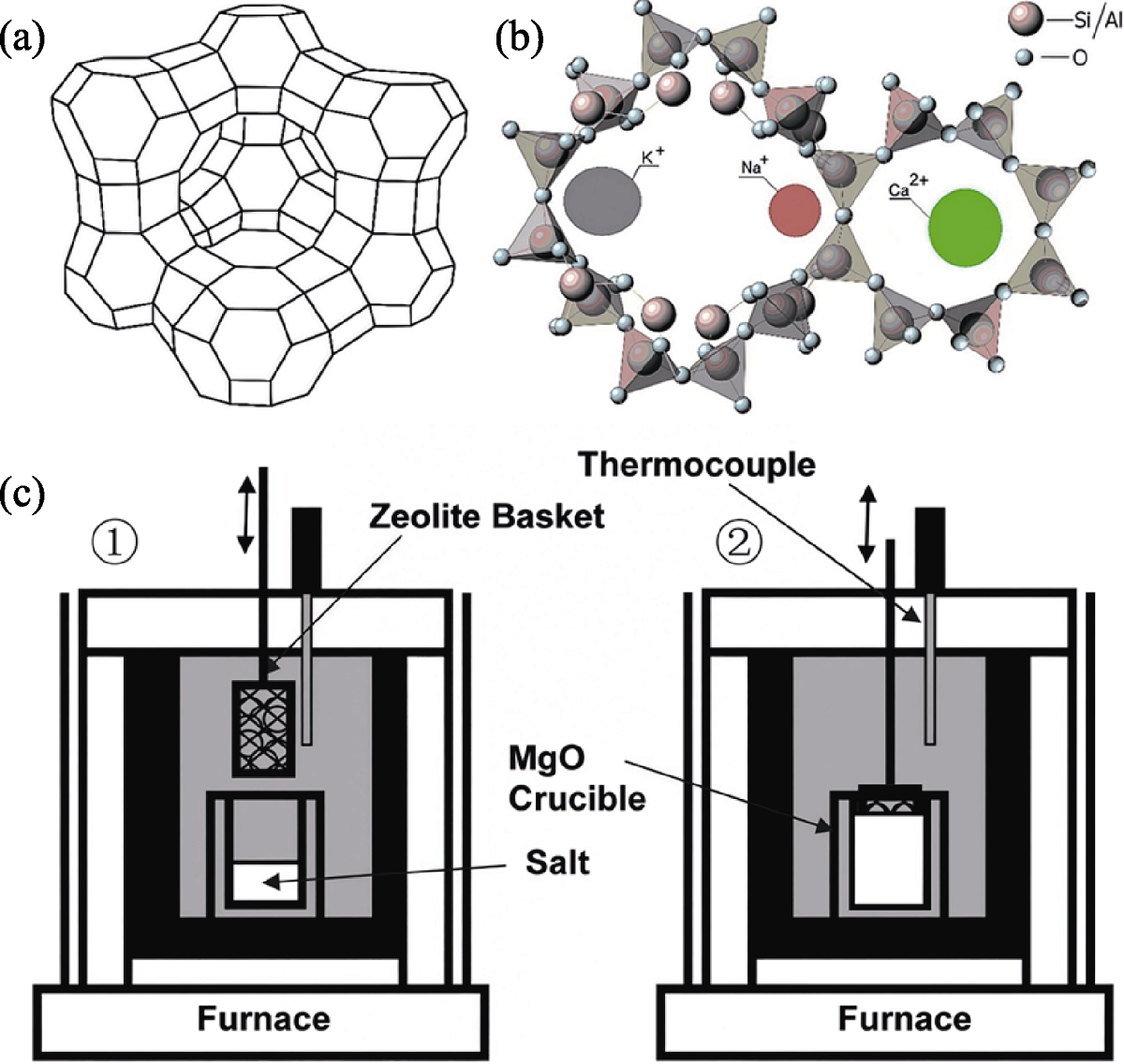
图10 沸石结构及沸石离子交换装置示意图[52,56]
Fig. 10 Schematic diagrams of zeolite structure and zeolite ion exchange device[52,56] (a, b) Schematic structure of zeolite[52]; (c) System for molten salt-zeolite ion exchange tests[56]
| Method | Working salt | Nuclide | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|---|
| Physical method | Cold finger separation | LiCl | Sr, Cs | No impurities introduced | Difficult to scale application | [ |
| Zone-refining process | LiCl | Sr, Cs | No impurities introduced, high removal rate, easy accessibility | Long processing time | [ | |
| LiCl-KCl | [ | |||||
| Chemical method | Precipitation | LiCl-KCl | Sr, Cs | Short processing time, low cost | Low removal rate of Cs, introduction of impurities | [ |
| Electrolysis | LiCl-KCl | Sr | High efficiency | Low removal rate, high corrosivity to equipment | [ | |
| NaCl-KCl | [ | |||||
| Ion exchange | LiCl-KCl/ NaCl-KCl | Sr, Cs | Good selectivity, high removal efficiency | Introduction of new impurities | [ | |
表1 去除熔盐中Sr/Cs的可用方法及其优缺点
Table 1 Available methods for removing Sr and Cs from molten salts and their advantages and disadvantages
| Method | Working salt | Nuclide | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|---|
| Physical method | Cold finger separation | LiCl | Sr, Cs | No impurities introduced | Difficult to scale application | [ |
| Zone-refining process | LiCl | Sr, Cs | No impurities introduced, high removal rate, easy accessibility | Long processing time | [ | |
| LiCl-KCl | [ | |||||
