无机材料学报 ›› 2024, Vol. 39 ›› Issue (12): 1301-1315.DOI: 10.15541/jim20240240 CSTR: 32189.14.10.15541/jim20240240
所属专题: 【能源环境】储能电池(202506); 【能源环境】锂离子电池(202412)
• 综述 • 下一篇
文志朋1,2( ), 韦毅1,4, 侯向华1, 郭佳文1, 黎渠1, 朱满庆1, 张家浩1, 潘凯1(
), 韦毅1,4, 侯向华1, 郭佳文1, 黎渠1, 朱满庆1, 张家浩1, 潘凯1( ), 吴炼2,3(
), 吴炼2,3( )
)
收稿日期:2024-05-13
修回日期:2024-07-25
出版日期:2024-12-20
网络出版日期:2024-08-19
通讯作者:
吴 炼, 副研究员. E-mail: wulian@gdcri.com;作者简介:文志朋(1987-), 男, 高级工程师. E-mail: wenzhipeng_01@sina.com
基金资助:
WEN Zhipeng1,2( ), WEI Yi1,4, HOU Xianghua1, GUO Jiawen1, LI Qu1, ZHU Manqing1, ZHANG Jiahao1, PAN Kai1(
), WEI Yi1,4, HOU Xianghua1, GUO Jiawen1, LI Qu1, ZHU Manqing1, ZHANG Jiahao1, PAN Kai1( ), WU Lian2,3(
), WU Lian2,3( )
)
Received:2024-05-13
Revised:2024-07-25
Published:2024-12-20
Online:2024-08-19
Contact:
WU Lian, associate professor. E-mail: wulian@gdcri.com;About author:WEN Zhipeng (1987-), male, senior engineer. E-mail: wenzhipeng_01@sina.com
Supported by:摘要:
膨润土是一种储量丰富、廉价易得的天然黏土矿物, 其主要矿物成分为蒙脱石(MMT)。MMT因独特的二维层状纳米结构、丰富的孔隙结构和高比表面积而具有良好的离子交换性能、吸附性能和离子传输性能, 而且热稳定性、化学稳定性和机械稳定性优异。近年来, MMT因上述特性, 特别是其固有的金属离子(Li+、Na+、Zn2+等)传输特性, 引起了电化学储能领域研究人员的关注并被广泛用于电化学储能装置的关键部件(电极、聚合物电解质和隔膜), 展现出了良好的应用前景。本文首先概述了膨润土的结构及理化特性, 然后详细综述了膨润土基功能材料在电化学储能装置(主要包括金属负极、锂硫电池正极、固态/凝胶聚合物电解质、聚合物隔膜)中的应用研究进展, 在此基础上重点阐述了膨润土基功能材料在电化学储能过程中促进离子传输的作用机理。最后总结了当前膨润土基功能材料在电化学储能装置领域所面临的问题和挑战, 并对未来的研究方向进行了展望, 以期为今后设计开发膨润土基电化学储能功能材料提供有益指导。
中图分类号:
文志朋, 韦毅, 侯向华, 郭佳文, 黎渠, 朱满庆, 张家浩, 潘凯, 吴炼. 膨润土基功能材料在电化学储能中的研究进展[J]. 无机材料学报, 2024, 39(12): 1301-1315.
