无机材料学报 ›› 2025, Vol. 40 ›› Issue (5): 497-503.DOI: 10.15541/jim20240490 CSTR: 32189.14.jim20240490
所属专题: 【能源环境】储能电池(202506)
万俊池( ), 杜路路(
), 杜路路( ), 张永上, 李琳, 刘建德, 张林森(
), 张永上, 李琳, 刘建德, 张林森( )
)
收稿日期:2024-11-20
修回日期:2025-01-13
出版日期:2025-05-20
网络出版日期:2025-02-13
通讯作者:
杜路路, 讲师. E-mail: 2024007@zzuli.edu.cn;作者简介:万俊池(1995-), 男, 硕士研究生. E-mail: 875801788@qq.com
基金资助:
WAN Junchi( ), DU Lulu(
), DU Lulu( ), ZHANG Yongshang, LI Lin, LIU Jiande, ZHANG Linsen(
), ZHANG Yongshang, LI Lin, LIU Jiande, ZHANG Linsen( )
)
Received:2024-11-20
Revised:2025-01-13
Published:2025-05-20
Online:2025-02-13
Contact:
DU Lulu, lecturer. E-mail: 2024007@zzuli.edu.cn;About author:WAN Junchi (1995-), male, Master candidate. E-mail: 875801788@qq.com
Supported by:摘要:
开发低成本和长寿命的钠离子电池(SIBs)正极材料是实现大规模储能的关键。铁基磷酸盐正极材料具有高理论容量、良好的结构稳定性和丰富的储量, 近年来备受关注。本研究通过溶胶凝胶技术以及热处理过程, 制备了一系列Na4FexP4O12+x/C(x=2.6~3.3)电极材料, 探究了Na4FexP4O12+x/C电极材料的相结构对电化学性能的影响。研究发现Na4FexP4O12+x/C电极材料主要存在Na2FeP2O7(NFPO)相、Na4Fe3(PO4)2P2O7(NFPP)相以及NaFePO4(NFP)相。Na4Fe3.1P4O15.1/C电极材料中NFPP相的含量最高, 具有电子和钠离子传导快的特点, 表现出最佳的电化学性能。以Na4Fe3.1P4O15.1/C为正极的SIB表现出较高的可逆容量, 在0.1C(1C=129 mAh·g-1)电流密度下放电比容量达到102.8 mAh·g-1, 过700圈循环后容量保持率为88.7%。同时, 该电池具有出色的倍率性能, 在5C电流密度下放电比容量为61.5 mAh·g-1。
中图分类号:
万俊池, 杜路路, 张永上, 李琳, 刘建德, 张林森. Na4FexP4O12+x/C钠离子电池正极材料的结构演变及其电化学性能[J]. 无机材料学报, 2025, 40(5): 497-503.
WAN Junchi, DU Lulu, ZHANG Yongshang, LI Lin, LIU Jiande, ZHANG Linsen. Structural Evolution and Electrochemical Performance of Na4FexP4O12+x/C Cathode Materials for Sodium-ion Batteries[J]. Journal of Inorganic Materials, 2025, 40(5): 497-503.
