无机材料学报 ›› 2024, Vol. 39 ›› Issue (4): 374-382.DOI: 10.15541/jim20230432 CSTR: 32189.14.10.15541/jim20230432
所属专题: 【能源环境】氢能材料(202506)
收稿日期:2023-09-22
修回日期:2023-11-23
出版日期:2024-04-20
网络出版日期:2023-12-04
通讯作者:
吕功煊, 教授. E-mail: gxlu@lzb.ac.cn作者简介:杨 博(1989-), 男, 博士研究生. E-mail: yangbo18@licp.cas.cn
基金资助:
YANG Bo1,2,3( ), LÜ Gongxuan1(
), LÜ Gongxuan1( ), MA Jiantai3
), MA Jiantai3
Received:2023-09-22
Revised:2023-11-23
Published:2024-04-20
Online:2023-12-04
Contact:
LÜ Gongxuan, professor. E-mail: gxlu@lzb.ac.cnAbout author:YANG Bo (1989–), male, PhD candidate. E-mail: yangbo18@licp.cas.cn
Supported by:摘要:
本研究采用水热-磷化-电化学沉积法在磷化钴表面构筑了金属氢氧化物层, 制备了NiFeOH/CoP/NF复合电极, 考察了复合电极电解水制氢的性能。在1.0 molּ/L的KOH介质中, NiFeOH/CoP/NF复合电极表现出良好的催化电解水性能。