| Chemical method | Precipitation | LiCl-KCl | Sr, Cs | Short processing time, low cost | Low removal rate of Cs, introduction of impurities | [ |
| Electrolysis | LiCl-KCl | Sr | High efficiency | Low removal rate, high corrosivity to equipment | [ | |
| NaCl-KCl | [ | |||||
| Ion exchange | LiCl-KCl/ NaCl-KCl | Sr, Cs | Good selectivity, high removal efficiency | Introduction of new impurities | [ | |
| [1] | 丁煊涛, 焦立峰, 郭峃峄, 等. 核电规模化储能集成技术进展. 热力发电, 2025, 54: 12. |
| [2] | 王宏渊. 我国快堆闭式核燃料循环体系的现状及展望. 能源工程, 2013(5): 8. |
| [3] | 张东辉, 王松平, 代智文. 我国快堆的创新与发展. 核科学与工程, 2024, 44: 980. |
| [4] |
林如山, 何辉, 唐洪彬, 等. 我国乏燃料干法后处理技术研究现状与发展. 原子能科学技术, 2020, 54: 115.
DOI |
| [5] |
YIN T Q, XUE Y, YAN Y D, et al. Recovery and separation of rare earth elements by molten salt electrolysis. International Journal of Minerals, Metallurgy and Materials, 2021, 28(6): 899.
DOI |
| [6] | MIRZA M, ABDULAZIZ R, MASKELL W C, et al. Electrochemical processing in molten salts-a nuclear perspective. Energy & Environmental Science, 2023, 16(3): 952. |
| [7] |
WILLIAMSON M A, WILLIT J. Pyroprocessing flowsheets for recycling used nuclear fuel. Nuclear Engineering and Technology, 2011, 43: 329.
DOI URL |
| [8] |
YIN T, LIU Y, JIANG S, et al. Kinetic properties and electrochemical separation of uranium on liquid bismuth electrode in LiCl-KCl melt. Journal of the Electrochemical Society, 2021, 168(3): 032503.
DOI |
| [9] | 伍思达, 林如山, 张磊, 等. 干法后处理废盐中活泼裂片元素的净化工艺研究进展. 无机盐工业, 2022, 54: 81. |
| [10] |
IIZUKA M, UOZUMI K, OGATA T, et al. Development of an innovative electrorefiner for high uranium recovery rate from metal fast reactor fuels. Journal of Nuclear Science and Technology, 2009, 46(7): 699.