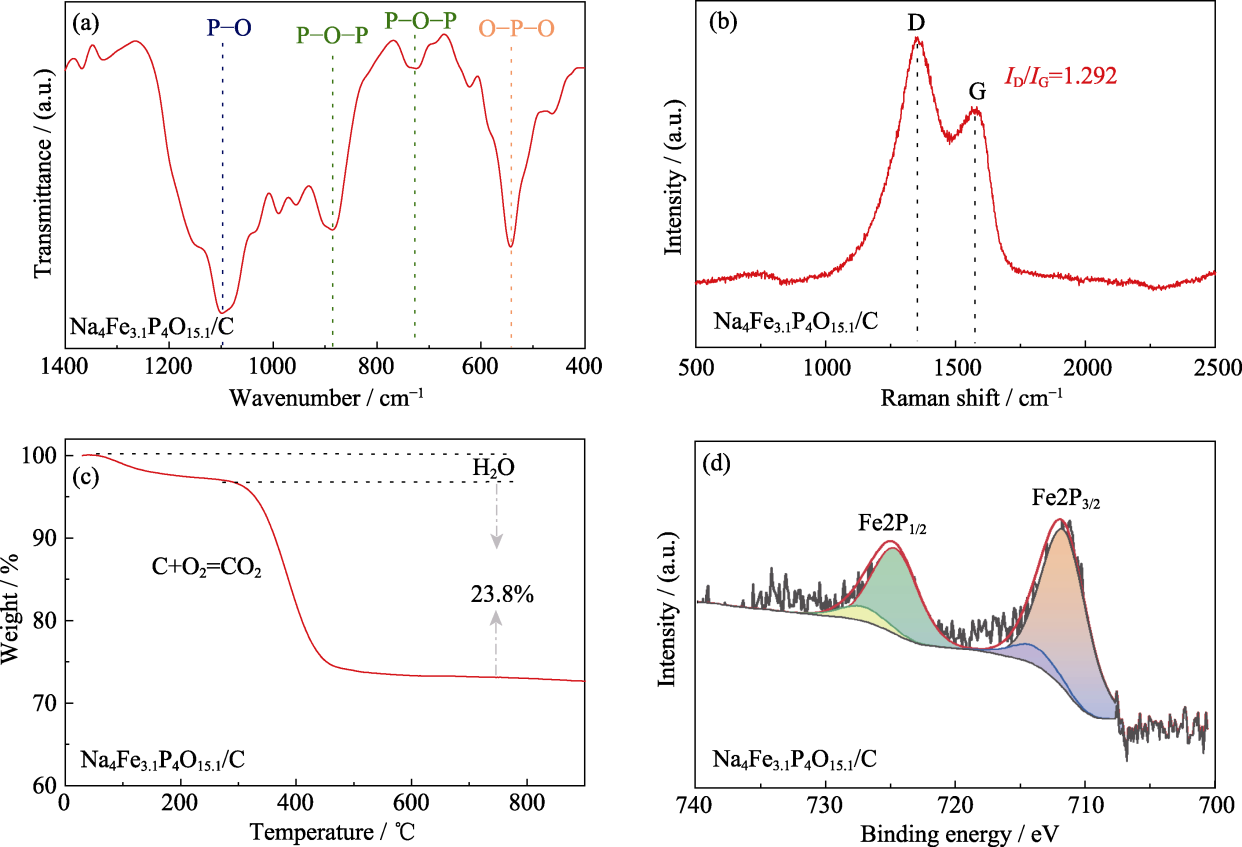
图2 Na4Fe3.1P4O15.1/C材料的(a) FT-IR谱图、(b) Raman光谱图、(c) TG曲线和(d) Fe2p XPS谱图
Fig. 2 (a) FT-IR spectrum, (b) Raman spectrum, (c) TG curve, and (d) Fe2p XPS spectrum of Na4Fe3.1P4O15.1/C material
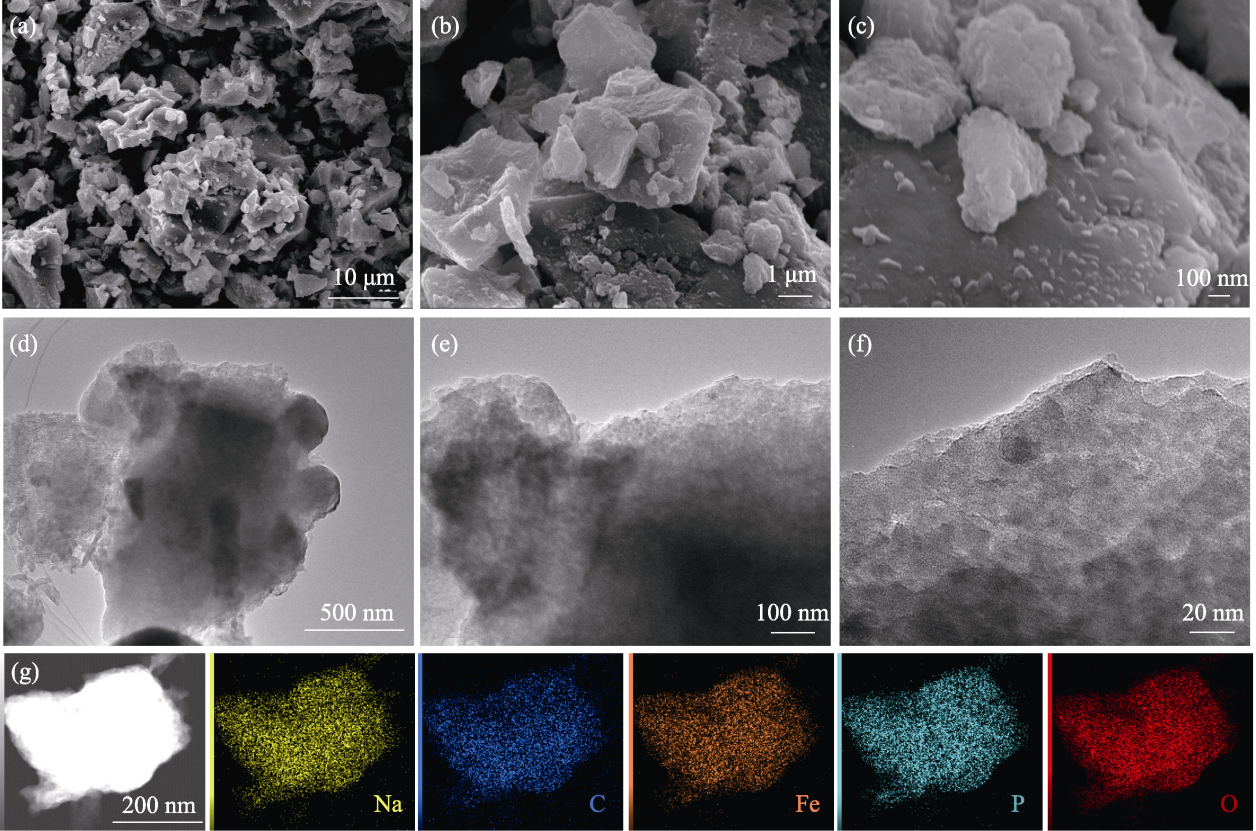
图3 Na4Fe3.1P4O15.1/C材料的(a~c) SEM照片、(d~f) TEM照片和(g) EDS元素分布图
Fig. 3 (a-c) SEM images, (d-f) TEM images and (g) EDS elemental mappings of Na4Fe3.1P4O15.1/C material
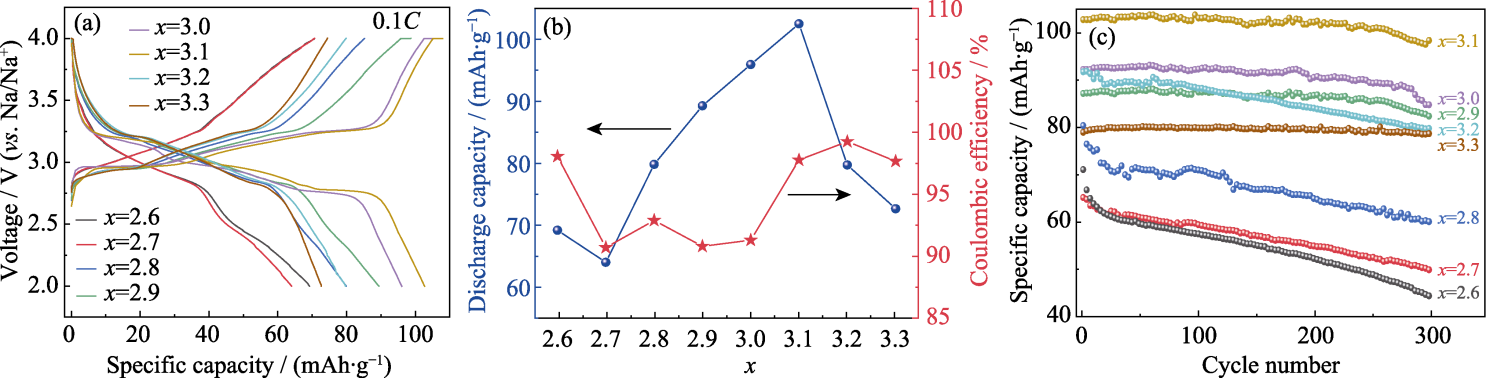
图5 Na4FexP4O12+x/C作为正极材料的SIBs的电化学性能
Fig. 5 Electrochemical performance of SIBs with Na4FexP4O12+x/C as cathodes (a) Initial charge-discharge curves; (b) Initial specific discharge capacities and Coulombic efficiencies; (c) Cycling test at 0.1C. Colorful figures are available on website
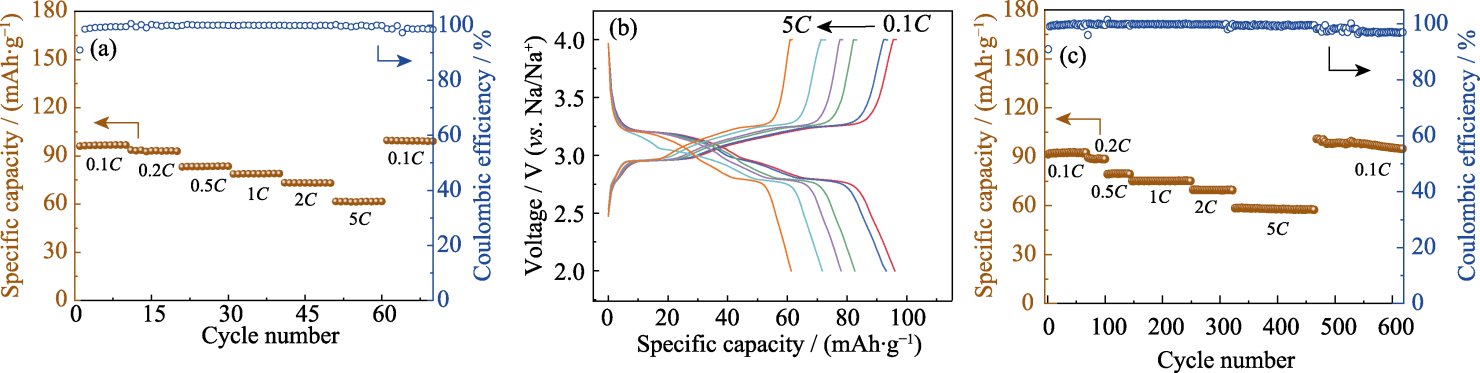
图6 Na4Fe3.1P4O15.1/C作为正极材料的SIBs的性能
Fig. 6 Performance of SIBs with Na4Fe3.1P4O15.1/C as cathodes (a) Rate performance; (b) Charge-discharge curves at different rates; (c) Cycling performance at different rates
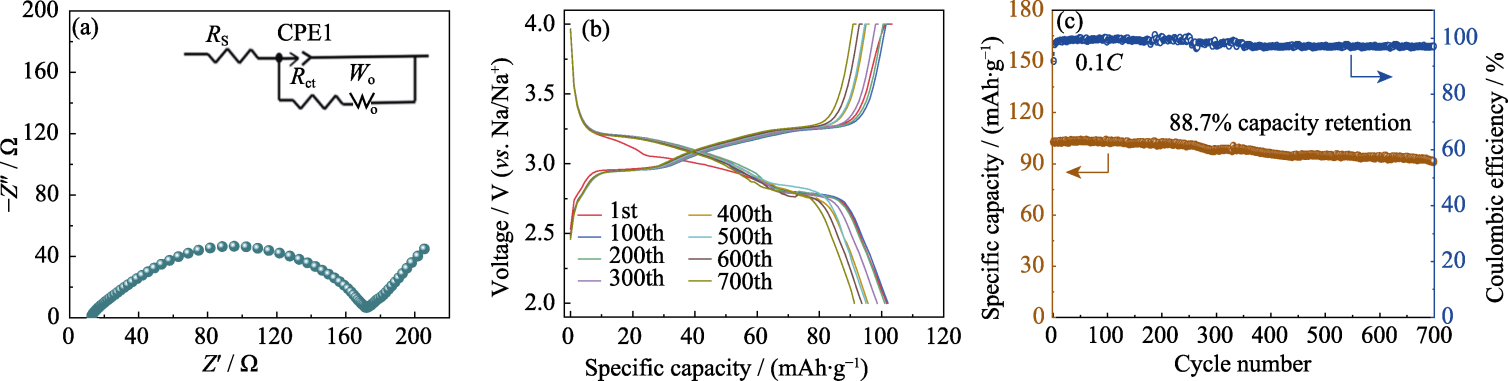
图7 Na4Fe3.1P4O15.1/C作为正极材料的SIBs的电化学性能
Fig. 7 Electrochemical performance of Na4Fe3.1P4O15.1/C as cathodes of SIBs (a) EIS plots; (b) Charge-discharge curves with different cycles at 0.1C; (c) Long-term cycling performance at 0.1C; Colorful figures are available on website
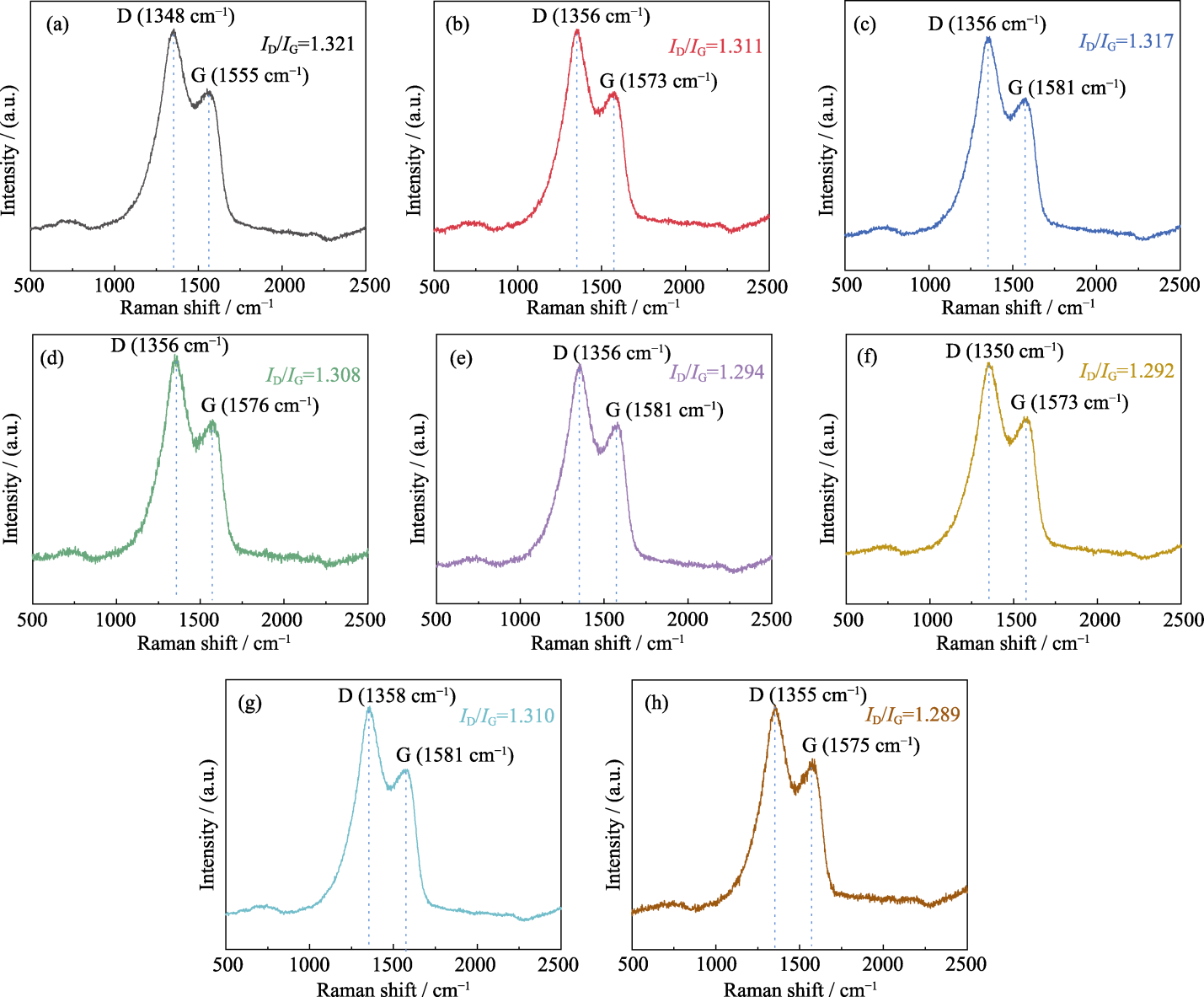
图S1 Na4FexP4O12+x/C材料的Raman光谱图
Fig. 8 S1 Raman spectra of Na4FexP4O12+x/C materials (a) x=2.6; (b) x=2.7; (c) x=2.8; (d) x=2.9; (e) x=3.0; (f) x=3.1; (g) x=3.2; (h) x=3.3