在电流密度为100 mA/cm2时, NiFeOH/CoP/NF复合电极电催化析氢(HER)和析氧反应(OER)所需的过电势分别为141和372 mV。在电流密度为10 mA/cm2时, NiFeOH/CoP/NF同时用作阴极和阳极电解水所需电压仅为1.61 V。NiFeOH保护层增强了CoP在电解水反应中的活性和稳定性, NiFeOH/CoP/NF复合电极在恒电流电解中表现出高的HER和OER稳定性, 活性可维持60000 s, 性能未见明显衰减。将NiFeOH/CoP/NF两电极电解池与GaAs太阳能电池组成光伏-电解水系统, 该系统在100 mW/cm2模拟光照条件下, 太阳能至氢能转化效率达到18.0%, 并可稳定运行200 h。
中图分类号:
杨博, 吕功煊, 马建泰. 镍铁氢氧化物-磷化钴复合电极电催化分解水研究[J]. 无机材料学报, 2024, 39(4): 374-382.
YANG Bo, LÜ Gongxuan, MA Jiantai. Electrocatalytic Water Splitting over Nickel Iron Hydroxide-cobalt Phosphide Composite Electrode[J]. Journal of Inorganic Materials, 2024, 39(4): 374-382.
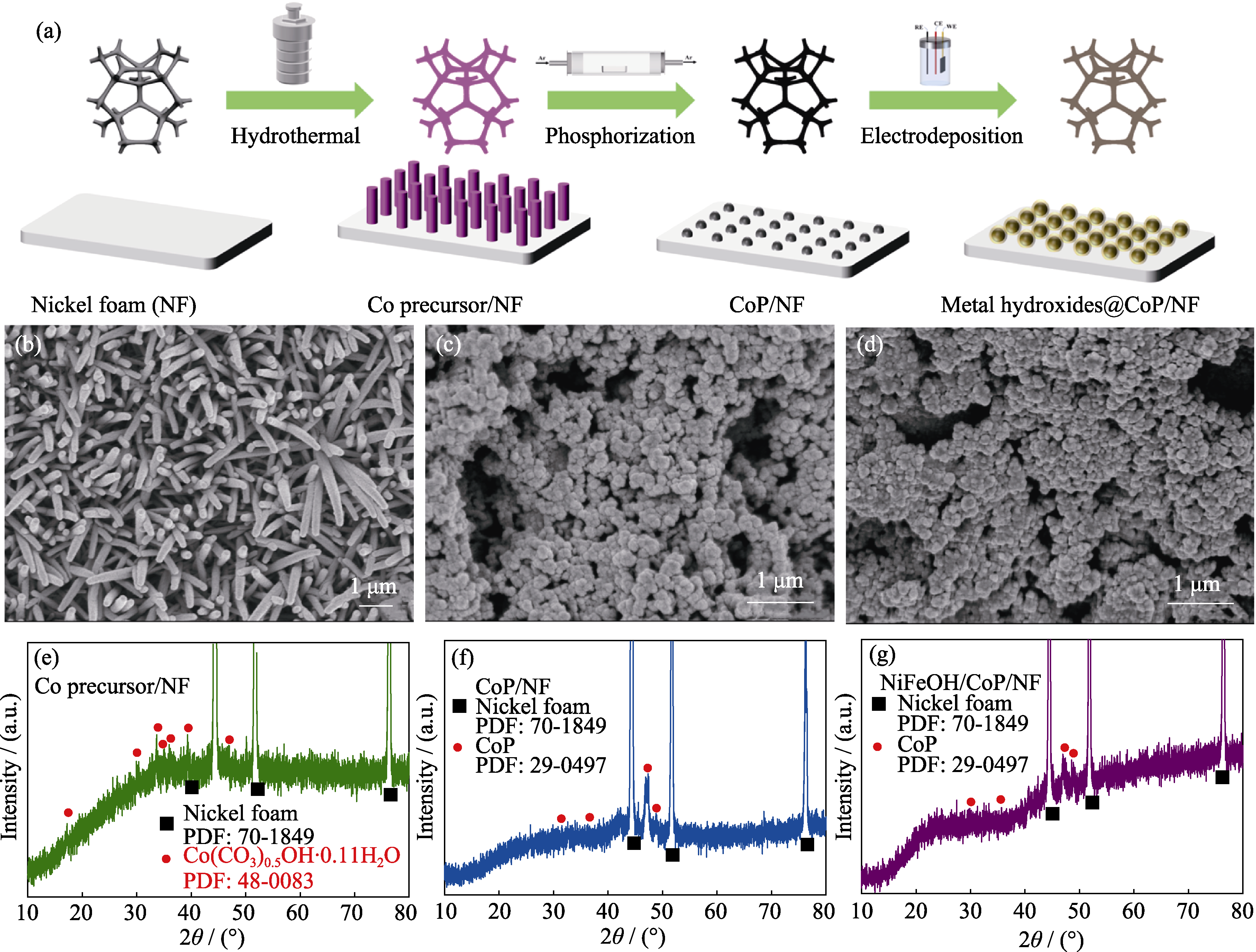
图1 电极制备过程示意图(a)及Co前驱体/NF(b, e)、CoP/NF(c, f)和NiFeOH/CoP/NF-200s(d, g)的SEM照片(b~d)和XRD谱图(e~g)