WEN Zhipeng, WEI Yi, HOU Xianghua, GUO Jiawen, LI Qu, ZHU Manqing, ZHANG Jiahao, PAN Kai, WU Lian. Research Progress of Bentonite-based Functional Materials in Electrochemical Energy Storage[J]. Journal of Inorganic Materials, 2024, 39(12): 1301-1315.
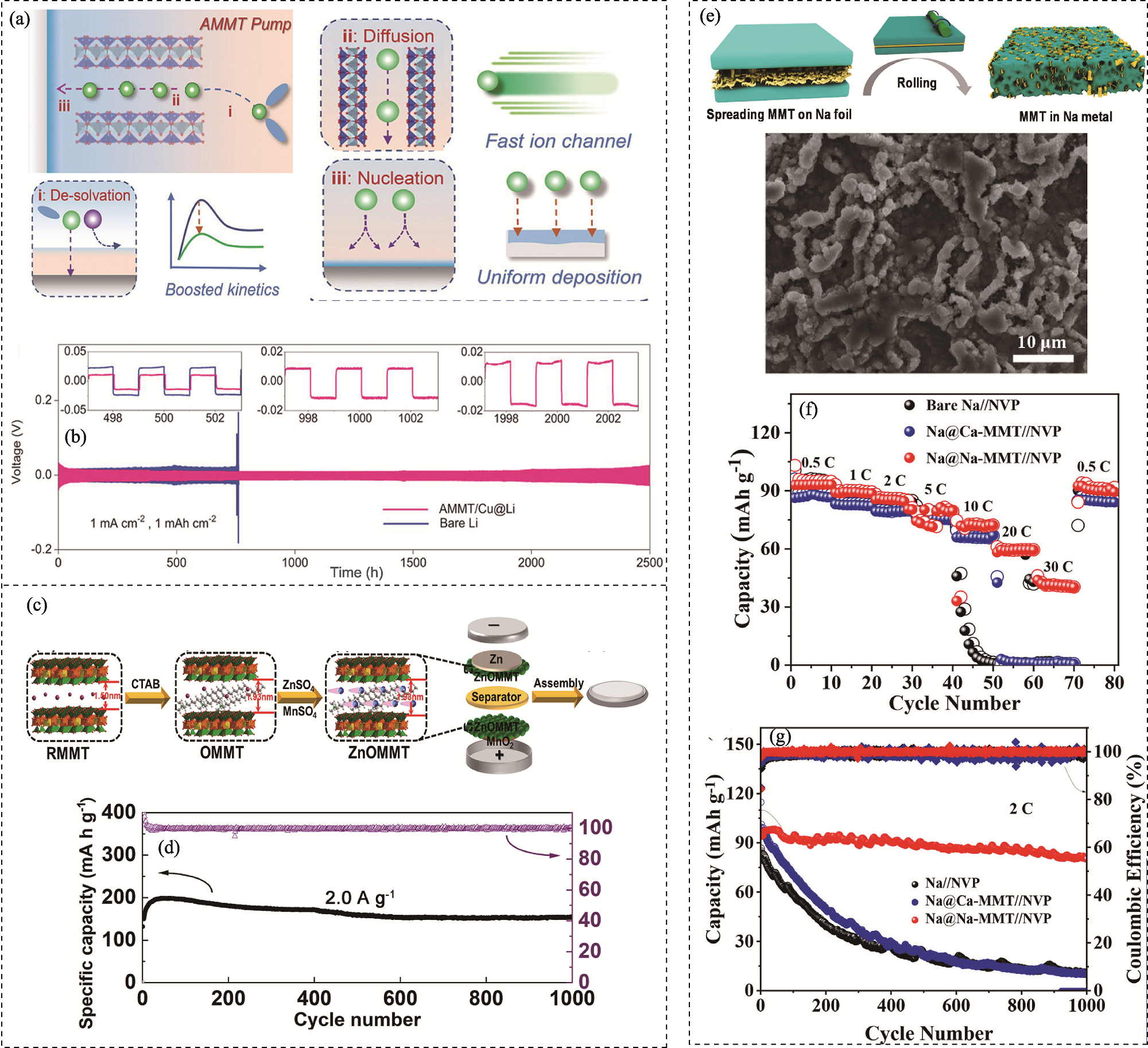
图3 膨润土功能材料修饰金属负极的应用
Fig. 3 Applications of bentonite-based functional materials in metal anodes (a) Schematic diagram of the lithium ion pumping effect in the interlayers of Ag-montmorillonite (AMMT); (b) Voltage profiles of the AMMT/Cu@Li||AMMT/Cu@Li and Li||Li symmetrical batteries under 1 mA·cm-2 and 1 mAh·cm-2 conditions with insets showing voltage profiles at different cycles[38]; (c) Preparation schematic of hexadecyl trimethyl ammonium bromide (CTAB)-pillared organic-montmorillonite (OMMT) mixed ZnSO4/MnSO4 liquid electrolyte as a protective layer (denoted as ZnOMMT) for zinc-ion batteries (ZIBs); (d) Long-term cycling performance of ZIBs with ZnOMMT protective layer at 2.0 A·g−1 [40]; (e) Preparation schematic and SEM image of the Na-montmorillonite (Na-MMT) modified Na metal anode (Na@Na-MMT); (f) Rate performance at different current densities and (g) cycling performance at 2C of the Na||Na3V2(PO4)3 (NVP), Na@Ca-montmorillonite (Ca-MMT)||NVP, and Na@Na-MMT||NVP batteries[42]
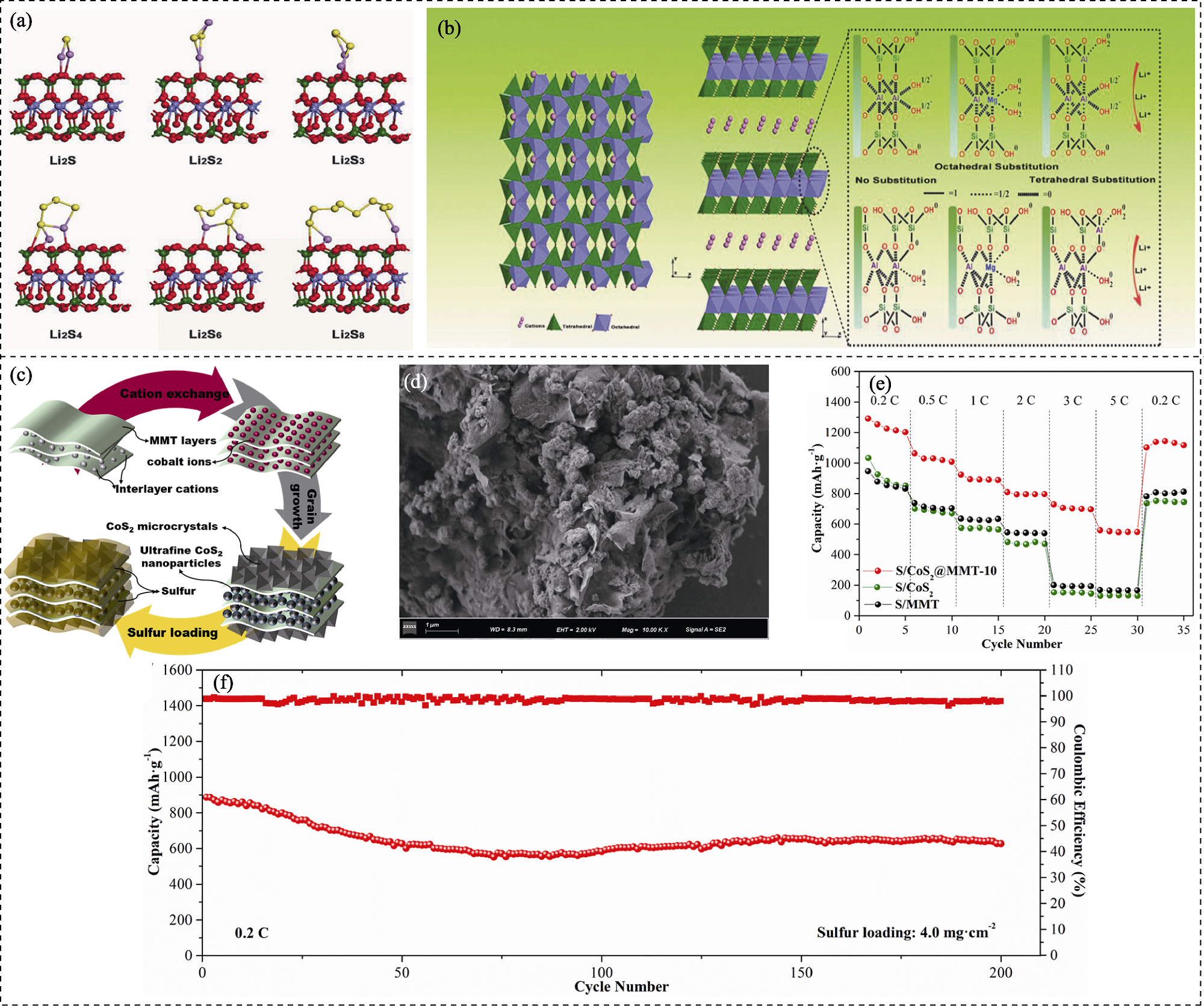
图4 膨润土基锂硫电池正极载体材料的制备、作用机理及电化学性能
