无机材料学报 ›› 2022, Vol. 37 ›› Issue (9): 991-1000.DOI: 10.15541/jim20210638 CSTR: 32189.14.10.15541/jim20210638
王红宁1( ), 黄丽1, 清江3, 马腾洲3(
), 黄丽1, 清江3, 马腾洲3( ), 黄维秋2, 陈若愚1(
), 黄维秋2, 陈若愚1( )
)
收稿日期:2021-10-18
修回日期:2022-02-18
出版日期:2022-09-20
网络出版日期:2022-03-15
通讯作者:
陈若愚, 教授. E-mail: chry@cczu.edu.cn;作者简介:王红宁(1980-), 女, 博士研究生. E-mail: 444873772@qq.com
基金资助:
WANG Hongning1( ), HUANG Li1, QING Jiang3, MA Tengzhou3(
), HUANG Li1, QING Jiang3, MA Tengzhou3( ), HUANG Weiqiu2, CHEN Ruoyu1(
), HUANG Weiqiu2, CHEN Ruoyu1( )
)
Received:2021-10-18
Revised:2022-02-18
Published:2022-09-20
Online:2022-03-15
Contact:
CHEN Ruoyu, professor. E-mail: chry@cczu.edu.cn;About author:WANG Hongning (1980-), female, PhD candidate. E-mail: 444873772@qq.com
Supported by:摘要:
介孔有机-无机复合氧化硅空心球(MOSs)在碱性条件下以反向胶束为模板经过正硅酸乙酯(TEOS)和1,2-双(三乙氧基甲硅烷基)乙烷(BTSE)共缩合被成功合成, 并通过不同手段对样品的结构和性能进行表征。MOSs用于去除挥发性有机物(VOCs), 研究其对水蒸气、正己烷、甲苯和92#汽油的静态吸附性能, 并以商业硅胶(SG)和活性炭(AC)为参考。实验结果发现, 初始BTSE/(BTSE+TEOS) 摩尔比为10%时, (MOS-10%)的样品具有均匀的中空介观结构和最大的VOCs吸附容量(1.28 g·g-1正己烷, 1.25 g·g-1甲苯和1.14 g·g-1 92#汽油), 静态水蒸气吸附量最小(0.630 g·g-1)。通过穿透曲线评估单一组分VOC(正己烷或甲苯)在MOS-10%上的动态吸附行为, 动态正己烷和甲苯吸附结果以及高湿度条件下的正己烷吸附性能表明, 与商业吸附剂相比, MOS-10%具有最佳的穿透时间、吸附能力和疏水性。对于二元组分同时吸附(正己烷和甲苯), MOS-10%的正己烷吸附性能优于甲苯。介孔有机-无机复合氧化硅空心球的动态VOCs吸附容量较大归因于有机基团、表面积和孔体积的共同作用。MOSs的VOCs去除能力强和可回收性优良, 显示出巨大的VOCs捕获潜力。
中图分类号:
王红宁, 黄丽, 清江, 马腾洲, 黄维秋, 陈若愚. 有机-无机氧化硅空心球的合成及VOCs吸附应用[J]. 无机材料学报, 2022, 37(9): 991-1000.
WANG Hongning, HUANG Li, QING Jiang, MA Tengzhou, HUANG Weiqiu, CHEN Ruoyu. Mesoporous Organic-inorganic Hybrid Siliceous Hollow Spheres: Synthesis and VOCs Adsorption[J]. Journal of Inorganic Materials, 2022, 37(9): 991-1000.
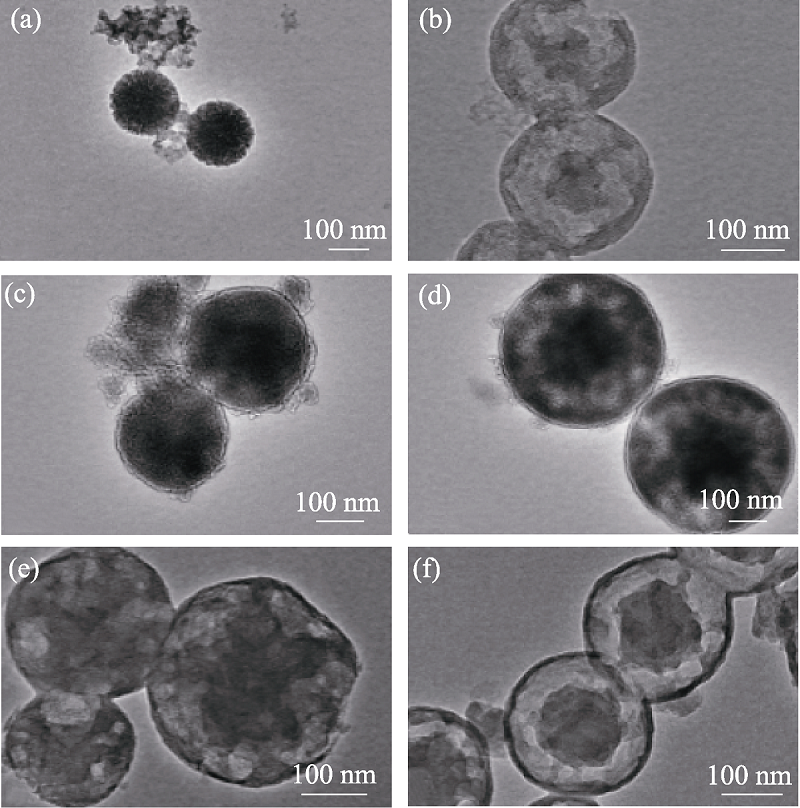
图1 不同有机硅源量MOSs的TEM照片
Fig. 1 TEM images of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (a) MOS-0; (b) MOS-5%; (c) MOS-7.5%; (d) MOS-10%; (e) MOS-12.5%; (f) MOS-15%
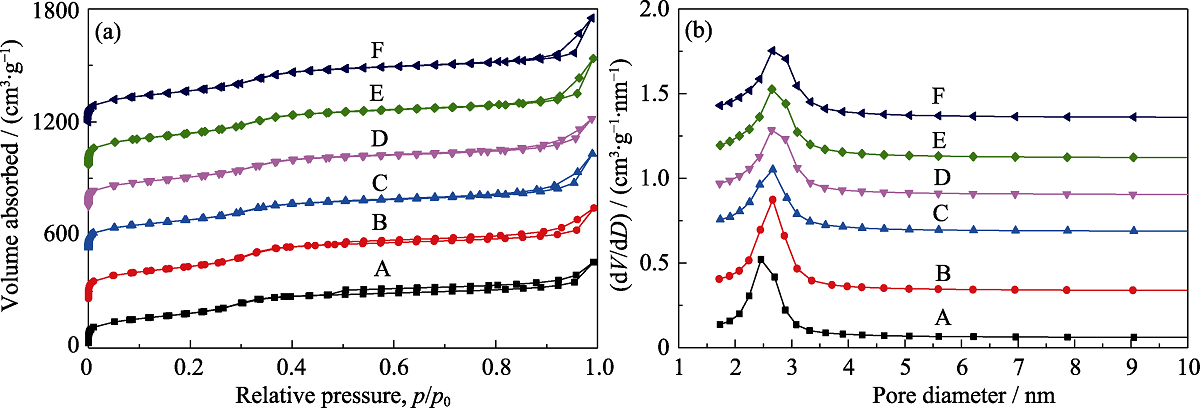
图2 不同样品的N2吸附脱附等温线(a)和孔径分布图(b)
Fig. 2 N2 sorption isotherms (a) and pore size distributions (b) of MOSs with different initial BTSE/(BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E)MOS-12.5%; (F) MOS-15%. In (a), the Y-axis values of (B-F) are 300, 600, 800, 1000, and 1300 m2·g-1, respectively. In (b), the Y-axis values of (B-F) are 0.1, 0.4, 0.8, 1.0, 1.2, and 1.4 cm3·g-1, respectively
| Sample | SBET/ (m2·g-1) | Sm/ (m2·g-1) | Vt/ (cm3·g-1) | Vm/ (cm3·g-1) | Pore size/ nm |