DOI URL |
| [11] |
VOLKOVICH V A, GRIFFITHS T R, THIED R C. Treatment of molten salt wastes by phosphate precipitation: removal of fission product elements after pyrochemical reprocessing of spent nuclear fuels in chloride melts. Journal of Nuclear Materials, 2003, 323(1): 49.
DOI URL |
| [12] | SIMPSON M F. Projected salt waste production from a commercial pyroprocessing facility. Science and Technology of Nuclear Installations, 2013, 2013(1): 945858. |
| [13] |
CHOI E Y, WON C Y, KANG D S, et al. Production of uranium metal via electrolytic reduction of uranium oxide in molten LiCl and salt distillation. Journal of Radioanalytical and Nuclear Chemistry, 2015, 304(2): 535.
DOI URL |
| [14] |
KIM S W, JEON M K, CHOI E Y. Electrolytic behavior of SrCl2 and BaCl2 in LiCl molten salt during oxide reduction in pyroprocessing. Journal of Radioanalytical and Nuclear Chemistry, 2019, 321(1): 361.
DOI |
| [15] |
CHO Y Z, PARK G H, LEE H S, et al. Concentration of cesium and strontium elements involved in a LiCl waste salt by a melt crystallization process. Nuclear Technology, 2010, 171(3): 325.
DOI URL |
| [16] |
VANCE E R, DAVIS J, OLUFSON K, et al. Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel. Journal of Nuclear Materials, 2012, 420(1/2/3): 396.
DOI URL |
| [17] |
WANG D D, LIU Y L, JIANG S L, et al. Separation of uranium from lanthanides (La, Ce, Nd) and purification of waste salt via aluminum electrodes with different structures in LiCl-KCl eutectic. Separation and Purification Technology, 2025, 353: 128328.
DOI URL |
| [18] |
WANG D D, LIU Y L, YANG D W, et al. Separation of uranium from lanthanides (La, Sm) with sacrificial Li anode in LiCl-KCl eutectic salt. Separation and Purification Technology, 2022, 292: 121025.
DOI URL |
| [19] |
YANG D W, JIANG S L, LIU Y L, et al. Electrochemical extraction kinetics of Nd on reactive electrodes. Separation and Purification Technology, 2022, 281: 119853.
DOI URL |
| [20] |
YANG M C, ZHONG Y K, WANG D D, et al. Rapid and efficient extraction of cerium by forming Al-Ce alloys in LiCl-KCl molten salts. Separation and Purification Technology, 2024, 341: 126868.
DOI URL |
| [21] |
FIGUEIREDO B R, CARDOSO S P, PORTUGAL I, et al. Inorganic ion exchangers for cesium removal from radioactive wastewater. Separation and Purification Reviews, 2018, 47(4): 306.
DOI URL |
| [22] |
VINCENT T, VINCENT C, BARRE Y, et al. Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: synthesis and characterization. Journal of Materials Chemistry A, 2014, 2(26): 10007.
DOI URL |
| [23] |
CHEN S, HU J, HAN S, et al. A review on emerging composite materials for cesium adsorption and environmental remediation on the latest decade. Separation and Purification Technology, 2020, 251: 117340.
DOI URL |
| [24] |
LIZAGA I, GASPAR L, QUIJANO L, et al. NDVI, 137Cs and nutrients for tracking soil and vegetation development on glacial landforms in the Lake Paron Catchment (Cordillera Blanca, Peru). Science of the Total Environment, 2019, 651: 250.
DOI URL |
| [25] |
MENENDEZ-DUARTE R, FERNANDEZ S, SOTO J. The application of 137Cs to post-fire erosion in north-west Spain. Geoderma, 2009, 150(1/2): 54.
DOI URL |
| [26] | KIM G Y, JANG J, PAEK S, et al. Electrochemical removal of rare earth element in LiCl-KCl molten salt. Science and Technology of Nuclear Installations, 2020, 2020: 2392489. |
| [27] |
JANG J, LEE M, KIM G Y, et al. Cesium and strontium recovery from LiCl-KCl eutectic salt using electrolysis with liquid cathode. Nuclear Engineering and Technology, 2022, 54(10): 3957.