Fig. 4 Preparation, action mechanism and electrochemical performance of bentonite-based host materials for lithium-sulfur battery cathodes (a) Adsorption conformations of Li-montmorillonite (Li-MMT) on various lithium polysulfides (Li2S, Li2S2, Li2S3, Li2S4, Li2S6, and Li2S8); (b) Crystal structure of Li-MMT[28]; (c) Preparation schematic of the S/CoS2@montmorillonite (S/CoS2@MMT) composite materials; (d) SEM image of CoS2@MMT; (e) Rate performance of the S/CoS2@MMT, S/CoS2, and S/MMT cathodes; (f) Cycling performance of the S/CoS2@MMT with a sulfur loading of 4.0 mg·cm-2[47]
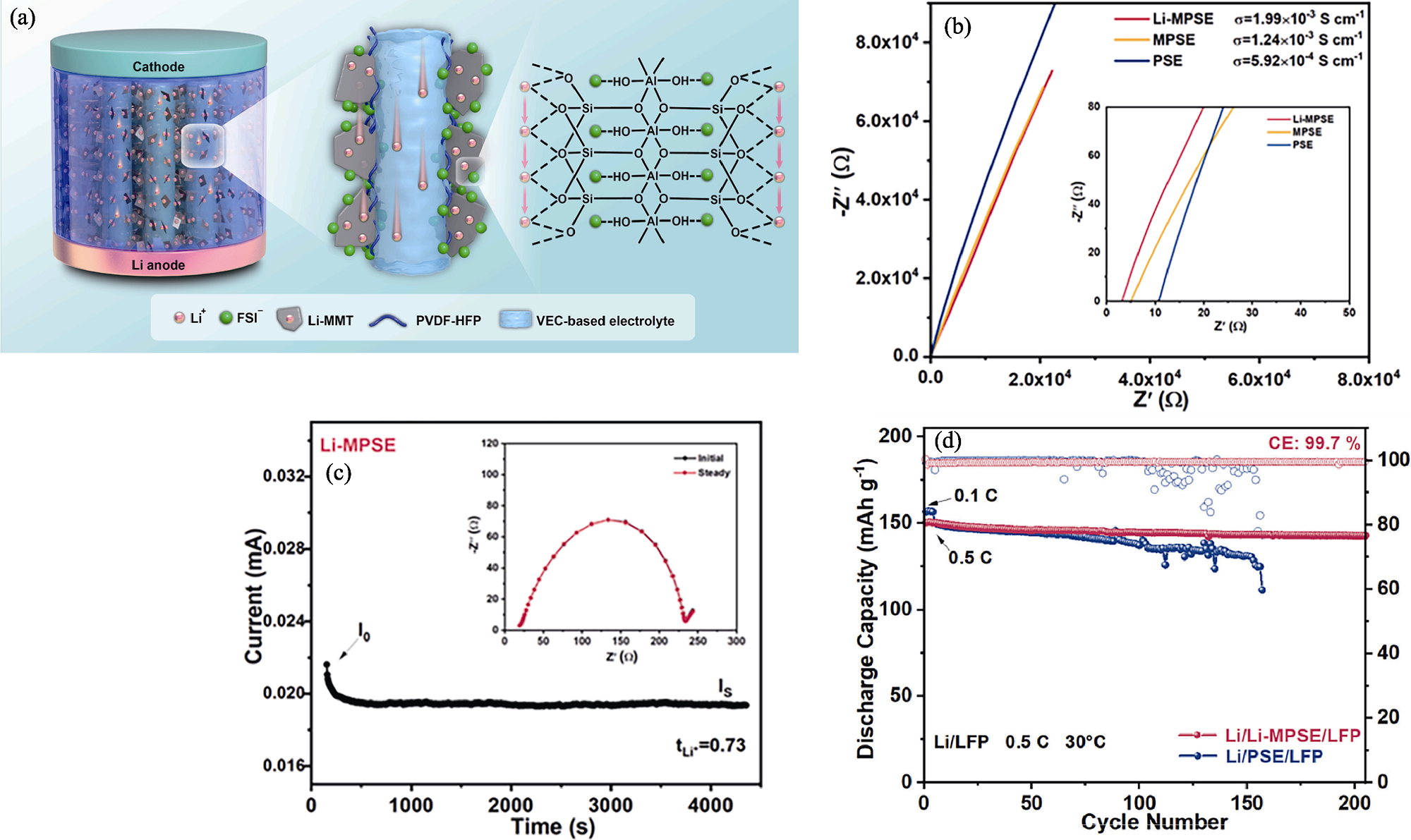
图5 膨润土纳米片在固态聚合物电解质中构筑的快速锂离子传输通道结构及其锂离子传输性能[74]
Fig. 5 Structure and Li ion transport properties of fast Li ion transport path constructed by bentonite nanosheets in solid polymer electrolytes[74] (a) Schematic illustration of dual Li+ transportation pathways in the Li-montmorillonite (Li-MMT)/polyvinylidene difluoride-hexafluoropropylene (PVDF-HFP) solid electrolyte (Li-MPSE); (b) Electrochemical impedance spectra (EIS) of PVDF-HFP solid electrolyte (PSE), montmorillonite/PVDF-HFP solid electrolyte (MPSE), and Li-MPSE; (c) Chronoamperometry curve of Li/Li-MPSE/Li with 10 mV at room temperature with inset showing the initial and steady-state EIS of the cell; (d) Cycling performance of Li/Li-MPSE/LiFePO4 (LFP) and Li/PSE/LFP batteries
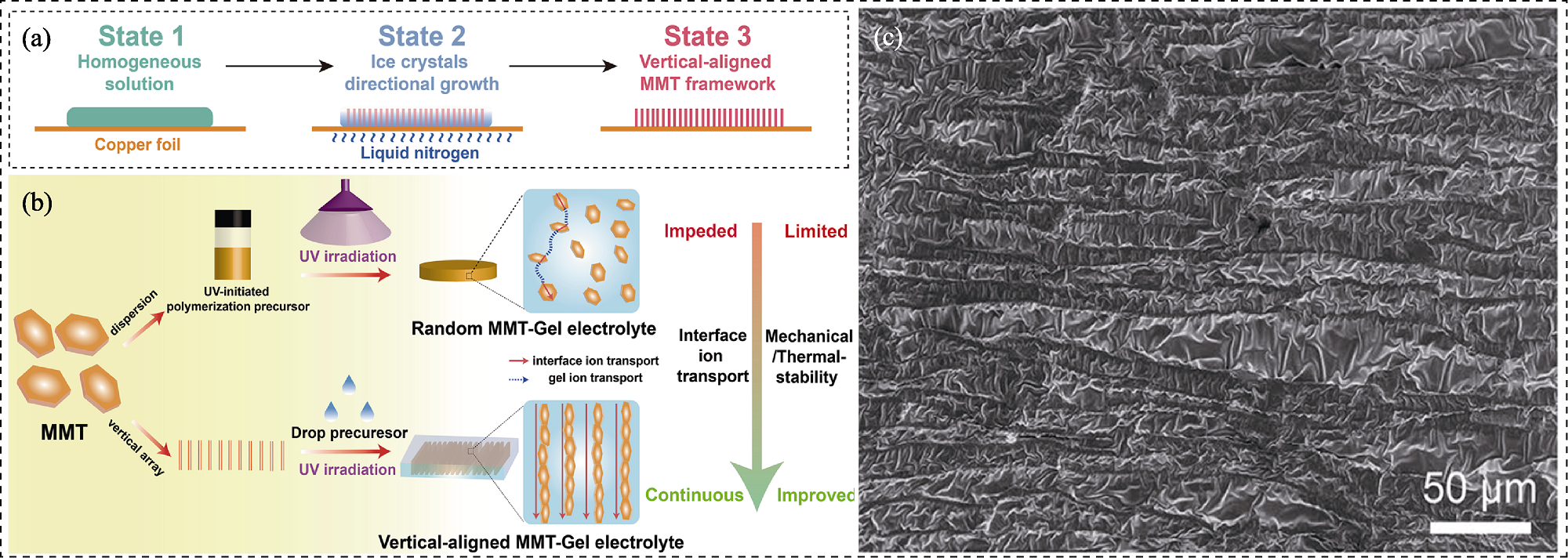
图6 膨润土纳米片在凝胶聚合物电解质(GPEs)中垂直排布构筑定向离子传输通道[75]
Fig. 6 Directional ion transport pathways constructed by vertically arranging bentonite nanosheets in gel polymer electrolytes (GPEs)[75] (a, b) Schematic illustration of (a) vertical-aligned montmorillonite prepared by directional freezing technology and (b) fabrication process of GPEs with/without vertical-aligned ion pathways; (c) Top view SEM image of the ion-conducting arrays GPEs