|---|---|---|---|---|---|
| MOS-0 | 591 | 0 | 0.721 | 0 | 2.5 |
| MOS-5% | 612 | 0 | 0.722 | 0 | 2.7 |
| MOS-7.5% | 612 | 0 | 0.776 | 0 | 2.8 |
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.6 |
| MOS-12.5% | 655 | 0 | 0.873 | 0 | 2.7 |
| MOS-15% | 648 | 0 | 0.857 | 0 | 2.7 |
表1 不同有机硅源量MOSs样品的结构参数
Table 1 Structural parameters of MOSs with different initial BTSE/(BTSE+TEOS) molar ratios
| Sample | SBET/ (m2·g-1) | Sm/ (m2·g-1) | Vt/ (cm3·g-1) | Vm/ (cm3·g-1) | Pore size/ nm |
|---|---|---|---|---|---|
| MOS-0 | 591 | 0 | 0.721 | 0 | 2.5 |
| MOS-5% | 612 | 0 | 0.722 | 0 | 2.7 |
| MOS-7.5% | 612 | 0 | 0.776 | 0 | 2.8 |
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.6 |
| MOS-12.5% | 655 | 0 | 0.873 | 0 | 2.7 |
| MOS-15% | 648 | 0 | 0.857 | 0 | 2.7 |
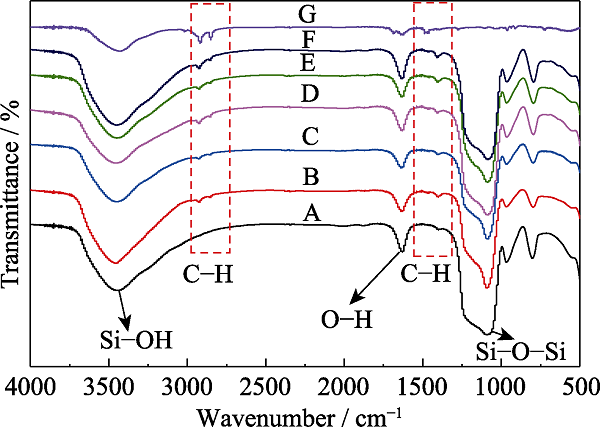
图3 不同有机硅源量MOSs的红外光谱图
Fig. 3 FT-IR spectra of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%; (G) CTAB
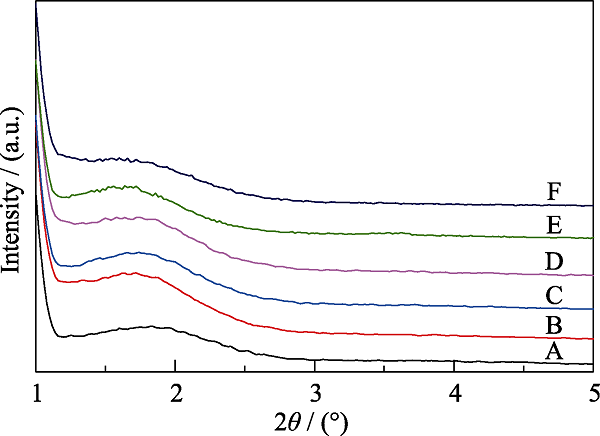
图4 不同有机硅源量MOSs的XRD图谱
Fig. 4 XRD patterns of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%
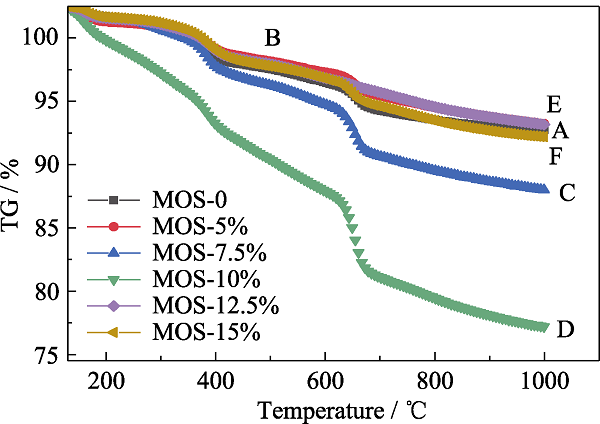
图5 不同有机硅源量MOSs的热重曲线
Fig. 5 TGA curves of MOSs with different initial BTSE/ (BTSE+TEOS) molar ratios (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%;

图6 不同样品对正己烷、甲苯和汽油的静态吸附容量(a, c, e)和解吸效率(b, d, f)
Fig. 6 Histograms of static VOCs (n-hexane, toluene and 92# gasoline) adsorption capacities (a, c, e) and desorption efficiencies (b, d, f) of different samples (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS-12.5%; (F) MOS-15%; (G) SG; (H) AC
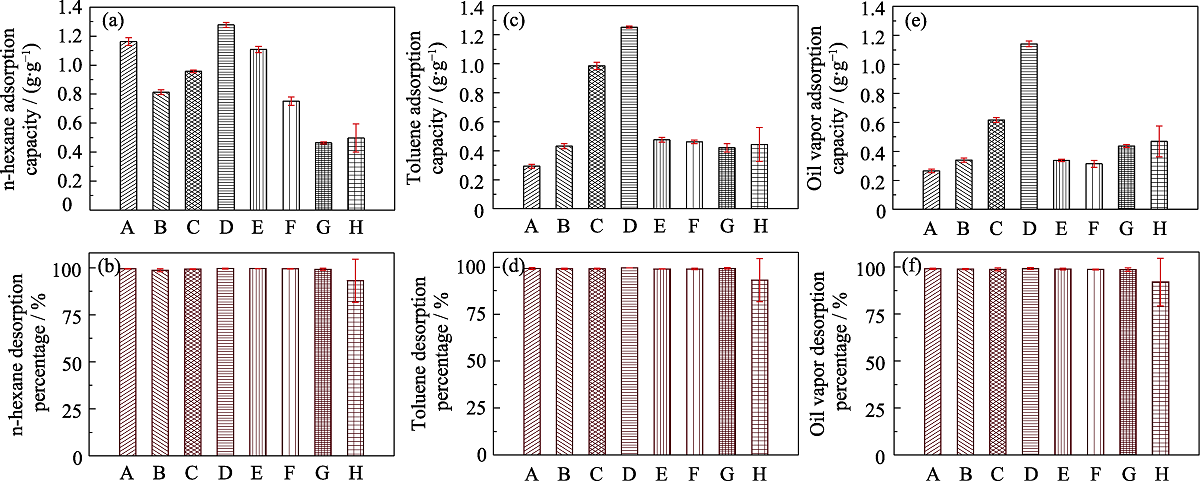
图6 不同样品对正己烷、甲苯和汽油的静态吸附容量(a, c, e)和解吸效率(b, d, f)
Fig. 6 Histograms of static VOCs (n-hexane, toluene and 92# gasoline) adsorption capacities (a, c, e) and desorption efficiencies (b, d, f) of different samples (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS-12.5%; (F) MOS-15%; (G) SG; (H) AC
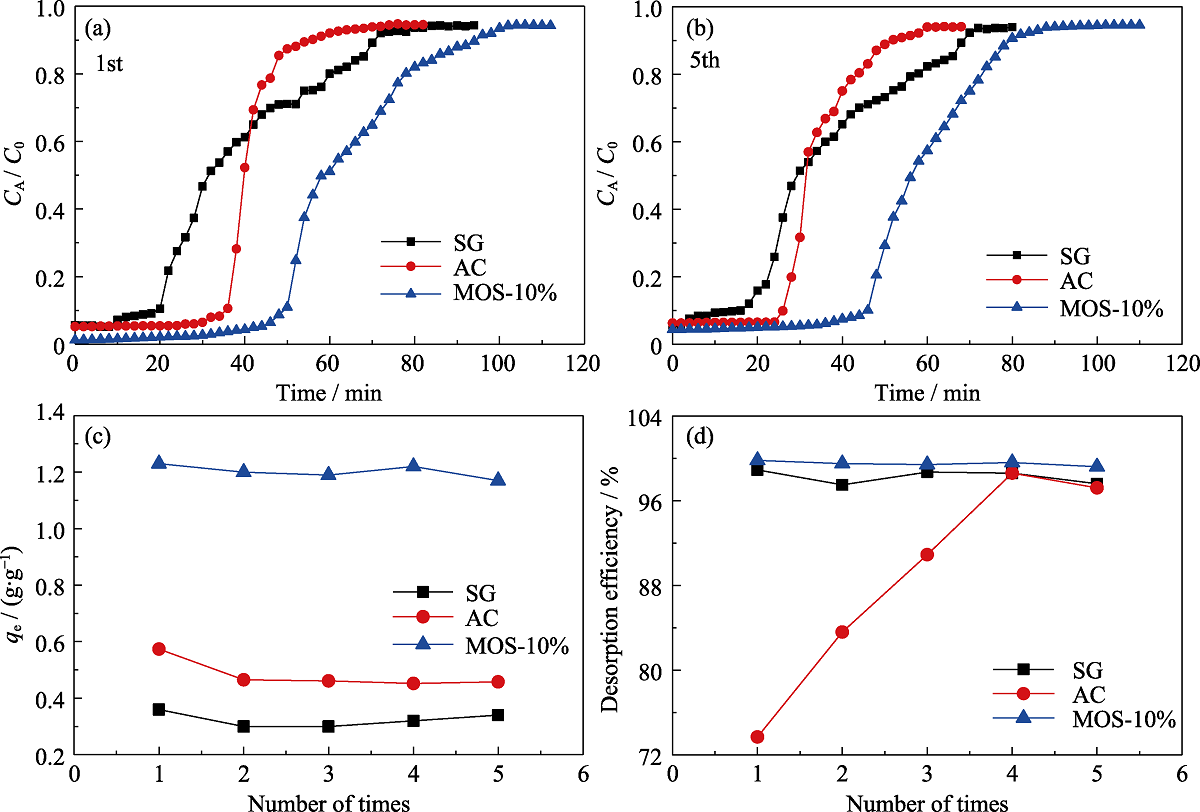
图8 SG(■), AC(●)和MOS-10%(▲)的正己烷穿透曲线(第一次(a), 第五次(b)), 五次的平衡吸附容量的比较(c)和五次解吸效率的比较(d)
Fig. 8 Breakthrough curves for n-hexane of SG (■), AC (●) and MOS-10% (▲) under dry condition for the (a) first time and (b) fifth time and comparison of the qe (c) and desorption efficiency (d) of 5 times
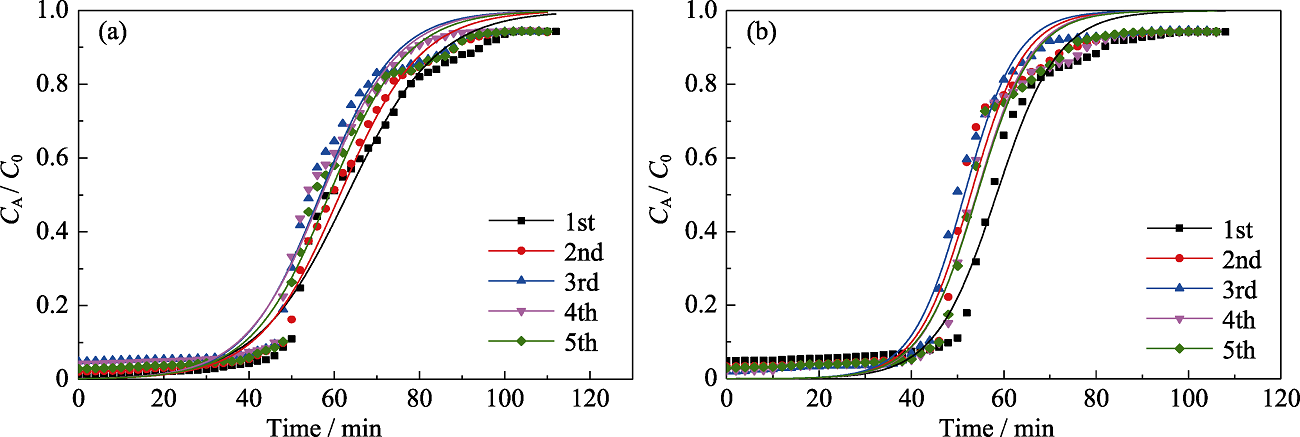
图10 MOS-10%五次干燥条件下正己烷(a)和甲苯(b)动态吸附的Yoon and Nelson模拟