图S2 Na4FexP4O12+x/C材料的TG曲线
Fig. 9 S2 TG curves of Na4FexP4O12+x/C materials (a) x=2.6; (b) x=2.7; (c) x=2.8; (d) x=2.9; (e) x=3.0; (f) x=3.1; (g) x=3.2; (h) x=3.3
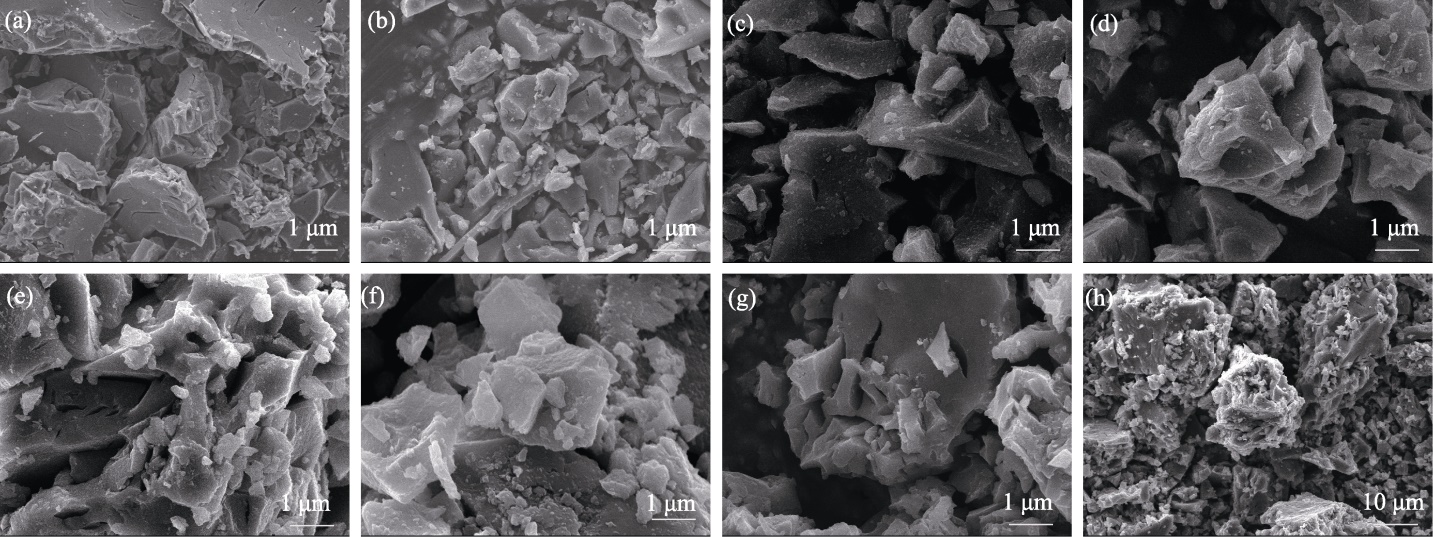
图S3 Na4FexP4O12+x/C材料的SEM照片
Fig. 10 S3 SEM images of Na4FexP4O12+x/C materials (a) x=2.6; (b) x=2.7; (c) x=2.8; (d) x=2.9; (e) x=3.0; (f) x=3.1; (g) x=3.2; (h) x=3.3
| Material | Initial discharge capacity/ (mAh·g-1) | (Reversible capacity/(mAh·g-1))/ cycle number | Capacity retention | Ref. |
|---|---|---|---|---|
| Na4Fe3.1P4O15.1/C | 102.8 | 101.2/200; 95.0/500 | 98.8%; 92.4% | This work |
| Na4Fe2.9Mn0.14(PO4)2(P2O7)@C | 94.5 | 92.1/100 | 97.4% | [S1] |
| Na4Fe3(PO4)2(P2O7)/C | 95.3 | 90/100 | 94.7% | [S2] |
| NaNi0.4Fe0.2Mn0.4O2 | 100 | 70/100 | 70% | [S3] |
| Na4Fe3(PO4)2(P2O7)@C/Ti3C2Tx | 93.4 | 91.1/200 | 97.5% | [S4] |
| Na3V2(PO4)3/C | 102 | 94/500 | 92.1% | [S5] |
| Na0.44MnO2/C | 114 | 74.1/1000 | 65% | [S6] |
表S1 本工作与文献报道的聚阴离子类钠离子电池正极材料的循环性能对比[S1-S6]
Table S1 Cycle performance comparison of polyanionic cathode materials of sodium-ion batteries in this work and literature[S1-S6]
| Material | Initial discharge capacity/ (mAh·g-1) | (Reversible capacity/(mAh·g-1))/ cycle number | Capacity retention | Ref. |
|---|---|---|---|---|
| Na4Fe3.1P4O15.1/C | 102.8 | 101.2/200; 95.0/500 | 98.8%; 92.4% | This work |
| Na4Fe2.9Mn0.14(PO4)2(P2O7)@C | 94.5 | 92.1/100 | 97.4% | [S1] |
| Na4Fe3(PO4)2(P2O7)/C | 95.3 | 90/100 | 94.7% | [S2] |