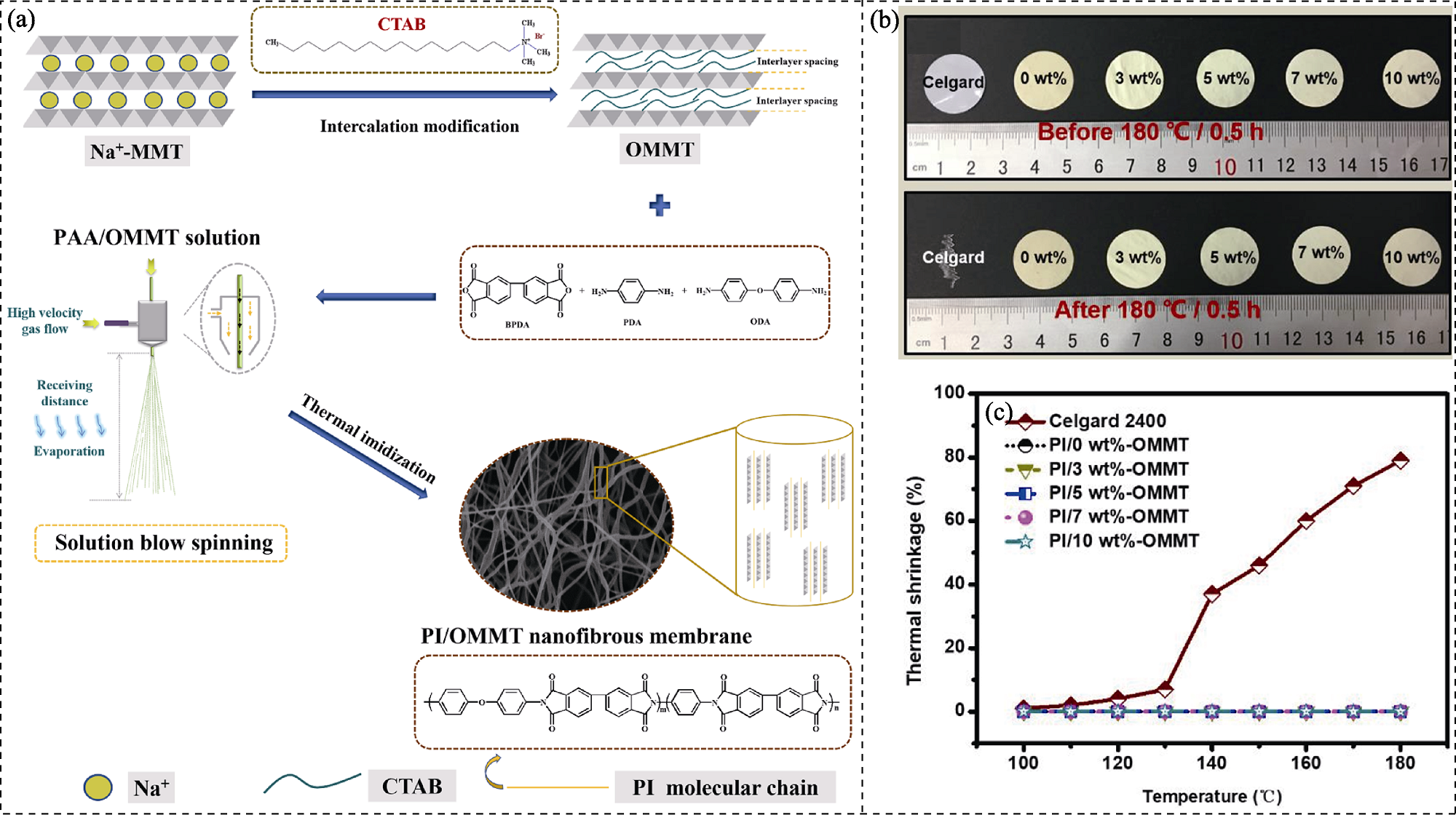
图7 膨润土基功能材料填充改性的聚合物隔膜制备过程及其热稳定性能[93]
Fig. 7 Preparation process and thermostability of bentonite-based functional material filling modified polymer separator[93] (a) Preparation of organic-montmorillonite (OMMT) via the intercalation modification of Na-montmorillonite (Na-MMT) by hexadecyl trimethyl ammonium bromide (CTAB) and preparation of polyimide (PI)/OMMT nanofibrous membrane via the thermal imidization of polyamide acid (PAA)/OMMT; (b) Photographs of Celgard 2400 and PI/OMMT hybrid separators before and after exposure to 180 ℃ for 0.5 h and (c) the corresponding thermal shrinkage changes at different OMMT mass fractions of 0, 3%, 5%, 7%, and 10% (denoted as PI/0 wt%-OMMT, PI/3 wt%-OMMT, PI/5 wt%-OMMT, PI/7 wt%-OMMT, and PI/10 wt%-OMMT, respectively)
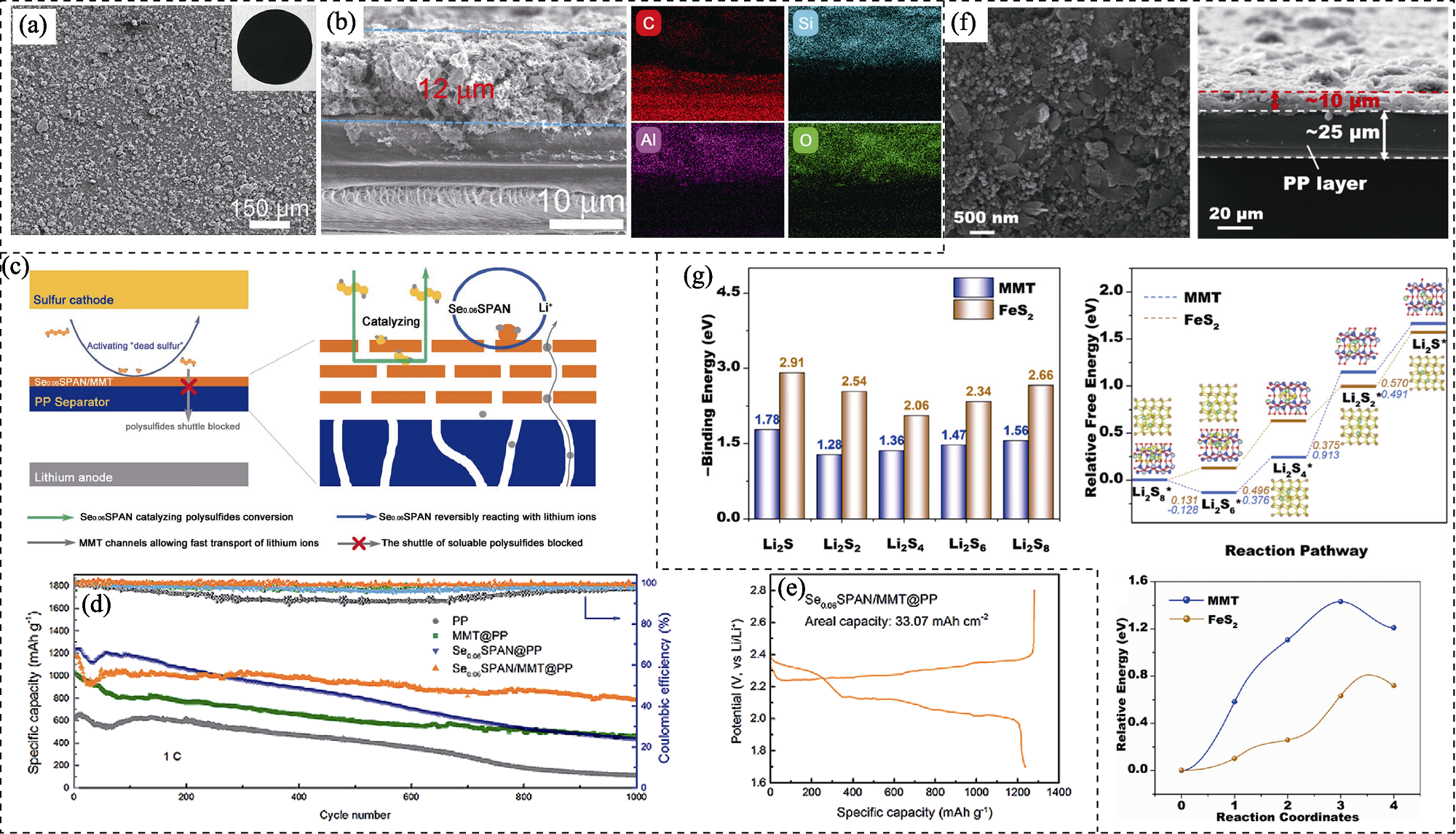
图8 膨润土基锂硫电池隔膜表面功能涂层结构、作用机理及电化学性能
Fig. 8 Structure, action mechanism and electrochemical performance of bentonite-based functional coating on the surface of separators for lithium sulfur batteries (a) SEM image of Keggin Al13-pillared montmorillonite (AlMMT)@polypropylene (PP) separator with inset showing its optical image; (b) Cross- sectional SEM image and EDS mappings of AlMMT@PP separator[99]; (c) Schematic illustration of functions of the selenium-doped sulfurized-polyacrylonitrile (Se0.06SPAN)/montmorillonite (MMT) coating layer; (d) Cycling performance at 1C of