Fig. 10 Yoon and Nelson model fitting for 5 times adsorption of n-hexane (a) and toluene (b) on MOS-10% under dry condition Colorful figures are available on website
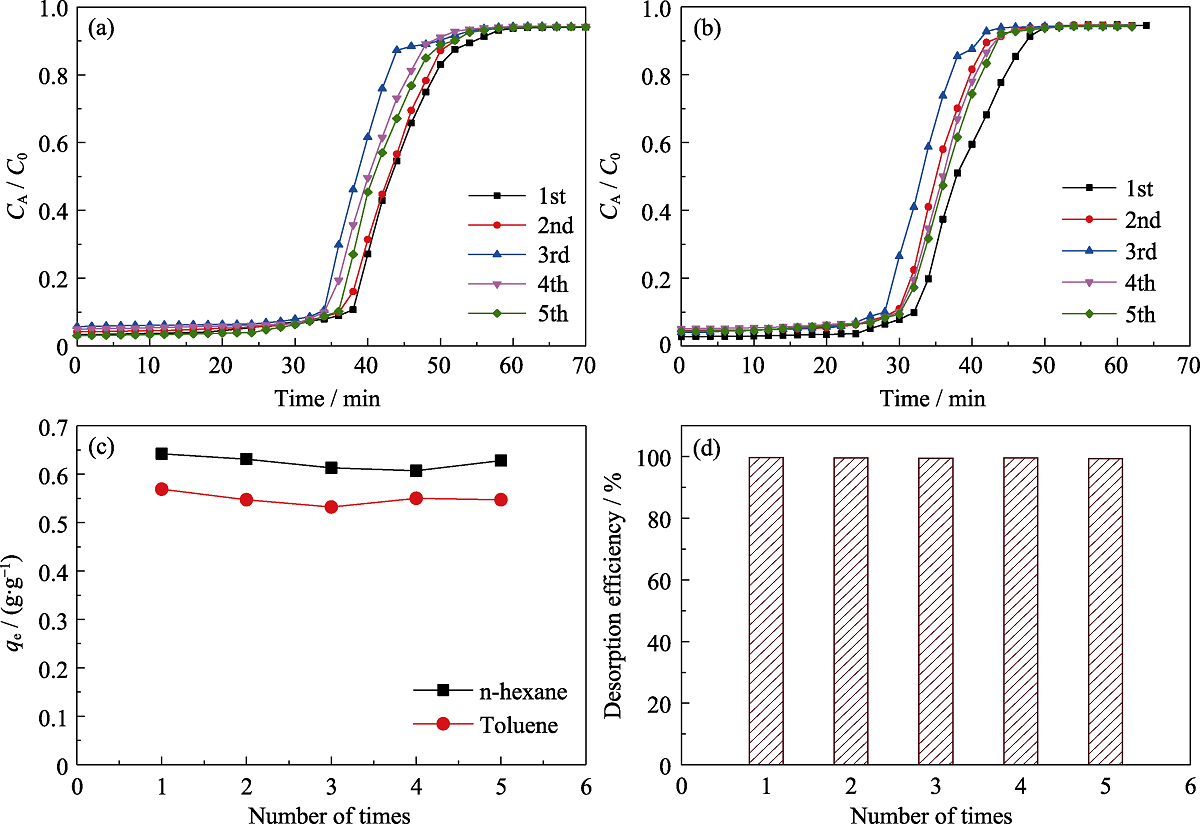
图12 干燥条件下MOS-10%上正己烷与甲苯五次动态同时吸附的穿透曲线正己烷(a)和甲苯(b), 以及五次平衡吸附容量的比较(c)和五次解吸效率的比较(d)
Fig. 12 Simultaneous breakthrough adsorption n-hexane (a) and toluene (b) on MOS-10% under dry condition, comparison of the equilibrium adsorption capacities (c) and desorption efficiency (d) for 5 times, respectively
| Sample | SBET/(m2 ·g-1) | Sm/(m2 ·g-1) | Vt/(cm3 ·g-1) | Vm/(cm3 ·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| AC | 1451 | 973 | 1.03 | 0.48 | 5.6 |
| SG | 430 | 15 | 0.710 | 0.010 | 6.9 |
表S1 商业化AC、SG的结构参数
Table S1 Structural parameters of AC and SG
| Sample | SBET/(m2 ·g-1) | Sm/(m2 ·g-1) | Vt/(cm3 ·g-1) | Vm/(cm3 ·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| AC | 1451 | 973 | 1.03 | 0.48 | 5.6 |
| SG | 430 | 15 | 0.710 | 0.010 | 6.9 |
| Sample | First event | Second event | Residual mass /% | ||
|---|---|---|---|---|---|
| (Tons-Tf )/℃ | Δm/% | (Tons-Tf )/℃ | Δm/% | ||
| MOS-0 | 30-200 | 1.7 | 200-900 | 8.1 | 90.2 |
| MOS-5% | 30-200 | 1.7 | 200-900 | 7.6 | 90.7 |
| MOS-7.5% | 30-200 | 1.9 | 200-900 | 12.6 | 85.5 |
| MOS-10% | 30-200 | 5.3 | 200-900 | 20.1 | 74.6 |
| MOS-12.5% | 30-200 | 1.5 | 200-900 | 7.9 | 90.6 |
| MOS-15% | 30-200 | 1.4 | 200-900 | 9.0 | 89.6 |
表S2 不同有机硅源量MOSs在30~900 ℃, 氮气气氛(10 ℃/min)下获得的热重结果
Table S2 TGA results of different MOSs under nitrogen atmosphere in the range of 30-900 ℃ (10 ℃/min)
| Sample | First event | Second event | Residual mass /% | ||
|---|---|---|---|---|---|
| (Tons-Tf )/℃ | Δm/% | (Tons-Tf )/℃ | Δm/% | ||
| MOS-0 | 30-200 | 1.7 | 200-900 | 8.1 | 90.2 |
| MOS-5% | 30-200 | 1.7 | 200-900 | 7.6 | 90.7 |
| MOS-7.5% | 30-200 | 1.9 | 200-900 | 12.6 | 85.5 |
| MOS-10% | 30-200 | 5.3 | 200-900 | 20.1 | 74.6 |
| MOS-12.5% | 30-200 | 1.5 | 200-900 | 7.9 | 90.6 |
| MOS-15% | 30-200 | 1.4 | 200-900 | 9.0 | 89.6 |
| Sample | Adsorption | Desorption | -OH/(×1020, g-1) | ||
|---|---|---|---|---|---|
| Average/(g·g-1) | STDEa/% | Average/% | STDEa/% | ||
| MOS-0% | 0.903 | 0.0168 | 99.4 | 0.205 | 3.11 |
| MOS-5% | 0.667 | 0.0177 | 99.3 | 0.329 | 2.35 |
| MOS-7.5% | 0.656 | 0.0165 | 99.4 | 0.283 | 2.31 |