DOI URL |
| [28] | NIGL T P, LICHTENSTEIN T, KONG Y, et al. Electrochemical separation of alkaline-earth elements from molten salts using liquid metal electrodes. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14818. |
| [29] |
CHEN X, ZHANG Y, QU J, et al. Integrating preparation of borides and separation of alkaline- and rare-earth ions through an electrochemical alloying approach in molten salts. Separation and Purification Technology, 2022, 285: 120391.
DOI URL |
| [30] |
YUAN Y, ZHANG Y, CHEN X, et al. Electrochemical purification of waste salt from pyro-processing of spent nuclear fuels. Separation and Purification Technology, 2023, 326: 124805.
DOI URL |
| [31] |
VERSEY J R, PHONGIKAROON S, SIMPSON M F. Separation of CsCl from LiCl-CsCl molten salt by cold finger melt crystallization. Nuclear Engineering and Technology, 2014, 46(3): 395.
DOI URL |
| [32] |
CHOI J H, CHO Y Z, LEE T K, et al. Inclusion behavior of Cs, Sr, and Ba impurities in LiCl crystal formed by layer-melt crystallization: combined first-principles calculation and experimental study. Journal of Crystal Growth, 2013, 371: 84.
DOI URL |
| [33] |
LEE B, KIM G Y, CHOI J H, et al. Reactive-crystallization method for purification of LiCl-KCl eutectic salt waste. Journal of Radioanalytical and Nuclear Chemistry, 2024, 333(12): 6331.
DOI |
| [34] |
LEE H S, OH G H, LEE Y S, et al. Concentrations of CsCl and SrCl2 from a simulated LiCl Salt waste generated by pyroprocessing by using Czochralski method. Journal of Nuclear Science and Technology, 2009, 46(4): 392.
DOI URL |
| [35] |
SHIM M, CHOI H G, CHOI J H, et al. Separation of Cs and Sr from LiCl-KCl eutectic salt via a zone-refining process for pyroprocessing waste salt minimization. Journal of Nuclear Materials, 2017, 491: 9.
DOI URL |
| [36] |
CHO Y Z, LEE T K, CHOI J H, et al. Eutectic (LiCl-KCl) waste salt treatment by sequential separation process. Nuclear Engineering and Technology, 2013, 45(5): 675.
DOI URL |
| [37] |
RODRIGUEZ-LAGUNA M D R, TOLMAN K R, KROPP M T, et al. Separation of fission products from high-level waste salt systems by partial crystallization: CsCl-NaCl-LiCl-KCl study. Separation and Purification Technology, 2024, 332: 125602.
DOI URL |
| [38] |
WILLIAMS A N, PHONGIKAROON S, SIMPSON M F. Separation of CsCl from a ternary CsCl-LiCl-KCl salt via a melt crystallization technique for pyroprocessing waste minimization. Chemical Engineering Science, 2013, 89: 258.
DOI URL |
| [39] |
CHOI H G, SHIM M, LEE J H, et al. Numerical analysis of impurity separation from waste salt by investigating the change of concentration at the interface during zone refining process. Journal of Crystal Growth, 2017, 474: 69.
DOI URL |
| [40] |
DIVAKARAN S, JOSEPH J, MANOHARAN M, et al. CsCl enrichment during solidification of molten LiCl-KCl-CsCl salt mixture. Nuclear Engineering and Technology, 2024, 56(11): 4716.
DOI URL |
| [41] | 付海英, 耿俊霞, 杨洋, 等. 乏燃料干法后处理中的熔盐减压蒸馏技术. 核技术, 2018, 41: 5. |
| [42] |
EUN H C, YANG H C, CHO Y Z, et al. Vacuum distillation of a mixture of LiCl-KCl eutectic salts and RE oxidative precipitates and a dechlorination and oxidation of RE oxychlorides. Journal of Hazardous Materials, 2008, 160(2): 634.
DOI PMID |
| [43] |
EUN H C, CHOI J H, KIM N Y, et al. A reactive distillation process for the treatment of LiCl-KCl eutectic waste salt containing rare earth chlorides. Journal of Nuclear Materials, 2016, 480: 69.