different separators (PP, MMT@PP, Se0.06SPAN@PP, and Se0.06SPAN/MMT@PP) with a sulfur loading of 0.8 mg·cm-2; (e) First cycle charge and discharge curves of Li-S battery with Se0.06SPAN/MMT@PP separator at a sulfur loading of 26.75 mg·cm-2[101]; (f) Surface and cross-sectional SEM images of FeS2@MMT/PP separator; (g) Binding energies of lithium polysulfides (Li2Sx, x=1, 2, 4, 6, 8) on MMT and FeS2 (200) surface (left), energy profiles for the reactions from Li2S8 to Li2S (upper right), and energy barrier profiles of Li2S decomposition on MMT and FeS2 (200) surface (lower right)[102]
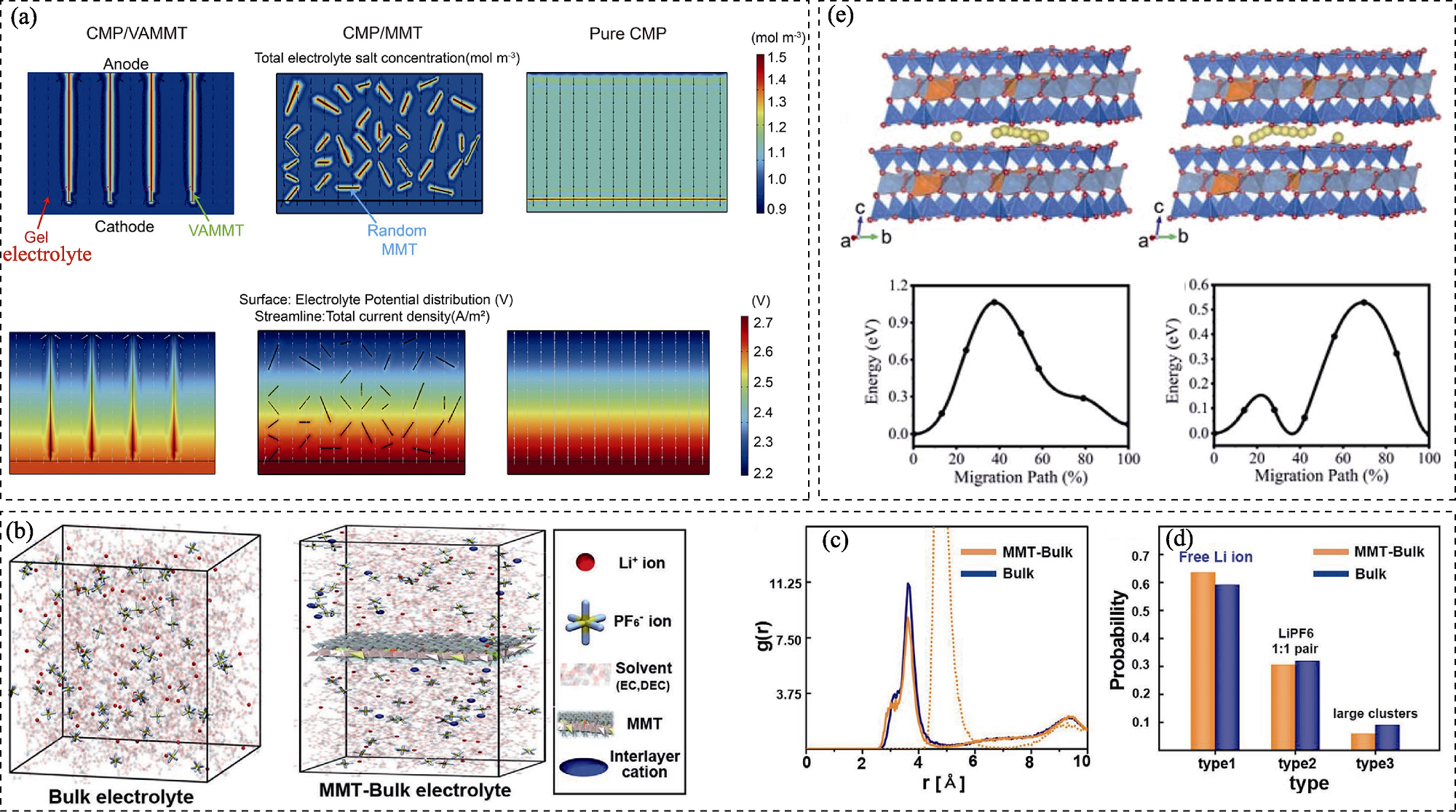
图9 膨润土基功能材料促进电化学储能过程的离子传输机理
Fig. 9 Promotion mechanism of ion transport by bentonite-based functional materials during electrochemical energy storage process (a) Finite element simulations of the current distribution in cross-linked methoxy poly(ethylene glycol) acrylate (CMP)/vertical-aligned montmorillonite (VAMMT), CMP/MMT, and CMP GPEs[75]; (b) Molecular dynamics simulations of the electrolyte system (LiPF6 in ethylene carbonate (EC)/diethyl carbonate (DEC)) with/without MMT, and the corresponding (c) radial distribution function and (d) distribution of Li ion clusters[69]; (e) Migration pathways and energy barriers of Zn2+ along the a axis and b axis of the Zn-MMT (001) surface[105]
| [1] | CHEN Z, ZHI C. MXene based zinc ion batteries: recent development and prospects. Journal of Inorganic Materials, 2024, 39(2):204. |
| [2] |
潘新慧, 陈人杰, 吴锋. 电化学储能技术发展研究. 中国工程科学, 2023, 25(6):225.