| MOS-10% | 0.630 | 0.0137 | 99.4 | 0.134 | 2.23 |
| MOS-12.5% | 0.697 | 0.0156 | 99.4 | 0.230 | 2.44 |
| MOS-15% | 0.697 | 0.0136 | 99.4 | 0.365 | 2.45 |
| SG | 0.433 | 0.0984 | 97.7 | 0.867 | 1.72 |
| AC | 0.482 | 0.0305 | 93.5 | 11.1 | 1.77 |
表S3 不同样品的水蒸气吸附容量、解吸百分率和表面羟基含量
Table S3 Water vapor adsorption capacities, desorption efficiencies and the densities of surface hydroxyl groups of different samples
| Sample | Adsorption | Desorption | -OH/(×1020, g-1) | ||
|---|---|---|---|---|---|
| Average/(g·g-1) | STDEa/% | Average/% | STDEa/% | ||
| MOS-0% | 0.903 | 0.0168 | 99.4 | 0.205 | 3.11 |
| MOS-5% | 0.667 | 0.0177 | 99.3 | 0.329 | 2.35 |
| MOS-7.5% | 0.656 | 0.0165 | 99.4 | 0.283 | 2.31 |
| MOS-10% | 0.630 | 0.0137 | 99.4 | 0.134 | 2.23 |
| MOS-12.5% | 0.697 | 0.0156 | 99.4 | 0.230 | 2.44 |
| MOS-15% | 0.697 | 0.0136 | 99.4 | 0.365 | 2.45 |
| SG | 0.433 | 0.0984 | 97.7 | 0.867 | 1.72 |
| AC | 0.482 | 0.0305 | 93.5 | 11.1 | 1.77 |
| Sample | tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% |
|---|---|---|---|---|
| MOS-10%-1st | 50 | 104 | 1.23 | 99.8 |
| MOS-10%-2nd | 48 | 102 | 1.20 | 99.5 |
| MOS-10%-3rd | 46 | 100 | 1.19 | 99.4 |
| MOS-10%-4th | 48 | 102 | 1.22 | 99.6 |
| MOS-10%-5th | 48 | 102 | 1.21 | 99.2 |
| SG-1st | 16 | 72 | 0.361 | 98.9 |
| SG-2nd | 14 | 70 | 0.321 | 97.5 |
| SG-3rd | 14 | 70 | 0.334 | 98.7 |
| SG-4th | 12 | 72 | 0.321 | 98.6 |
| SG-5th | 10 | 72 | 0.343 | 97.6 |
| AC-1st | 38 | 50 | 0.574 | 73.7 |
| AC-2nd | 28 | 44 | 0.465 | 83.6 |
| AC-3rd | 28 | 42 | 0.461 | 90.9 |
| AC-4th | 26 | 44 | 0.452 | 98.6 |
| AC-5th | 24 | 42 | 0.458 | 97.2 |
表S4 干燥条件下, 不同样品对正己烷吸附的五次动态吸附参数比较
Table S4 Comparison of dynamic n-hexane adsorption parameters on different samples for 5 times under dry condition
| Sample | tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% |
|---|---|---|---|---|
| MOS-10%-1st | 50 | 104 | 1.23 | 99.8 |
| MOS-10%-2nd | 48 | 102 | 1.20 | 99.5 |
| MOS-10%-3rd | 46 | 100 | 1.19 | 99.4 |
| MOS-10%-4th | 48 | 102 | 1.22 | 99.6 |
| MOS-10%-5th | 48 | 102 | 1.21 | 99.2 |
| SG-1st | 16 | 72 | 0.361 | 98.9 |
| SG-2nd | 14 | 70 | 0.321 | 97.5 |
| SG-3rd | 14 | 70 | 0.334 | 98.7 |
| SG-4th | 12 | 72 | 0.321 | 98.6 |
| SG-5th | 10 | 72 | 0.343 | 97.6 |
| AC-1st | 38 | 50 | 0.574 | 73.7 |
| AC-2nd | 28 | 44 | 0.465 | 83.6 |
| AC-3rd | 28 | 42 | 0.461 | 90.9 |
| AC-4th | 26 | 44 | 0.452 | 98.6 |