Fig. 1 Synthesis diagram of electrodes (a), SEM images (b-d) and XRD patterns (e-g) of Co precursor/NF (b, e), CoP/NF (c, f), and NiFeOH/CoP/NF-200s (d, g)
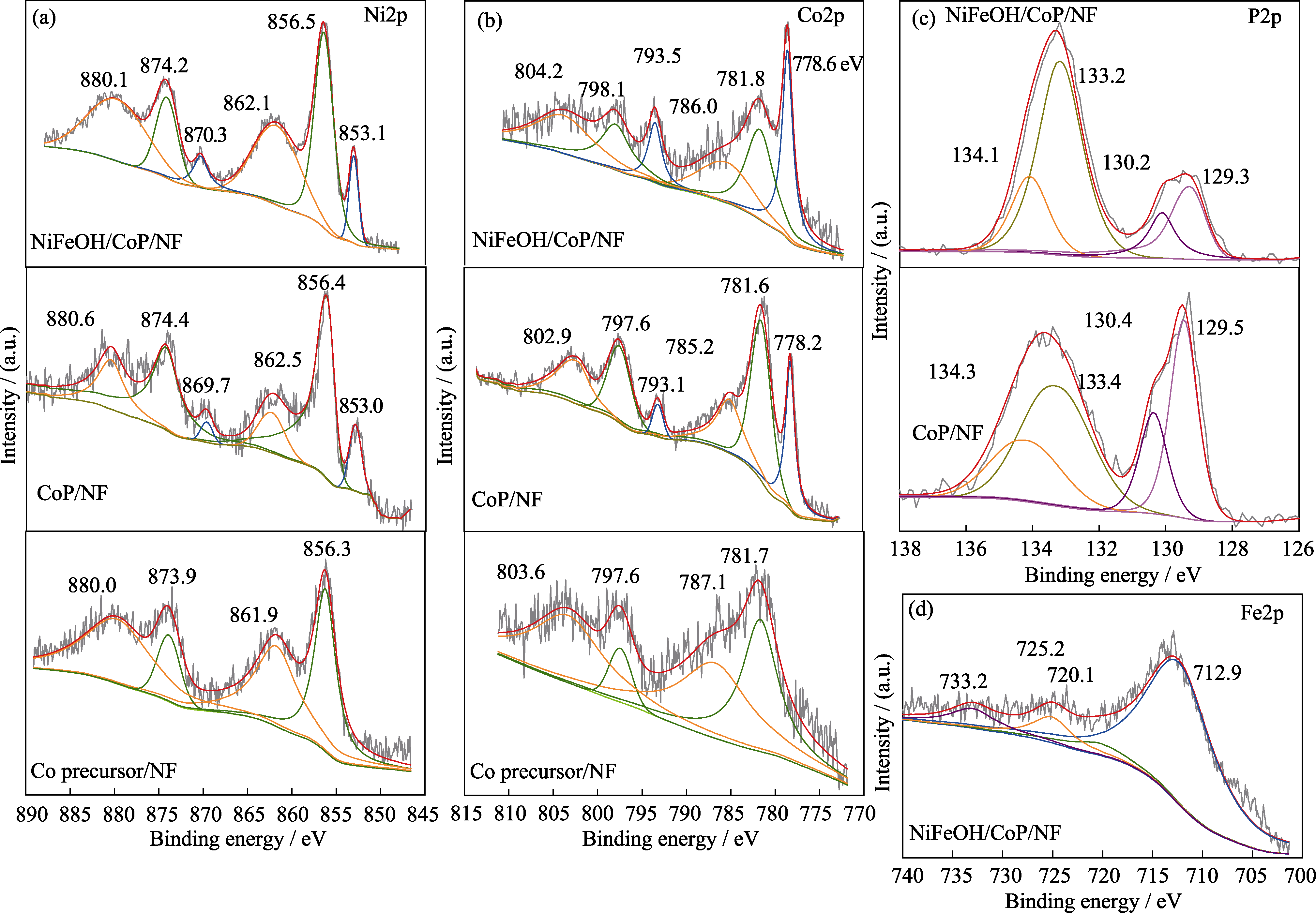
图2 Co前驱体/NF、CoP/NF和NiFeOH/CoP/NF-200s的Ni2p(a)、Co2p(b)、P2p(c)和Fe2p(d) XPS谱图
Fig. 2 Ni2p(a), Co2p(b), P2p(c), and Fe2p(d) XPS spectra of Co precursor/NF, CoP/NF, and NiFeOH/CoP/NF-200s
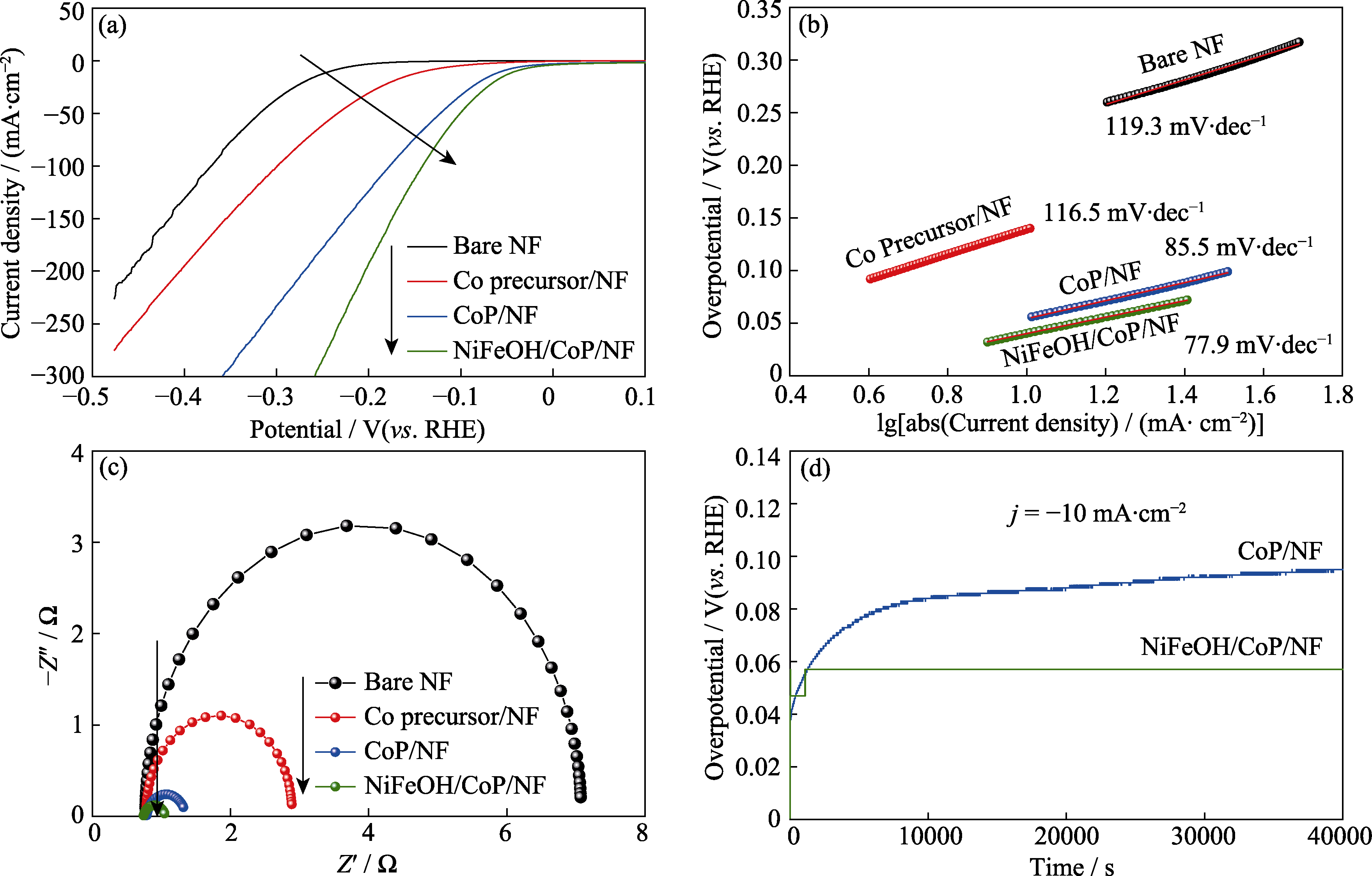
图3 单独NF、Co前驱体/NF、CoP/NF和NiFeOH/CoP/NF-200s的HER电化学性质
Fig. 3 HER electrocatalytic properties of bare NF, Co precursor/NF, CoP/NF, and NiFeOH/CoP/NF-200s (a) LSV curves; (b) Corresponding Tafel plots; (c) Nyquist plots at -0.20 V (vs. RHE); (d) Chronopotentiometry plots of CoP/NF and NiFeOH/CoP/NF-200s at the current density of -10 mA/cm2