DOI |
| [3] | YANG Y, MENG G, WANG H, et al. Efficient polysulfides trapping and redox enabled by Co/N-carbon implanted Li+-montmorillonite for advanced lithium-sulfur batteries. Chemical Engineering Journal, 2023, 451: 138914. |
| [4] | YANG M, LI Z, CHEN W, et al. Carbon-intercalated montmorillonite as efficient polysulfide mediator for enhancing the performance of lithium-sulfur batteries. Energy & Fuels, 2020, 34(7):8947. |
| [5] | DENG J, MA F, GAO X, et al. Effect of directional arrangement one-dimensional Fe3O4-coated sepiolite structure on the Li+conduction of PEO-based polymer electrolyte. Journal of Alloys and Compounds, 2024, 976: 173240. |
| [6] | YANG Z, ZHANG Z, LIU Y, et al. Enhancement mechanism of the comprehensive performance of all-solid-state polymer electrolytes by halloysite nanotubes: construction of efficient lithium-ion conduction channels. Journal of Power Sources, 2024, 603: 234391. |
| [7] | ZENG Z, DONG Y, YUAN S, et al. Natural mineral compounds in energy-storage systems: development, challenges, prospects. Energy Storage Materials, 2022, 45: 442. |
| [8] | WU L, HE X, ZHAO Y, et al. Montmorillonite-based materials for electrochemical energy storage. Green Chemistry, 2024, 26(2):678. |
| [9] | GHOSH P K, BARD A J. Clay-modified electrodes. Journal of the American Chemical Society, 1983, 105(17):5691. |
| [10] | WANG D, YU W, ZHU B. A special solid electrolyte-montmorillonite. Solid State Ionics, 1989, 34(4):219. |
| [11] | RIAZ U, ASHRAF S M. Microwave-assisted solid state in situ polymerization and intercalation of poly(carbazole) between bentonite layers: effect of microwave irradiation and gallery confinement on the spectral, fluorescent, and morphological properties. The Journal of Physical Chemistry C, 2012, 116(22):12366. |
| [12] | ZHAO Y, WANG Y. Tailored solid polymer electrolytes by montmorillonite with high ionic conductivity for lithium-ion batteries. Nanoscale Research Letters, 2019, 14: 1. |
| [13] | NUNES-PEREIRA J, LOPES A, COSTA C, et al. Porous membranes of montmorillonite/poly(vinylidene fluoride-trifluorethylene) for Li-ion battery separators. Electroanalysis, 2012, 24(11): 2147. |
| [14] | TIAN S, HWANG T, ESTALAKI S M, et al. A low-cost quasi-solid-state “water-in-swelling-clay” electrolyte enabling ultrastable aqueous zinc-ion batteries. Advanced Energy Materials, 2023, 13(30):2300782. |
| [15] | NEELAMMA M, HOLLA S R, SELVAKUMAR M, et al. Bentonite clay liquid crystals for high-performance supercapacitors. Journal of Electronic Materials, 2022, 51(5): 2192. |
| [16] | JI S, QIN J, YANG S, et al. A mechanically durable hybrid hydrogel electrolyte developed by controllable accelerated polymerization mechanism towards reliable aqueous zinc-ion battery. Energy Storage Materials, 2023, 55: 236. |
| [17] | LAN Y, LIU Y, LI J, et al. Natural clay-based materials for energy storage and conversion applications. Advanced Science, 2021, 8(11):2004036. |
| [18] | YANG C, GAO R, YANG H. Application of layered nanoclay in electrochemical energy: current status and future. EnergyChem, 2021, 3(5):100062. |
| [19] | YAN H, LI S, NAN Y, et al. Ultrafast zinc-ion-conductor interface toward high-rate and stable zinc metal batteries. Advanced Energy Materials, 2021, 11(18):2100186. |
| [20] | YI H, ZHAN W Q, ZHAO Y L, et al. A novel core-shell structural montmorillonite nanosheets/stearic acid composite PCM for great promotion of thermal energy storage properties. Solar Energy Materials and Solar Cells, 2019, 192: 57. |
| [21] | LONG X, LUO Z H, ZHOU W H, et al. Two-dimensional montmorillonite-based heterostructure for high-rate and long-life lithium-sulfur batteries. Energy Storage Materials, 2022, 52: 120. |
| [22] | WU L, DAI Y, ZENG W, et al. Effective ion pathways and 3D conductive carbon networks in bentonite host enable stable and high-rate lithium-sulfur batteries. Nanotechnology Reviews, 2021, 10(1):20. |
| [23] | 杨茂.基于蒙脱土改性的锂硫电池隔膜设计及其界面离子调控机理研究. 成都: 电子科技大学博士学位论文, 2021. |
| [24] | CAGLAR B, AFSIN B, TABAK A, et al. Characterization of the cation-exchanged bentonites by XRPD, ATR, DTA/TG analyses and BET measurement. Chemical Engineering Journal, 2009, 149(1/2/3):242. |
| [25] | BANANEZHAD B, ISLAMI M R, GHONCHEPOUR E, et al. Bentonite clay as an efficient substrate for the synthesis of the super stable and recoverable magnetic nanocomposite of palladium (Fe3O4/bentonite-Pd). Polyhedron, 2019, 162: 192. |
| [26] | 宁超凡, 潘俊安, 程豪, 等. 膨润土/硫复合材料在锂硫电池中的应用. 功能材料, 2016, 47(11):11087. |
| [27] | FAN P, LIU H, MAROSZ V, et al. High performance composite polymer electrolytes for lithium-ion batteries. Advanced Functional Materials, 2021, 31(23):2101380. |
| [28] | CHEN W, LEI T, LÜ W, et al. Atomic interlamellar ion path in high sulfur content lithium-montmorillonite host enables high-rate and stable lithium-sulfur battery. Advanced Materials, 2018, 30(40):1804084. |
| [29] | UMMARTYOTIN S, BUNNAK N, MANUSPIYA H. A comprehensive review on modified clay based composite for energy based materials. Renewable and Sustainable Energy Reviews, 2016, 61: 466. |
| [30] | TANG H, SUN M, WANG C. 2D silicate materials for composite polymer electrolytes. Chemistry — An Asian Journal, 2021, 16(19):2842. |
| [31] |
LIU F, CHUAN X. Recent developments in natural mineral-based separators for lithium-ion batteries. RSC Advances, 2021, 11(27):16633.
DOI PMID |
| [32] | BALKANLOO P G, MARJANI A P, ZANBILI F, et al. Clay mineral/polymer composite: characteristics, synthesis, and application in Li-ion batteries: a review. Applied Clay Science, 2022, 228: 106632. |
| [33] | CHEN C H, MA Y Z, WANG C L. Investigation of electrochemical performance of montmorillonite clay as Li-ion battery electrode. Sustainable Materials and Technologies, 2018, 17: e00086. |
| [34] | BAKHMATYUK B P, GRYGORCHAK I I, PIDLUZHNA A Y, et al. Intercalation of bentonite: thermodynamics, kinetics, and practical applications. Inorganic Materials, 2007, 43(5):537. |
| [35] | CHEN M S, FU W, HU Y, et al. Controllable growth of carbon nanosheets in the montmorillonite interlayers for high-rate and stable anode in lithium ion battery. Nanoscale, 2020, 12(30):16262. |
| [36] | FENG L, SONG J, SUN C, et al. Improving the performance of SiOx/carbon materials for high energy density commercial lithium-ion batteries based on montmorillonite. ChemElectroChem, 2020, 7(2):445. |
| [37] | TANG H, ZHAO S, WENG Q, et al. Fast Li-ion conductor additive toward high-rate lithium storage capacity for Li2ZnTi3O8 in lithium-ion batteries. Ionics, 2023, 29(8):3001. |
| [38] | FENG Y, ZHONG B, ZHANG R, et al. Achieving high-power and dendrite-free lithium metal anodes via interfacial ion-transport-rectifying pump. Advanced Energy Materials, 2023, 13(12):2203912. |
| [39] | ZENG T, YAN Y, HE M, et al. A single-ion-conducting lithium- based montmorillonite interfacial layer for stable lithium-metal batteries. Journal of Materials Chemistry A, 2022, 10(44):23712. |
| [40] | WANG Y, FAN Y, LIAO D, et al. Highly Zn2+-conductive and robust modified montmorillonite protective layer of electrodes toward high-performance rechargeable zinc-ion batteries. Energy Storage Materials, 2022, 51: 212. |
| [41] | HAN Y, WANG F, ZHANG B, et al. Building block effect induces horizontally oriented bottom Zn(002) deposition for a highly stable zinc anode. Energy Storage Materials, 2023, 62: 102928. |
| [42] | LUO C, WANG H, QIAN Y, et al. Montmorillonite as a sodium- ion-conductor interface for stable sodium metal anodes. Journal of Power Sources, 2022, 548: 232038. |
| [43] |
陈丹, 杨蓉, 张卫华, 等. 有机金属骨架材料在电化学储能领域中的研究进展. 化工进展, 2018, 37(2):628.