| AC-5th | 24 | 42 | 0.458 | 97.2 |
| tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% | |
|---|---|---|---|---|
| 1st | 48 | 100 | 1.21 | 99.7 |
| 2nd | 46 | 98 | 1.18 | 99.5 |
| 3rd | 44 | 96 | 1.17 | 99.3 |
| 4th | 46 | 98 | 1.18 | 99.4 |
| 5th | 46 | 98 | 1.18 | 99.1 |
表S5 干燥条件下, MOS-10%的五次甲苯动态吸附参数比较
Table S5 Comparison of dynamic toluene adsorption parameters on MOS-10% for 5 times under dry condition
| tb/min | te/min | qe/(g·g-1) | Desorption efficiency/% | |
|---|---|---|---|---|
| 1st | 48 | 100 | 1.21 | 99.7 |
| 2nd | 46 | 98 | 1.18 | 99.5 |
| 3rd | 44 | 96 | 1.17 | 99.3 |
| 4th | 46 | 98 | 1.18 | 99.4 |
| 5th | 46 | 98 | 1.18 | 99.1 |
| Sample | SBET/(m2·g-1) | Sm/(m2·g-1) | Vt/(cm3·g-1) | Vm/(cm3·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.64 |
| MOS-10%-5th | 638 | 0 | 0.830 | 0 | 2.64 |
| AC | 1654 | 652 | 1.06 | 0.48 | 5.58 |
| AC-5th | 1310 | 395 | 0.880 | 0.38 | 5.51 |
表S6 在干燥条件下MOS-10%和AC吸附前和五次动态吸附正己烷后的孔结构参数
Table S6 Structural parameters of MOS-10% and AC before and after 5 times dynamic n-hexane adsorption under dry condition
| Sample | SBET/(m2·g-1) | Sm/(m2·g-1) | Vt/(cm3·g-1) | Vm/(cm3·g-1) | Pore size/nm |
|---|---|---|---|---|---|
| MOS-10% | 696 | 0 | 0.887 | 0 | 2.64 |
| MOS-10%-5th | 638 | 0 | 0.830 | 0 | 2.64 |
| AC | 1654 | 652 | 1.06 | 0.48 | 5.58 |
| AC-5th | 1310 | 395 | 0.880 | 0.38 | 5.51 |
| n-hexane | Toluene | |||||
|---|---|---|---|---|---|---|
| τ0/min | K׳/min-1 | R2 | τ0/min | K׳/min-1 | R2 | |
| 1st | 63.1 | 0.150 | 0.984 | 58.4 | 0.0948 | 0.979 |
| 2nd | 61.3 | 0.172 | 0.990 | 52.8 | 0.105 | 0.977 |
| 3rd | 56.7 | 0.174 | 0.979 | 51.5 | 0.114 | 0.987 |
| 4th | 57.1 | 0.169 | 0.986 | 54.1 | 0.111 | 0.978 |
| 5th | 58.9 | 0.165 | 0.984 | 54.2 | 0.110 | 0.982 |
表S7 MOS-10%s在干燥条件下, 五次单组份正己烷和甲苯动态吸附的拟合参数
Table S7 Simulation parameters of 5 times dynamic n-hexane and toluene adsorption on MOS-10% under dry condition
| n-hexane | Toluene | |||||
|---|---|---|---|---|---|---|
| τ0/min | K׳/min-1 | R2 | τ0/min | K׳/min-1 | R2 | |
| 1st | 63.1 | 0.150 | 0.984 | 58.4 | 0.0948 | 0.979 |
| 2nd | 61.3 | 0.172 | 0.990 | 52.8 | 0.105 | 0.977 |
| 3rd | 56.7 | 0.174 | 0.979 | 51.5 | 0.114 | 0.987 |
| 4th | 57.1 | 0.169 | 0.986 | 54.1 | 0.111 | 0.978 |
| 5th | 58.9 | 0.165 | 0.984 | 54.2 | 0.110 | 0.982 |