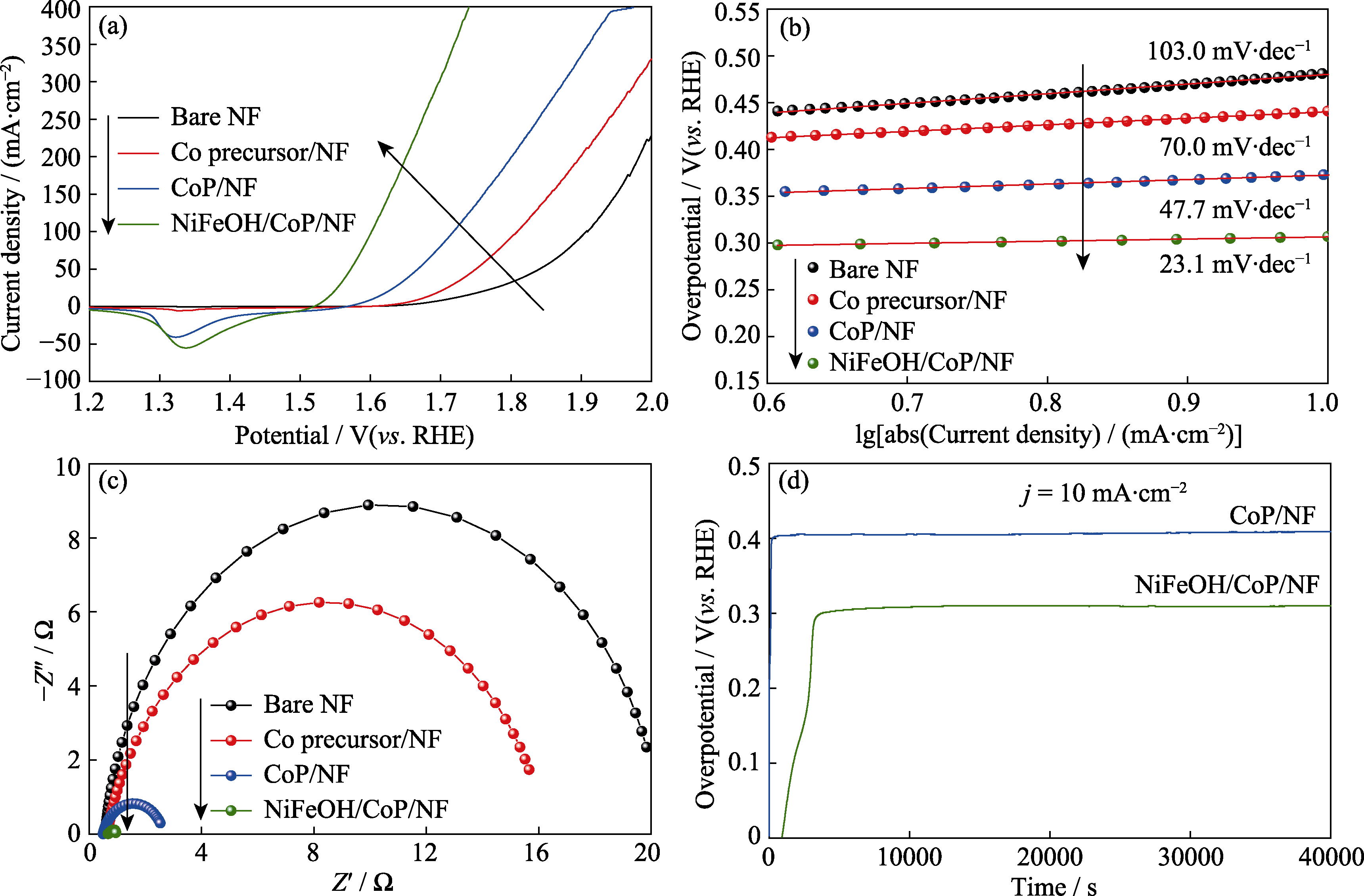
图4 单独NF、Co前驱体/NF、CoP/NF和NiFeOH/CoP/NF-200s的OER电化学性质
Fig. 4 OER electrocatalytic properties of bare NF, Co precursor/NF, CoP/NF, and NiFeOH/CoP/NF-200s (a) LSV curves; (b) Corresponding Tafel plots; (c) Nyquist plots at the potential of 1.60 V (vs. RHE); (d) Chronopotentiometry plots of CoP/NF and NiFeOH/CoP/NF-200s at the current density of 10 mA/cm2
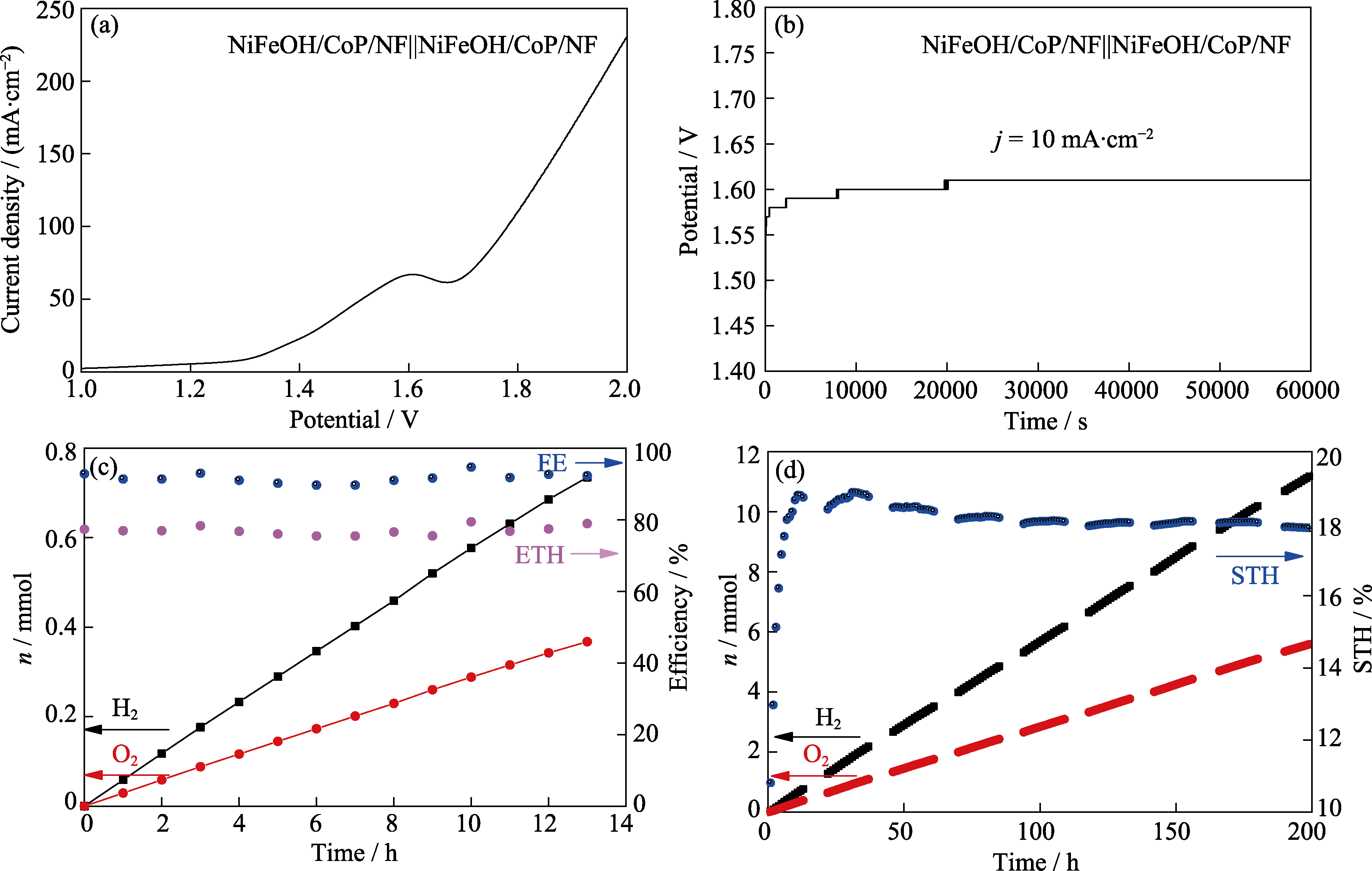
图5 NiFeOH/CoP/NF-200s||NiFeOH/CoP/NF-200s两电极全分解水的电化学性质, 以及两电极电解池与GaAs太阳能电池组成光伏-电催化系统的太阳能制氢效率
Fig. 5 Electrocatalytic overall water splitting properties of NiFeOH/CoP/NF-200s||NiFeOH/CoP/NF-200s two electrode system and hydrogen production efficiency of the photovoltaic-electrocatalytic system