| NaNi0.4Fe0.2Mn0.4O2 | 100 | 70/100 | 70% | [S3] |
| Na4Fe3(PO4)2(P2O7)@C/Ti3C2Tx | 93.4 | 91.1/200 | 97.5% | [S4] |
| Na3V2(PO4)3/C | 102 | 94/500 | 92.1% | [S5] |
| Na0.44MnO2/C | 114 | 74.1/1000 | 65% | [S6] |
| [1] | DUNN B, KAMATH H, TARASCON J M. Electrical energy storage for the grid: a battery of choices. Science, 2011, 334(6058): 928. |
| [2] | ARMAND M, TARASCON J M. Building better batteries. Nature, 2008, 451(7179): 652. |
| [3] | YANG Z, ZHANG J, KINTNER-MEYER M C W, et al. Electrochemical energy storage for green grid. Chemical Reviews, 2011, 111(5): 3577. |
| [4] | CRABTREE G. Perspective: the energy-storage revolution. Nature, 2015, 526(7575): S92. |
| [5] | ABAS N, KALAIR A, KHAN N. Review of fossil fuels and future energy technologies. Futures, 2015, 69: 31. |
| [6] | ZU C X, LI H. Thermodynamic analysis on energy densities of batteries. Energy & Environmental Science, 2011, 4(8): 2614. |
| [7] | DE LA LLAVE E, BORGEL V, PARK K J, et al. Comparison between Na-ion and Li-ion cells: understanding the critical role of the cathodes stability and the anodes pretreatment on the cells behavior. ACS Applied Materials & Interfaces, 2016, 8(3): 1867. |
| [8] | HU M F, HUANG L P, LI H, et al. Research progress on hard carbon anode for Li/Na-ion batteries. Journal of Inorganic Materials, 2024, 39(1): 32. |
| [9] | HWANG J Y, MYUNG S T, SUN Y K. Sodium-ion batteries: present and future. Chemical Society Reviews, 2017, 46(12): 3529. |