| tb/min | te/min | qe,hexane /g·g-1 | qe,t /g·g-1 adsorbent | qe,water /g·g-1 adsorbent | qe,hexane/ qe,water | Desorption efficiency /% | |
|---|---|---|---|---|---|---|---|
| 1st | 46 | 100 | 1.23 | 1.23 | 0.006 | 205 | 99.6 |
| 2nd | 44 | 98 | 1.20 | 1.20 | 0.003 | 400 | 99.7 |
| 3rd | 42 | 96 | 1.19 | 1.19 | 0.006 | 199 | 99.6 |
| 4th | 42 | 96 | 1.21 | 1.21 | 0.003 | 404 | 99.4 |
| 5th | 44 | 98 | 1.17 | 1.20 | 0.008 | 147 | 99.2 |
表S8 在RH 95%的条件下MOS-10%五次正己烷动态吸附参数
Table S8 Dynamic n-hexane adsorption parameters on MOS-10% for 5 times under 95% RH
| tb/min | te/min | qe,hexane /g·g-1 | qe,t /g·g-1 adsorbent | qe,water /g·g-1 adsorbent | qe,hexane/ qe,water | Desorption efficiency /% | |
|---|---|---|---|---|---|---|---|
| 1st | 46 | 100 | 1.23 | 1.23 | 0.006 | 205 | 99.6 |
| 2nd | 44 | 98 | 1.20 | 1.20 | 0.003 | 400 | 99.7 |
| 3rd | 42 | 96 | 1.19 | 1.19 | 0.006 | 199 | 99.6 |
| 4th | 42 | 96 | 1.21 | 1.21 | 0.003 | 404 | 99.4 |
| 5th | 44 | 98 | 1.17 | 1.20 | 0.008 | 147 | 99.2 |
| Dynamic adsorption capacity, qe /(g·g-1) | |||||
|---|---|---|---|---|---|
| Single component | Bi-component | ||||
| n-hexane | Toluene | n-hexane | Toluene | Total VOCs | |
| 1st | 1.23 | 1.21 | 0.642 | 0.569 | 1.21 |
| 2nd | 1.20 | 1.18 | 0.631 | 0.547 | 1.18 |
| 3rd | 1.19 | 1.17 | 0.613 | 0.532 | 1.15 |
| 4th | 1.22 | 1.18 | 0.607 | 0.550 | 1.16 |
| 5th | 1.21 | 1.18 | 0.628 | 0.547 | 1.18 |
表S9 干燥条件下MOS-10%样品正己烷-甲苯五次动态同时吸附参数
Table S9 Comparison of simultaneous adsorption n-hexane and toluene parameters on MOS-10% for 5 times under dry condition
| Dynamic adsorption capacity, qe /(g·g-1) | |||||
|---|---|---|---|---|---|
| Single component | Bi-component | ||||
| n-hexane | Toluene | n-hexane | Toluene | Total VOCs | |
| 1st | 1.23 | 1.21 | 0.642 | 0.569 | 1.21 |
| 2nd | 1.20 | 1.18 | 0.631 | 0.547 | 1.18 |
| 3rd | 1.19 | 1.17 | 0.613 | 0.532 | 1.15 |
| 4th | 1.22 | 1.18 | 0.607 | 0.550 | 1.16 |
| 5th | 1.21 | 1.18 | 0.628 | 0.547 | 1.18 |
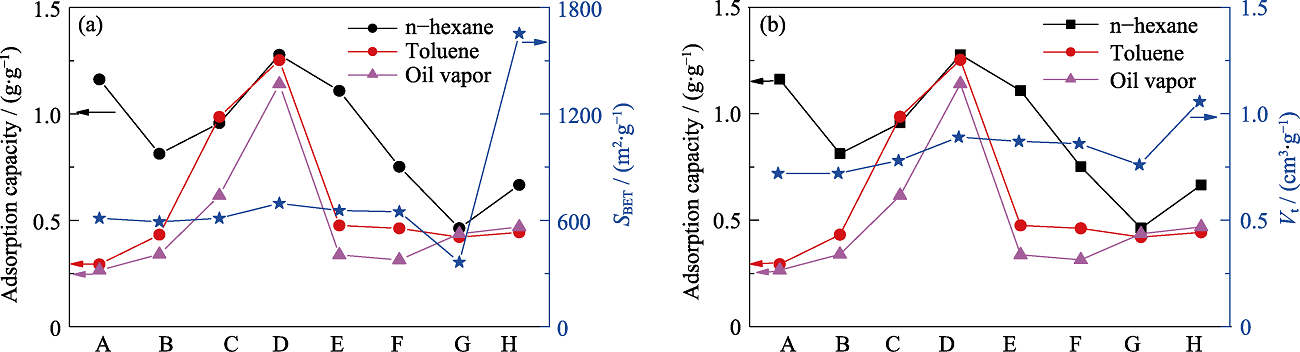
图S2 不同样品的正己烷、甲苯、汽油静态吸附容量与其结构参数的关系图
Fig. S2 Relationship between VOCs adsorption capacities and structure parameters of different samples (A) MOS-0; (B) MOS-5%; (C) MOS-7.5%; (D) MOS-10%; (E) MOS- 12.5%; (F) MOS-15%; (G) SG; (H) AC
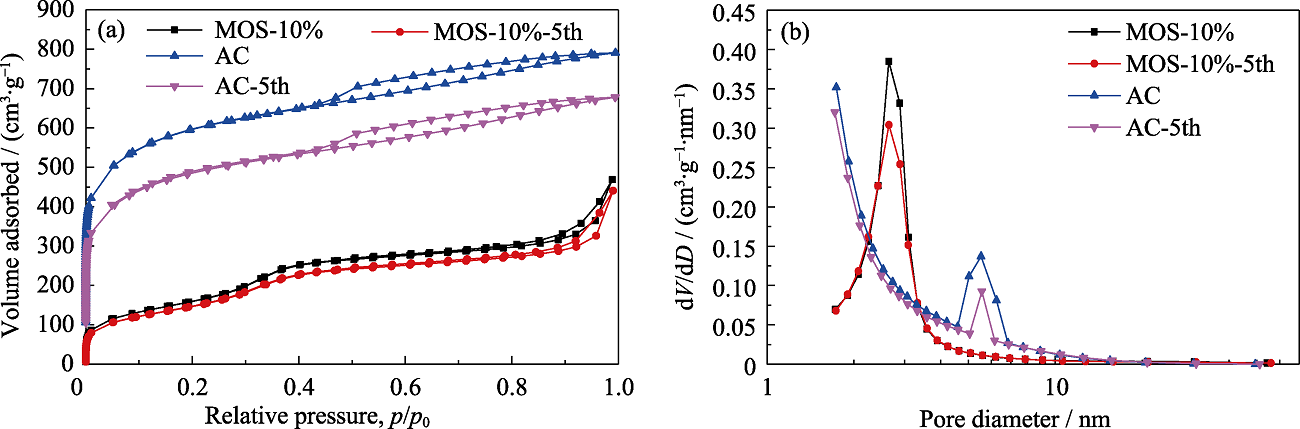
图S3 MOS-10%和AC以及五次动态吸附正己烷后MOS-10%和AC的N2吸附-解吸等温线(a)和孔径分布图(b)