DOI URL |
| [44] |
EUN H C, CHOI J H, KIM N Y, et al. A study of separation and solidification of group II nuclides in waste salt delivered from the pyrochemical process of used nuclear fuel. Journal of Nuclear Materials, 2017, 491: 149.
DOI URL |
| [45] |
WESTPHAV B R, MARSDEN K C, PRICE J C, et al. On the development of a distillation process for the electrometallurgical treatment of irradiated spent nuclear fuel. Nuclear Engineering and Technology, 2008, 40(3): 163.
DOI URL |
| [46] |
CHO Y Z, PARK G H, YANG H C, et al. Minimization of eutectic salt waste from pyroprocessing by oxidative precipitation of lanthanides. Journal of Nuclear Science and Technology, 2009, 46(10): 1004.
DOI URL |
| [47] |
CHO Y Z, LEE T K, EUN H C, et al. Purification of used eutectic (LiCl-KCl) salt electrolyte from pyroprocessing. Journal of Nuclear Materials, 2013, 437(1/2/3): 47.
DOI URL |
| [48] |
GRIFFITHS T R, VOLKOVICH V A, YAKIMOV S M, et al. Reprocessing spent nuclear fuel using molten carbonates and subsequent precipitation of rare earth fission products using phosphate. Journal of Alloys and Compounds, 2006, 418(1): 116.
DOI URL |
| [49] |
HAN W, ZHANG Y, LIU R, et al. Purification of spent electrolyte by sequential precipitation method and its on-line monitoring. Ionics, 2021, 27(11): 4829.
DOI |
| [50] |
HAN W, ZHANG Y, LIU R, et al. Removal of RE3+, Cs+, Sr2+, Ba2+ from molten salt electrolyte by precipitation and solidification of glass- ceramics. Journal of Non-Crystalline Solids, 2023, 606: 122208.
DOI URL |
| [51] |
UOZUMI K, IIZUKA M, OMORI T. Removal of rare-earth fission products from molten chloride salt used in pyroprocessing by precipitation for consolidation into glass-bonded sodalite waste form. Journal of Nuclear Materials, 2021, 547: 152784.
DOI URL |
| [52] |
LONIN A Y, LEVENETS V V, OMELNIK O P, et al. Removal of a mixture of Cs, Sr and Co cations from an aqueous solution using composite sorbents based on natural and synthetic zeolites. Journal of Radioanalytical and Nuclear Chemistry, 2022, 331(12): 5517.
DOI |
| [53] |
HAO W, YAN N, XIE M, et al. Origin of the exceptional selectivity of NaA zeolite for the radioactive isotope 90Sr2+. Inorganic Chemistry Frontiers, 2022, 9(23): 6258.
DOI URL |
| [54] |
YANG H M, PARK C W, KIM I, et al. Sulfur-modified chabazite as a low-cost ion exchanger for the highly selective and simultaneous removal of cesium and strontium. Applied Surface Science, 2021, 536: 147776.
DOI URL |
| [55] | LEXA D, JOHNSON I. Occlusion and ion exchange in the molten (lithium chloride-potassium chloride-alkali metal chloride) salt plus zeolite 4A system with alkali metal chlorides of sodium, rubidium, and cesium. Metallurgical and Materials Transactions B-Process Metallurgy and Materials Processing Science, 2001, 32(3): 429. |
| [56] |
SACHDEV P, SIMPSON M F, FRANK S M, et al. Selective separation of Cs and Sr from LiCl-based salt for electrochemical processing of oxide spent nuclear fuel. Separation Science and Technology, 2008, 43(9/10): 2709.
DOI URL |
| [57] |
PARK H S, KIM I T, CHO Y J, et al. Removal behavior of Cs from molten salt by using zeolitic materials. Journal of Radioanalytical and Nuclear Chemistry, 2010, 283(2): 267.
DOI URL |
| [58] |
SHALTRY M, PHONGIKAROON S, SIMPSON M F. Ion exchange kinetics of fission products between molten salt and zeolite-A. Microporous and Mesoporous Materials, 2012, 152: 185.