DOI |
| [44] | ZHANG C, HE Y, WANG Y, et al. CoFe2O4 nanoparticles loaded N-doped carbon nanofibers networks as electrocatalyst for enhancing redox kinetics in Li-S batteries. Applied Surface Science, 2021, 560: 149908. |
| [45] |
CHEN M, JIANG S, HUANG C, et al. Honeycomb-like nitrogen and sulfur dual-doped hierarchical porous biomass-derived carbon for lithium-sulfur batteries. ChemSusChem, 2017, 10(8): 1803.
DOI PMID |
| [46] | DONG W, JI L, ZHAO M, et al. Nitrogen-doped nanotubes and few-layer montmorillonite composites as an effective polysulfides adsorbent for lithium-sulfur batteries. Diamond and Related Materials, 2023, 139: 110265. |
| [47] | WU L, YU Y, DAI Y, et al. Multisize CoS2 particles intercalated/ coated-montmorillonite as efficient sulfur host for high-performance lithium-sulfur batteries. ChemSusChem, 2022, 15(1):e202101991. |
| [48] | MOLAHALLI V, BHAT V S, SHETTY A, et al. ZnO doped SnO2 nano flower decorated on graphene oxide/polypyrrole nanotubes for symmetric supercapacitor applications. Journal of Energy Storage, 2023, 69: 107953. |
| [49] | ARVAS M B. Hydrothermal synthesis of polypyrrole/dye- functionalized carbon cloth electrode for wide potential window supercapacitor. Synthetic Metals, 2023, 293: 117275. |
| [50] | GE W, MA Q, AI Z, et al. Three-dimensional reduced graphene oxide/montmorillonite nanosheet aerogels as electrode material for supercapacitor application. Applied Clay Science, 2021, 206: 106022. |
| [51] | JIANG D, ZHENG M, YOU Y, et al. β-NiOH2/nickel-cobalt layered double hydroxides coupled with fluorine-modified graphene as high-capacitance supercapacitor electrodes with improved cycle life. Journal of Alloys and Compounds, 2021, 875: 159929. |
| [52] | XU G, WANG M, BAO H, et al. Design of Ni(OH)2/M-MMT nanocomposite with higher charge transport as a high capacity supercapacitor. Frontiers in Chemistry, 2022, 10: 916860. |
| [53] | LUO X F, HSU F Y, GAN Y H, et al. Intercalation of Fe-montmorillonite for developing nacre-inspired flexible all-solid- state supercapacitor with circular economy approach. Chinese Journal of Physics, 2023, 84: 405. |
| [54] | HAMIDOUCHE F, GHEBACHE Z, LEPRETRE J C, et al. Montmorillonite/poly(pyrrole) for low-cost supercapacitor electrode hybrid materials. Polymers, 2024, 16(7):919. |
| [55] | RATHNAYAKE D T, KARUNADASA K S, WIJEKOON A S, et al. Polyaniline-conjugated graphite-montmorillonite composite electrode prepared by in situ electropolymerization for supercapacitor applications. Chemical Papers, 2023, 77(5):2923. |
| [56] |
张小颂, 夏永高. 锂离子电池电解液的安全性研究进展. 储能科学与技术, 2018, 7(6):1016.
DOI |
| [57] | 肖琴, 岳勇, 任世杰. 锂离子电池用化学交联型凝胶聚合物电解质的研究进展. 功能高分子学报, 2019, 32(1):28. |
| [58] | AN Y, HAN X, LIU Y, et al. Progress in solid polymer electrolytes for lithium-ion batteries and beyond. Small, 2022, 18(3):2103617. |
| [59] | ZHU B, WANG D Z, YU W H. The study of structure and electrical properties of montmorillonite solid electrolyte. Solid State Ionics, 1989, 36(1/2):15. |
| [60] | RUIZ-HITZKY E, ARANDA P. Polymer-salt intercalation complexes in layer silicates. Advanced Materials, 1990, 2(11):545. |
| [61] | CHOUDHARY S, SENGWA R. Effect of different anions of lithium salt and MMT nanofiller on ion conduction in melt-compounded PEO-LiX-MMT electrolytes. Ionics, 2012, 18(4):379. |
| [62] | VAIA R A, VASUDEVAN S, KRAWIEC W, et al. New polymer electrolyte nanocomposites: melt intercalation of poly(ethylene oxide) in mica-type silicates. Advanced Materials, 1995, 7(2):154. |
| [63] | CHEN H W, CHANG F C. The novel polymer electrolyte nanocomposite composed of poly(ethylene oxide), lithium triflate and mineral clay. Polymer, 2001, 42(24):9763. |
| [64] | MORENO M, QUIJADA R, SANTA ANA M A, et al. Electrical and mechanical properties of poly(ethylene oxide)/intercalated clay polymer electrolyte. Electrochimica Acta, 2011, 58: 112. |
| [65] | NATH A K, SHARMA B, BORAH B J, et al. Structural and electrochemical properties of montmorillonite-poly(ethylene oxide) intercalated nanocomposites for lithium-ion batteries. International Journal of Polymer Analysis and Characterization, 2023, 28(3):279. |
| [66] | TIAN X, ZOU S, LV R, et al. Well-dispersed polydopamine in situ coated lithium montmorillonite nanofillers composite polyethylene oxide-based solid electrolyte for all-solid-state lithium batteries. Energy Technology, 2024, 12(4):2300980. |
| [67] | MA Y, LI L B, GAO G X, et al. Effect of montmorillonite on the ionic conductivity and electrochemical properties of a composite solid polymer electrolyte based on polyvinylidenedifluoride/ polyvinyl alcohol matrix for lithium ion batteries. Electrochimica Acta, 2016, 187: 535. |
| [68] | MASOUD E M. Montmorillonite incorporated polymethylmethacrylate matrix containing lithium trifluoromethanesulphonate (LTF) salt: thermally stable polymer nanocomposite electrolyte for lithium-ion batteries application. Ionics, 2019, 25(6):2645. |
| [69] | JEON Y M, KIM S, LEE M, et al. Polymer-clay nanocomposite solid-state electrolyte with selective cation transport boosting and retarded lithium dendrite formation. Advanced Energy Materials, 2020, 10(47):2003114. |
| [70] | LI L, SHAN Y, YANG X. New insights for constructing solid polymer electrolytes with ideal lithium-ion transfer channels by using inorganic filler. Materials Today Communications, 2021, 26: 101910. |
| [71] | ZHOU S, HAN Z, WANG X, et al. Low-cost and high-safety montmorillonite-based solid electrolyte for lithium metal batteries. Applied Clay Science, 2024, 251: 107329. |
| [72] | ZHU Y, ZHENG Y, LIU J, et al. Molecular coupling strategy achieving in situ synthesis of agglomeration-free solid composite electrolytes. The Journal of Physical Chemistry Letters, 2024, 15(3):733. |
| [73] | ZHAO Y, LI L, SHAN Y, et al. In situ construction channels of lithium-ion fast transport and uniform deposition to ensure safe high-performance solid batteries. Small, 2023, 19(39):e2301572. |
| [74] | WANG L, YI S, LIU Q, et al. Bifunctional lithium-montmorillonite enabling solid electrolyte with superhigh ionic conductivity for high-performanced lithium metal batteries. Energy Storage Materials, 2023, 63: 102961. |
| [75] | LI X, WANG Y, XI K, et al. Quasi-solid-state ion-conducting arrays composite electrolytes with fast ion transport vertical- aligned interfaces for all-weather practical lithium-metal batteries. Nano-Micro Letters, 2022, 14(1):210. |
| [76] | RILEY M, FEDKIW P S, KHAN S A. Transport properties of lithium hectorite-based composite electrolytes. Journal of the Electrochemical Society, 2002, 149(6):A667. |