consisting of two electrode cell and GaAs solar cell (a) LSV curve; (b) Long term chronopotentiometry plot at current density of 10 mA/cm2; (c) Galvanostatic electrocatalytic hydrogen and oxygen evolution curves, Faradic efficiency (FE), and electricity to hydrogen efficiency curves (ETH); (d) Hydrogen, oxygen evolution and corresponding solar to hydrogen efficiency curves of a photovoltaic-electrocatalytic system consisting of NiFeOH/CoP/NF-200s||NiFeOH/CoP/NF-200s electrocatalytic cell and GaAs solar cell
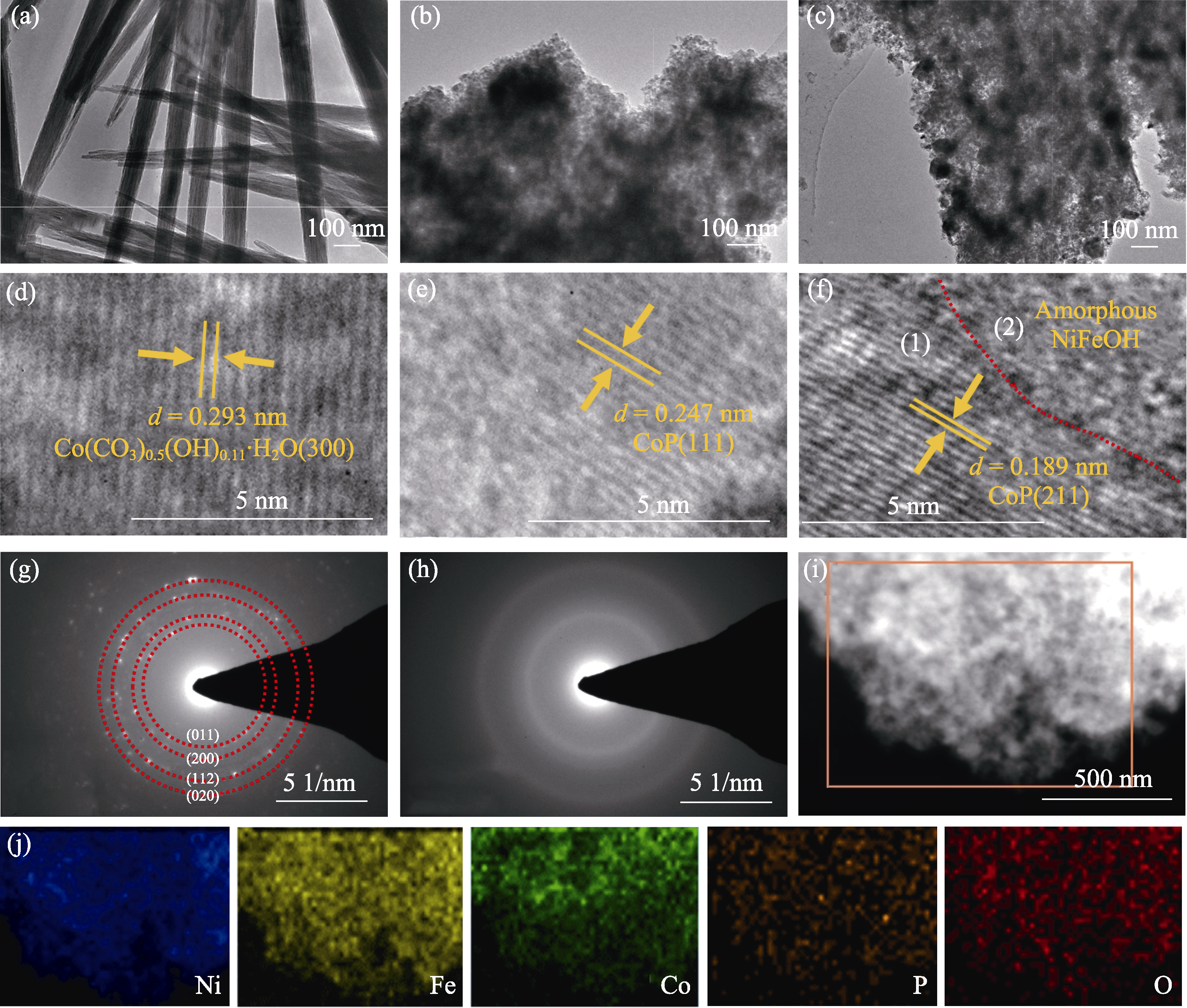
图S1 Co前驱体/NF、CoP/NF和NiFeOH/CoP/NF-200s的TEM表征
Fig. S1 TEM characterization of Co precursor/NF, CoP/NF, and NiFeOH/CoP/NF-200s (a-c) TEM images of (a) Co precursor/NF, (b) CoP/NF, and (c) NiFeOH/CoP/NF-200s; (d-f) HRTEM images of (d) Co precursor/NF, (e) CoP/NF, and (f) NiFeOH/CoP/NF-200s; (g, h) SAED images in crystalline (1) and amorphous (2) areas of (f); (i) HAADF-STEM image and (j) Ni, Fe, Co, P, O element mappings of NiFeOH/CoP/NF-200s
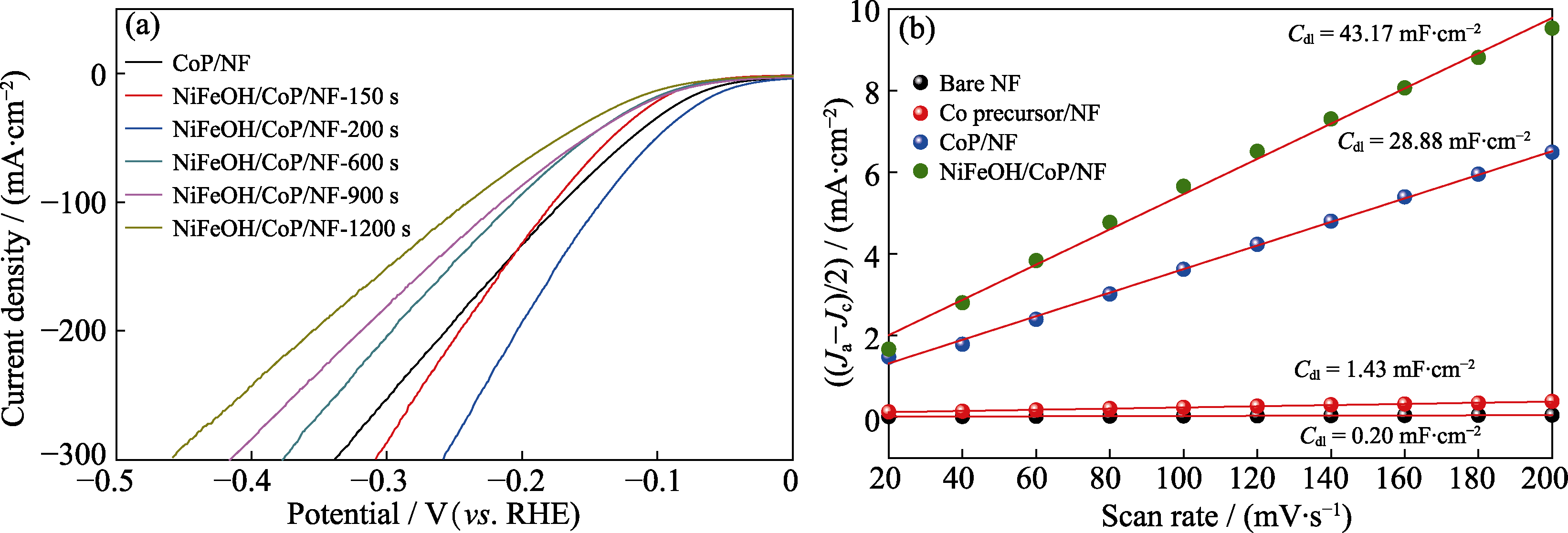
图S2 CoP/NF和不同电沉积时间制备的NiFeOH/CoP/NF-xs的阴极LSV曲线(a), 电流密度差对扫描速率作图计算单独NF、Co前驱体/NF、CoP/NF和NiFeOH/CoP/NF-200s的双层电化学电容(b)
Fig. S2 Cathode LSV curves of CoP/NF, and NiFeOH/CoP/NF-xs prepared with different electrodeposition time (a), and current density difference versus scan rate to calculate the double layer capacities (Cdls) of bare NF, Co precursor/NF, CoP/NF, and NiFeOH/CoP/NF-200s (b)
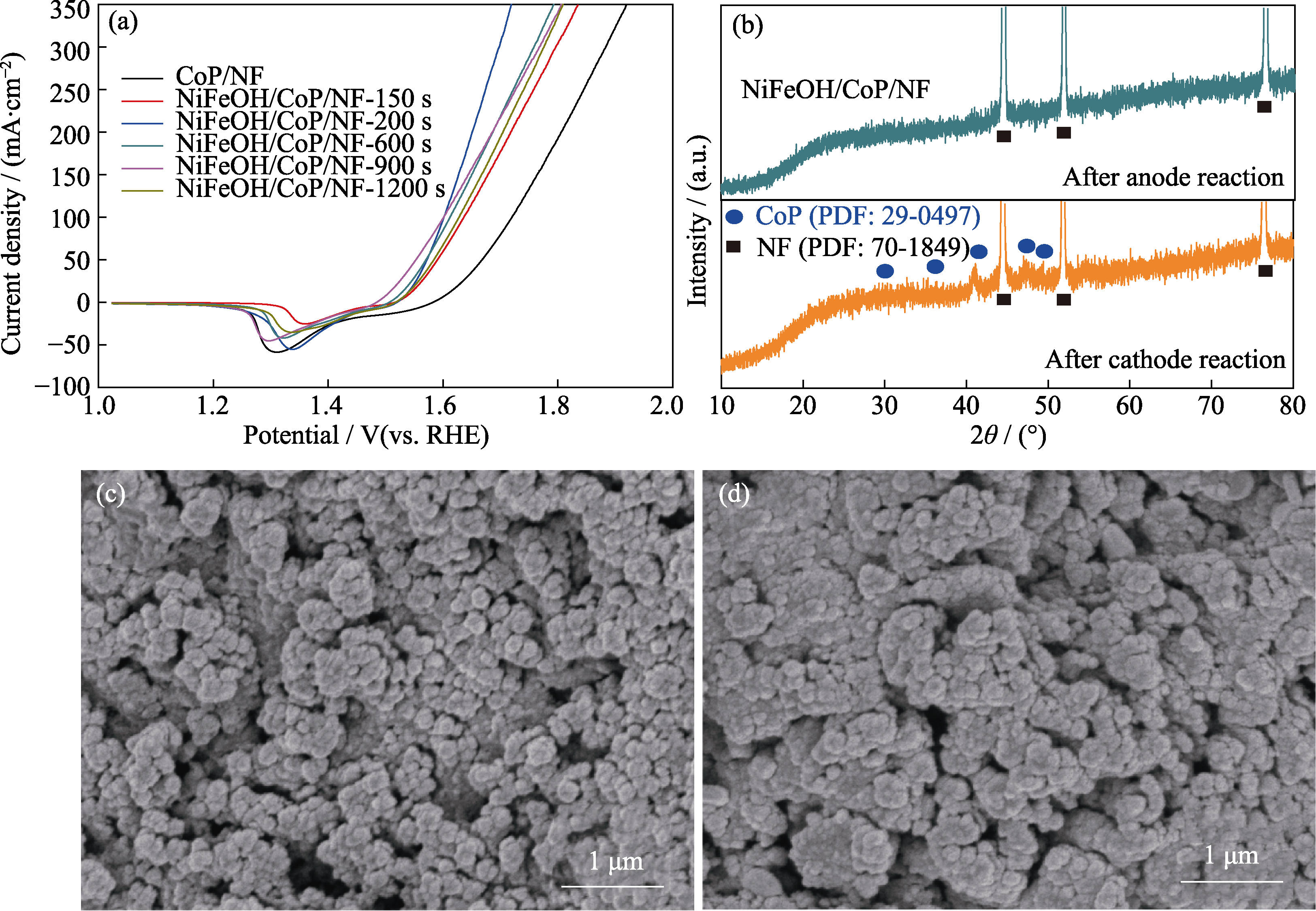