| [10] | GUO Y J, JIN R X, FAN M, et al. Sodium layered oxide cathodes: properties, practicality and prospects. Chemical Society Reviews, 2024, 53(15): 7828. |
| [11] | SU H, JAFFER S, YU H. Transition metal oxides for sodium-ion batteries. Energy Storage Materials, 2016, 5: 116. |
| [12] | LAN Y, YAO W, HE X, et al. Mixed polyanionic compounds as positive electrodes for low-cost electrochemical energy storage. Angewandte Chemie International Edition, 2020, 59(24): 9255. |
| [13] | KOSOVA N V, SHINDROV A A. Mixed polyoxyanion cathode materials. Energy Storage Materials, 2021, 42: 570. |
| [14] | BARPANDA P. Pursuit of sustainable iron-based sodium battery cathodes: two case studies. Chemistry of Materials, 2016, 28(4): 1006. |
| [15] | NI Q, BAI Y, WU F, et al. Polyanion-type electrode materials for sodium-ion batteries. Advanced Science, 2017, 4(3): 1600275. |
| [16] | FERGUS J W. Recent developments in cathode materials for lithium ion batteries. Journal of Power Sources, 2010, 195(4): 939. |
| [17] | HE L, LI H, GE X, et al. Iron-phosphate-based cathode materials for cost-effective sodium-ion batteries: development, challenges, and prospects. Advanced Materials Interfaces, 2022, 9(20): 2200515. |
| [18] | LI H, XU M, LONG H, et al. Stabilization of multicationic redox chemistry in polyanionic cathode by increasing entropy. Advanced Science, 2022, 9(25):2202082. |
| [19] | AHSAN Z, CAI Z, WANG S, et al. Recent development of phosphate based polyanion cathode materials for sodium-ion batteries. Advanced Energy Materials, 2024, 14(27): 2400373. |