| [77] | 胡拥军, 陈白珍, 李义兵. 基于OMMT/PVDF-HFP的锂离子电池用复合聚合物电解质. 复合材料学报, 2009, 26(6):54. |
| [78] | ZHANG Y G, ZHAO Y, BAKENOV Z, et al. Poly(vinylidene fluoride-co-hexafluoropropylene)/poly(methylmethacrylate)/nanoclay composite gel polymer electrolyte for lithium/sulfur batteries. Journal of Solid State Electrochemistry, 2014, 18(4):1111. |
| [79] | PORTHAULT H, CALBERG C, AMIRAN J, et al. Development of a thin flexible Li battery design with a new gel polymer electrolyte operating at room temperature. Journal of Power Sources, 2021, 482: 229055. |
| [80] | SAMUILOVA E O, SITNIKOVA V E, OLEKHNOVICH R O, et al. Studying the collapse of bentonite-containing composites based on acrylic copolymers. Russian Journal of Physical Chemistry A, 2018, 92(8):1602. |
| [81] | BASHIR S, HINA M, IQBAL J, et al. Self-healable poly(N, N-dimethylacrylamide)/poly(3, 4-ethylenedioxythiophene) polystyrene sulfonate composite hydrogel electrolytes for aqueous supercapacitors. Journal of Energy Storage, 2022, 45: 103760. |
| [82] | LIU Q, ZHAO A, HE X, et al. Full-temperature all-solid-state Ti3C2Tx/aramid fiber supercapacitor with optimal balance of capacitive performance and flexibility. Advanced Functional Materials, 2021, 31(22):2010944. |
| [83] | HU Q, SHI X, SUN K, et al. A super-stretchable and thermally stable hydrogel electrolyte for high performance supercapacitor with wide operation temperature. Journal of Alloys and Compounds, 2022, 909: 164646. |
| [84] | KAIBARTA B, DASMAHAPATRA A K. Carbon-based hierarchical mesoporous polyaniline/montmorillonite nanocomposites for high energy density supercapacitors. Journal of Energy Storage, 2024, 83: 110703. |
| [85] | XIA Y, MATHIS T S, ZHAO M Q, et al. Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes. Nature, 2018, 557(7705):409. |
| [86] | 张纳, 王飞, 宗传晖, 等. 聚合物电解质在电池中的应用及其研究进展. 山东理工大学学报(自然科学版), 2019, 33(5):14. |
| [87] | 付凤艳, 张杰, 程敬泉, 等. 氧化石墨烯在燃料电池质子交换膜中的应用. 化工进展, 2019, 38(5):2233. |
| [88] | DENG J, XIE J, ZHANG G, et al. Research progress of cross- linked fiber membranes for lithium-ion battery separators. Chemical Engineering Science, 2023, 280: 118970. |
| [89] | 王远铭, 史鑫然, 张信, 等. 锂离子电池隔膜改性研究进展. 高分子通报, 2023, 36(12):1634. |
| [90] | PARA M L, VERSACI D, AMICI J, et al. Synthesis and characterization of montmorillonite/polyaniline composites and its usage to modify a commercial separator. Journal of Electroanalytical Chemistry, 2021, 880: 114876. |
| [91] | FANG C J, YANG S L, ZHAO X F, et al. Electrospun montmorillonite modified poly(vinylidene fluoride) nanocomposite separators for lithium-ion batteries. Materials Research Bulletin, 2016, 79: 1. |
| [92] | KOH M J, HWANG H Y, KIM D J, et al. Preparation and characterization of porous PVdF-HFP/clay nanocomposite membranes. Journal of Materials Science & Technology, 2010, 26(7):633. |
| [93] | LI J, YU J, WANG Y, et al. Intercalated montmorillonite reinforced polyimide separator prepared by solution blow spinning for lithium-ion batteries. Industrial & Engineering Chemistry Research, 2020, 59(28):12879. |
| [94] | QIAO M, ZHANG G, DENG J, et al. Electrospun polyimide@organic-montmorillonite composite separator with enhanced mechanical and thermal performances for high-safety lithium-ion battery. Journal of Materials Science, 2022, 57(25):11796. |
| [95] | LI H, FENG T, LIANG Y, et al. Construction of PMIA@PAN/PVDF-HFP/TiO2 coaxial fibrous separator with enhanced mechanical strength and electrolyte affinity for lithium-ion batteries. Chinese Chemical Letters, 2023, 34(12):108350. |
| [96] | ZHANG Z, WANG J, QIN H, et al. Constructing an anion-braking separator to regulate local Li+ solvation structure for stabilizing lithium metal batteries. ACS Nano, 2024, 18(3):2250. |
| [97] | YANG M, JUE N, CHEN Y, et al. Improving cyclability of lithium metal anode via constructing atomic interlamellar ion channel for lithium sulfur battery. Nanoscale Research Letters, 2021, 16: 52. |
| [98] | AHN W, LIM S N, LEE D U, et al. Interaction mechanism between functionalized protection layer and dissolved polysulfide for extended cycle life of lithium sulfur batteries. Journal of Materials Chemistry A, 2015, 3(18):9461. |
| [99] | WANG Y, WU Y, MAO P, et al. A Keggin Al13-montmorillonite modified separator retards the polysulfide shuttling and accelerates Li-ion transfer in Li-S batteries. Small, 2023, 20(1):2304898. |
| [100] | ZHOU M X, ZHOU W H, LONG X, et al. A 2D montmorillonite- carbon nanotube interconnected porous network that prevents polysulfide shuttling. New Carbon Materials, 2023, 38(6):1070. |
| [101] | WANG W, XI K, LI B, et al. A sustainable multipurpose separator directed against the shuttle effect of polysulfides for high-performance lithium- sulfur batteries. Advanced Energy Materials, 2022, 12(19):2200160. |
| [102] | WU L, ZHAO Y, YU Y, et al. FeS2 intercalated montmorillonite as a multifunctional separator coating for high-performance lithium- sulfur batteries. Inorganic Chemistry Frontiers, 2023, 10(2):651. |
| [103] | WU L, ZHAO Y, DAI Y, et al. CoS2@montmorillonite as an efficient separator coating for high-performance lithium-sulfur batteries. Inorganic Chemistry Frontiers, 2022, 9(13):3335. |
| [104] | WANG H, LIANG C, LI Y, et al. A porous ceramic separator prepared from natural minerals: research on the mechanism of high liquid absorption and electrochemical properties of mineral material separator. Materials Chemistry and Physics, 2021, 272: 125032. |
| [105] | HONG L, WU X, MA C, et al. Boosting the Zn-ion transfer kinetics to stabilize the Zn metal interface for high-performance rechargeable Zn-ion batteries. Journal of Materials Chemistry A, 2021, 9(31):16814. |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 谭博文, 耿双龙, 张锴, 郑百林. 硅电极组分梯度设计抑制力-化学耦合劣化[J]. 无机材料学报, 2025, 40(7): 772-780. |
| [3] | 杨光, 张楠, 陈舒锦, 王义, 谢安, 严育杰. 基于多孔ITO电极的WO3薄膜的制备及其电致变色性能[J]. 无机材料学报, 2025, 40(7): 781-789. |
| [4] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [5] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [6] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [7] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [8] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [9] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [10] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [11] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [12] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [13] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [14] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [15] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||