DOI URL |
| [59] |
YOO T S, FRANK S M, SIMPSON M F, et al. Salt-zeolite ion-exchange equilibrium studies for a complete set of fission products in molten LiCl-KCl. Nuclear Technology, 2010, 171(3): 306.
DOI URL |
| [60] |
SIMPSON M F, GOUGAR M L D. Two-site equilibrium model for ion exchange between monovalent cations and zeolite-A in a molten salt. Industrial & Engineering Chemistry Research, 2003, 42(18): 4208.
DOI URL |
| [61] |
PHONGIKAROON S, SIMPSON M F. Equilibrium model for ion exchange between multivalent cations and zeolite-A in a molten salt. AICHE Journal, 2006, 52(5): 1736.
DOI URL |
| [62] |
TANG J H, JIN J C, LI W A, et al. Highly selective cesium(I) capture under acidic conditions by a layered sulfide. Nature Communications, 2022, 13: 658.
DOI |
| [63] |
QIU K, ZHANG Y, LI S, et al. Water-stable S-functionalized Ti3C2 MXene for high-performance Sr and Cs adsorption. Surfaces and Interfaces, 2024, 53: 105072.
DOI URL |
| [1] | 孙炼, 张磊磊, 薛泽旭, 吴坤, 陈晔, 李志远, 王鲁凯, 王尊刚. 面向辐射探测应用的零维金属卤化物闪烁体研究进展[J]. 无机材料学报, 2026, 41(2): 159-176. |
| [2] | 任先培, 李超, 胡启威, 向晖, 彭跃红. 金属/过渡金属化合物莫特-肖特基析氢催化剂研究进展[J]. 无机材料学报, 2026, 41(2): 137-149. |
| [3] | 范雨竹, 王媛, 王林燕, 向美玲, 鄢雨婷, 黎本慧, 李敏, 文志东, 王海超, 陈永福, 邱会东, 赵波, 周成裕. 氧化石墨烯基吸附材料去除水体中Pb(II): 制备、性能及机理[J]. 无机材料学报, 2026, 41(1): 12-26. |
| [4] | 徐锦涛, 高攀, 何唯一, 蒋圣楠, 潘秀红, 汤美波, 陈锟, 刘学超. 3C-SiC晶体制备研究进展[J]. 无机材料学报, 2026, 41(1): 1-11. |
| [5] | 余升阳, 苏海军, 姜浩, 余明辉, 姚佳彤, 杨培鑫. 激光增材制造超高温氧化物陶瓷孔隙缺陷形成及抑制研究进展[J]. 无机材料学报, 2025, 40(9): 944-956. |
| [6] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [7] | 肖晓琳, 王玉祥, 谷佩洋, 朱圳荣, 孙勇. 二维无机材料调控病损皮肤组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 860-870. |
| [8] | 马景阁, 吴成铁. 无机生物材料用于毛囊和毛发再生的研究[J]. 无机材料学报, 2025, 40(8): 901-910. |
| [9] | 张洪健, 赵梓壹, 吴成铁. 无机生物材料调控神经细胞功能及神经化组织再生的研究进展[J]. 无机材料学报, 2025, 40(8): 849-859. |
| [10] | 艾敏慧, 雷波. 微纳米生物活性玻璃: 功能化设计与血管化皮肤再生[J]. 无机材料学报, 2025, 40(8): 921-932. |
| [11] | 王宇彤, 常江, 徐合, 吴成铁. 硅酸盐生物陶瓷/玻璃促创面修复的研究进展:作用、机制和应用方式[J]. 无机材料学报, 2025, 40(8): 911-920. |
| [12] | 马文平, 韩雅卉, 吴成铁, 吕宏旭. 无机活性材料在类器官研究领域的应用[J]. 无机材料学报, 2025, 40(8): 888-900. |
| [13] | 罗晓民, 乔志龙, 刘颍, 杨晨, 常江. 无机生物活性材料调控心肌再生的研究进展[J]. 无机材料学报, 2025, 40(8): 871-887. |
| [14] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [15] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||