| [20] | SHI Y, JIANG P, WANG S, et al. Slight compositional variation- induced structural disorder-to-order transition enables fast Na+ storage in layered transition metal oxides. Nature Communications, 2022, 13: 7888. |
| [21] | WANG J, ZENG W, ZHU J, et al. Fe-rich pyrophosphate with prolonged high-voltage-plateaus and suppressed voltage decay as sodium-ion battery cathode. Nano Energy, 2023, 116: 108822. |
| [22] | ZHAO A, LIU C, JI F, et al. Revealing the phase evolution in Na4FexP4O12+x (2≤x≤4) cathode materials. ACS Energy Letters, 2023, 8(1): 753. |
| [23] | REN W, QIN M, ZHOU Y, et al. Electrospun Na4Fe3(PO4)2(P2O7) nanofibers as free-standing cathodes for ultralong-life and high-rate sodium-ion batteries. Energy Storage Materials, 2023, 54: 776. |
| [24] | SONG H J, KIM K H, KIM J C, et al. Superior sodium storage performance of reduced graphene oxide-supported Na3.12Fe2.44(P2O7)2/C nanocomposites. Chemical Communications, 2017, 53(67): 9316. |
| [25] | WANG J, XU S D, LU Z H, et al. Hollow-structured CoSe2/C anode materials: preparation and sodium storage properties for sodium-ion batteries. Journal of Inorganic Materials, 2022, 37(12): 1344. |
| [26] | YOU S, ZHANG Q, LIU J, et al. Hard carbon with an opened pore structure for enhanced sodium storage performance. Energy & Environmental Science, 2024, 17(21): 8189. |
| [27] | LIU Y, ZHANG N, WANG F, et al. Approaching the downsizing limit of maricite NaFePO4 toward high-performance cathode for sodium-ion batteries. Advanced Functional Materials, 2018, 28(30): 1801917. |