Fig. S3 N2 adsorption-desorption isotherms (a) and pore size distributions (b) of MOS-10% and AC, respectively
| [1] |
ZHAO X S, MA Q, LU G Q M. VOC removal: comparison of MCM-41 with hydrophobic zeolites and activated carbon. Energ. Fuel, 1998, 12(6): 1051-1054.
DOI URL |
| [2] | ZHANG G, FEIZBAKHSHAN M, ZHENG S, et al. Effects of properties of minerals adsorbents for the adsorption and desorption of volatile organic compounds (VOC). Appl. Clay Sci., 2019, 173: 88-96. |
| [3] | TRAN THANH T, MANH TRUNG T, FELLER J F, et al. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon, 2020, 162: 662-662. |
| [4] |
LIU S, PENG Y, YAN T, et al. Modified silica adsorbents for toluene adsorption under dry and humid conditions: impacts of pore size and surface chemistry. Langmuir, 2019, 35(27): 8927-8934.
DOI URL |
| [5] |
WANG X, HE Y, LIU C, et al. A controllable asymmetrical/ symmetrical coating strategy for architectural mesoporous organosilica nanostructures. Nanoscale, 2016, 8(28): 13581-13588.
DOI URL |
| [6] |
SUN Y, CHEN M, WU L. Controllable synthesis of hollow periodic mesoporous organosilica spheres with radial mesochannels and their degradable behavior. J. Mater. Chem. A, 2018, 6(26): 12323-12333.
DOI URL |
| [7] |
BATONNEAU-GENER I, YONLI A, TROUVE A, et al. Tailoring the hydrophobic character of mesoporous silica by silylation for VOC removal. Sep. Sci. Technol., 2010, 45(6): 768-775.
DOI URL |
| [8] |
DOU B, HU Q, LI J, et al. Adsorption performance of VOCs in ordered mesoporous silicas with different pore structures and surface chemistry. J. Hazard. Mater., 2011, 186(2/3): 1615-1624.
DOI URL |
| [9] |
ZHANG W, QU Z, LI X, et al. Comparison of dynamic adsorption/ desorption characteristics of toluene on different porous materials. J. Environ. Sci., 2012, 24(3): 520-528.
DOI URL |
| [10] |
HARTMANN M, BISCHOF C. Mechanical stability of mesoporous molecular sieve MCM-48 studied by adsorption of benzene, n-heptane, and cyclohexane. J. Phys. Chem. B, 1999, 103(30): 6230-6235.
DOI URL |
| [11] | YANG K, SUN Q, XUE F, et al. Adsorption of volatile organic compounds by metal-organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater., 2011, 195: 124-131. |
| [12] |
HU Q, LI J J, HAO Z P, et al. Dynamic adsorption of volatile organic compounds on organofunctionalized SBA-15 materials. Chem. Eng. J., 2009, 149(1/2/3): 281-288.