| [28] | ZHANG L M, HE X D, WANG S, et al. Hollow-sphere-structured Na4Fe3(PO4)2(P2O7)/C as a cathode material for sodium-ion batteries. ACS Applied Materials & Interfaces, 2021, 13(22): 25972. |
| [29] | WU X, ZHONG G, YANG Y. Sol-Gel synthesis of Na4Fe3(PO4)2(P2O7)/C nanocomposite for sodium ion batteries and new insights into microstructural evolution during sodium extraction. Journal of Power Sources, 2016, 327: 666. |
| [30] | KONG G Q, LENG M Z, ZHOU Z R, et al. Sb doped O3 type Na0.9Ni0.5Mn0.3Ti0.2O2 cathode material for Na-ion battery. Journal of Inorganic Materials, 2023, 38(6): 656. |
| [31] | YUAN T, WANG Y, ZHANG J, et al. 3D graphene decorated Na4Fe3(PO4)2(P2O7) microspheres as low-cost and high-performance cathode materials for sodium-ion batteries. Nano Energy, 2019, 56: 160. |
| [32] | PENG B, WAN G, AHMAD N, et al. Recent progress in the emerging modification strategies for layered oxide cathodes toward practicable sodium ion batteries. Advanced Energy Materials, 2023, 13(27): 2300334. |
| [33] | WANG C, LIU L, ZHAO S, et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium- ion battery. Nature Communications, 2021, 12: 2256. |
| [34] | LI M, QIU X, YIN Y, et al. O3-type Ni-Rich NaNi2/3Mn1/6Fe1/6O2: a high-performance cathode material for sodium-ion batteries. Journal of Alloys and Compounds, 2023, 969: 172406. |
| [1] | 闫共芹, 王晨, 蓝春波, 洪雨昕, 叶维超, 付向辉. Al掺杂P2型Na0.8Ni0.33Mn0.67-xAlxO2钠离子电池正极材料的制备与电化学性能[J]. 无机材料学报, 2025, 40(9): 1005-1012. |
| [2] | 薛柯, 蔡长焜, 谢满意, 李舒婷, 安胜利. 固体氧化物燃料电池Pr1+xBa1-xFe2O5+δ阴极材料的制备及电化学性能研究[J]. 无机材料学报, 2025, 40(4): 363-371. |
| [3] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [4] | 杨舒琪, 杨存国, 牛慧祝, 石唯一, 舒珂维. GeP3/科琴黑复合材料作为钠离子电池高性能负极材料[J]. 无机材料学报, 2025, 40(3): 329-336. |
| [5] | 朱志杰, 申明远, 吴涛, 李文翠. Cu和Mg协同取代抑制钠离子电池正极材料P2-Na2/3Ni1/3Mn2/3O2的P2-O2相变[J]. 无机材料学报, 2025, 40(2): 184-195. |
| [6] | 杨恒强, 张馨月, 马义初, 周青军. 铁基钙钛矿La0.25M0.75FeO3-δ (M=Ba, Sr, Ca)的制备及其作为固体氧化物燃料电池阴极材料的性能研究[J]. 无机材料学报, 2025, 40(12): 1365-1372. |
| [7] | 姜玥宏, 宋云峰, 张磊磊, 马季, 宋昭远, 龙文. 质子传导型固体氧化物燃料电池BaZr0.1Ce0.7Y0.1Yb0.1O3电解质的氟化研究[J]. 无机材料学报, 2025, 40(12): 1356-1364. |
| [8] | 凌意瀚, 郭胜, 曹志强, 田云峰, 刘方升, 金芳军, 高源. 固体氧化物电池直孔电极结构的制备技术与性能研究进展[J]. 无机材料学报, 2025, 40(12): 1311-1323. |
| [9] | 张宇婷, 李晓斌, 刘尊义, 李宁, 赵鹬. 复合蛋黄壳型NiCo2V2O8@TiO2@NC材料用作锂离子电池负极研究[J]. 无机材料学报, 2025, 40(11): 1221-1228. |
| [10] | 唐阳, 刘立敏, 周晓亮, 张搏, 蒋星洲, 贾浩义, 罗延麟庆. 质子陶瓷膜反应器的制备及低温氨分解性能研究[J]. 无机材料学报, 2025, 40(11): 1277-1284. |
| [11] | 王琨鹏, 刘兆林, 林存生, 王治宇. 基于低含水量普鲁士蓝正极的准固态钠离子电池[J]. 无机材料学报, 2024, 39(9): 1005-1012. |
| [12] | 陈正鹏, 金芳军, 李明飞, 董江波, 许仁辞, 徐韩昭, 熊凯, 饶睦敏, 陈创庭, 李晓伟, 凌意瀚. 双钙钛矿Sr2CoFeO5+δ阴极材料的制备及其中温固体氧化物燃料电池性能研究[J]. 无机材料学报, 2024, 39(3): 337-344. |
| [13] | 孔剑锋, 黄杰成, 刘兆林, 林存生, 王治宇. 基于DPEPA聚合物凝胶电解质的准固态钠离子电池[J]. 无机材料学报, 2024, 39(12): 1331-1338. |
| [14] | 周靖渝, 李兴宇, 赵晓琳, 王有伟, 宋二红, 刘建军. Ti和Cu掺杂β-NaMnO2正极材料:钠离子电池的倍率和循环性能[J]. 无机材料学报, 2024, 39(12): 1404-1412. |
| [15] | 胡梦菲, 黄丽萍, 李贺, 张国军, 吴厚政. 锂/钠离子电池硬碳负极材料的研究进展[J]. 无机材料学报, 2024, 39(1): 32-44. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||