DOI URL |
| [13] |
KUBO S, KOSUGE K. Salt-induced formation of uniform fiberlike SBA-15 mesoporous silica particles and application to toluene adsorption. Langmuir, 2007, 23(23): 11761-11768.
DOI URL |
| [14] | QIN Y, WANG Y, WANG H, et al. Effect of morphology and pore structure of SBA-15 on toluene dynamic adsorption/desorption performance. 4th International Symposium on Environmental Science and Technology (ISEST), Dalian, 2013:366-371. |
| [15] | LIU S, CHEN J, PENG Y, et al. Studies on toluene adsorption performance and hydrophobic property in phenyl functionalized KIT-6. Chem. Eng. J., 2018, 334: 191-197. |
| [16] |
DOU B, LI J, HU Q, et al. Hydrophobic micro/mesoporous silica spheres assembled from zeolite precursors in acidic media for aromatics adsorption. Micropor. Mesopor. Mat., 2010, 133(1/2/3): 115-123.
DOI URL |
| [17] |
WANG H, TANG M, HAN L, et al. Synthesis of hollow organosiliceous spheres for volatile organic compound removal. J. Mater. Chem. A, 2014, 2(45): 19298-19307.
DOI URL |
| [18] | WANG H, TANG M, ZHANG K, et al. Functionalized hollow siliceous spheres for VOCs removal with high efficiency and stability. J. Hazard. Mater., 2014, 268: 115-123. |
| [19] |
WANG H, RONG X, HAN L, et al. Controlled synthesis of hexagonal mesostructure silica and macroporous ordered siliceous foams for VOCs adsorption. RSC Adv., 2015, 5(8): 5695-5703.
DOI URL |
| [20] | WANG J, FENG S, SONG Y, et al. Synthesis of hierarchically porous carbon spheres with yolk-shell structure for high performance supercapacitors. Catal. Today, 2015, 243: 199-208. |
| [21] | ZHANG C, WU C, HAN W, et al. Controllable synthesis of multi-morphological hollow mesoporous SiO2 and adsorption reduction of Cu2+ by its composites. Chem. J. Chinese U., 2019, 40(11): 2412-2418. |
| [22] | 王小文, 胡芸, 黄晶. 等. 疏水性分子筛对焦化废水生物处理尾水的吸附过程解析. 环境科学学报, 2012, 3(2): 2058-2065. |
| [23] |
LIU W, MA N, LI S, et al. A one-step method for pore expansion and enlargement of hollow cavity of hollow periodic mesoporous organosilica spheres. J. Mater. Sci., 2017, 52(5): 2868-2878.
DOI URL |
| [24] |
GAO M, HAN S, HU Y, et al. A pH-driven molecular shuttle based on rotaxane-bridged periodic mesoporous organosilicas with responsive release of guests. RSC Adv., 2016, 6(33): 27922-27932.
DOI URL |
| [25] | MATYSIAK W, TANSKI T. Analysis of the morphology, structure and optical properties of 1D SiO2 nanostructures obtained with Sol-Gel and electrospinning methods. Appl. Surf. Sci., 2019, 489: 34-43. |
| [26] |
CHEN J, SUN C, HUANG Z, et al. Fabrication of functionalized porous silica nanocapsules with a hollow structure for high performance of toluene adsorption-desorption. ACS Omega, 2020, 5(11): 5805-5814.
DOI URL |
| [27] | YUAN W W, YUAN P, LIU D, et al. A hierarchically porous diatomite/silicalite-1 composite for benzene adsorption/desorption fabricated via a facile pre-modification in situ synthesis route. Chem. Eng. J., 2016, 294: 333-342. |
| [28] | RAJABI H, MOSLEH M H, PRAKOSO T, et al. Competitive adsorption of multicomponent volatile organic compounds on biochar. Chemosphere, 2021, 283: 131288. |
| [1] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [2] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [3] | 瞿牡静, 张淑兰, 朱梦梦, 丁浩杰, 段嘉欣, 代恒龙, 周国红, 李会利. CsPbBr3@MIL-53纳米复合荧光粉的合成、性能及其白光LEDs应用[J]. 无机材料学报, 2024, 39(9): 1035-1043. |
| [4] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [5] | 苗鑫, 闫世强, 韦金豆, 吴超, 樊文浩, 陈少平. Te基热电器件反常界面层生长行为及界面稳定性研究[J]. 无机材料学报, 2024, 39(8): 903-910. |
| [6] | 陈甜, 罗媛, 朱刘, 郭学益, 杨英. 有机-无机共添加增强柔性钙钛矿太阳能电池机械弯曲及环境稳定性能[J]. 无机材料学报, 2024, 39(5): 477-484. |
| [7] | 杨博, 吕功煊, 马建泰. 镍铁氢氧化物-磷化钴复合电极电催化分解水研究[J]. 无机材料学报, 2024, 39(4): 374-382. |
| [8] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [9] | 王煜, 熊浩, 黄孝坤, 江琳沁, 吴波, 黎健生, 杨爱军. 低剂量异辛酸亚锡调控两步法制备Sn-Pb混合钙钛矿太阳能电池[J]. 无机材料学报, 2024, 39(12): 1339-1347. |
| [10] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [11] | 方万丽, 沈黎丽, 李海艳, 陈薪羽, 陈宗琦, 寿春晖, 赵斌, 杨松旺. NiOx介孔层的成膜过程对碳电极钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 2023, 38(9): 1103-1109. |
| [12] | 陈雨, 林埔安, 蔡冰, 张文华. 钙钛矿太阳能电池无机空穴传输材料的研究进展[J]. 无机材料学报, 2023, 38(9): 991-1004. |
| [13] | 胡忠良, 傅赟天, 蒋蒙, 王连军, 江莞. Nb/Mg3SbBi界面层热稳定性研究[J]. 无机材料学报, 2023, 38(8): 931-937. |
| [14] | 刘建, 王凌坤, 许保亮, 赵倩, 王耀萱, 丁艺, 张胜泰, 段涛. 熔盐法低温合成掺钕ZrSiO4陶瓷的物相演变和化学稳定性[J]. 无机材料学报, 2023, 38(8): 910-916. |
| [15] | 肖娅妮, 吕嘉南, 李振明, 刘铭扬, 刘伟, 任志刚, 刘弘景, 杨东旺, 鄢永高. Bi2Te3基热电材料的湿热稳定性研究[J]. 无机材料学报, 2023, 38(7): 800-806. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||