图S3 CoP/NF和不同电沉积时间制备的NiFeOH/CoP/NF-xs电极的阳极LSV曲线(a)及NiFeOH/CoP/NF-200s阴、阳极恒电流反应后的XRD和SEM表征(b~d)
Fig. S3 Anode LSV curves of CoP/NF and NiFeOH/CoP/NF-xs prepared with different electrodeposition time (a), XRD and SEM characterization of NiFeOH/CoP/NF-200s after cathode and anode chronopotentiometry test (b-d) (b) XRD spectra after HER and OER; (c) SEM image after HER; (d) SEM image after OER
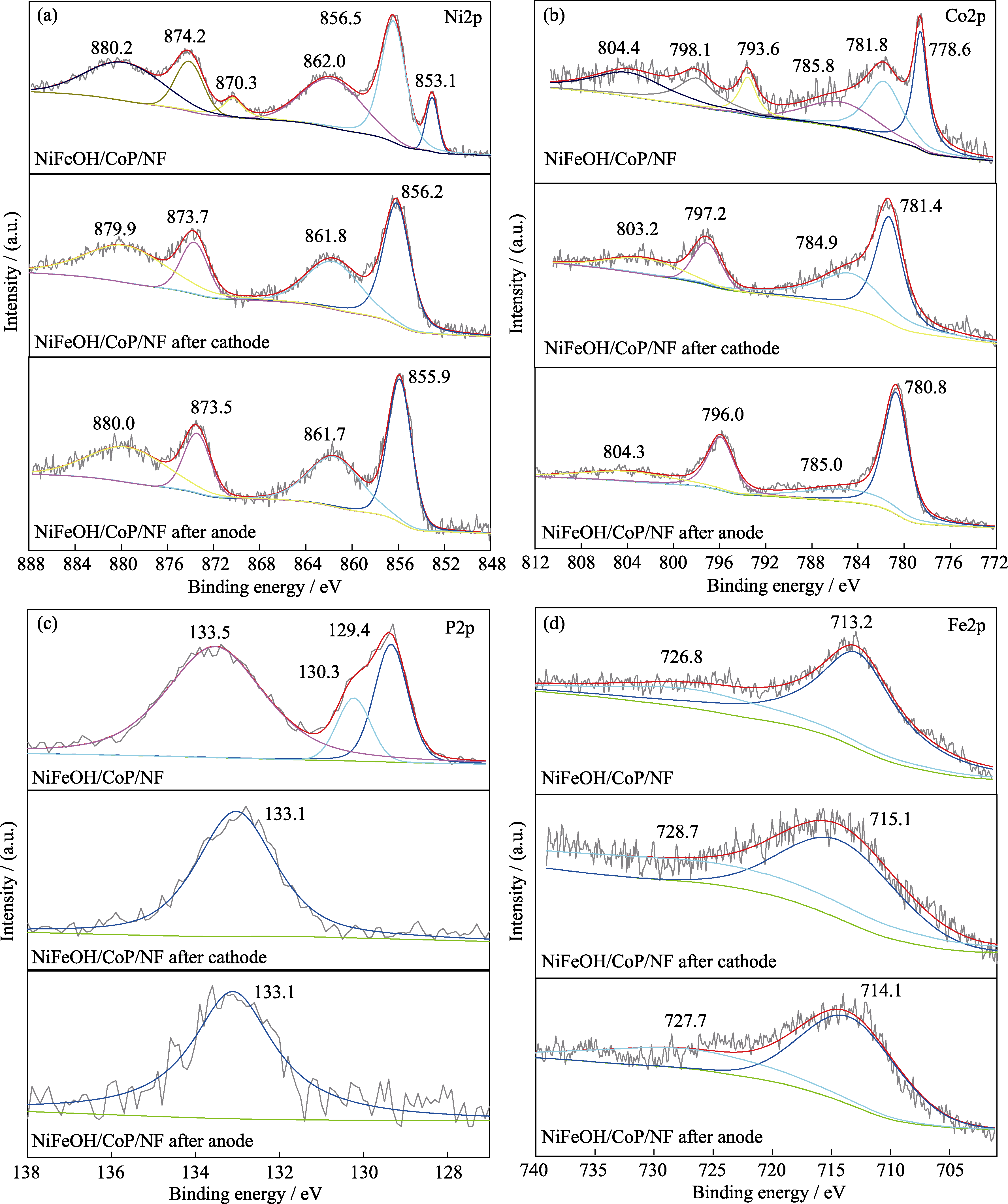
图S4 NiFeOH/CoP/NF-200s电极在HER和OER恒电流反应前后的XPS表征
Fig. S3 XPS characterization of NiFeOH/CoP/NF-200s before and after HER and OER chronopotentiometry test (a) Ni2p; (b) Co2p; (c) P2p; (d) Fe2p
| [1] |
YANG B, ZHEN W L, MA J T, et al. Corrosion inhibition and stability enhancement of cobalt phosphide in aqueous solution by coating TiO2 layer. International Journal of Hydrogen Energy, 2023, 48(94): 36784.
DOI URL |
| [2] | 叶朕, 罗皓霖, 江治, 等. 光催化还原二氧化碳全反应的研究进展. 分子催化, 2023, 37(2): 174. |
| [3] |
ZHEN W L, NING X F, YANG B J, et al. The enhancement of CdS photocatalytic activity for water splitting via anti-photocorrosion by coating Ni2P shell and removing nascent formed oxygen with artificial gill. Applied Catalysis B-Environmental, 2018, 221: 243.
DOI URL |
| [4] | 周飞. 石墨相氮化碳在光催化苯甲醛氧化耦合制氢领域的研究进展. 分子催化, 2023, 37(4): 397. |
| [5] |
MIN S X, LU G X. Dye-sensitized reduced graphene oxide photocatalysts for highly efficient visible-light-driven water reduction. Journal of Physical Chemistry C, 2011, 115(28): 13938.
DOI URL |
| [6] | 李博远, 何凤贵, 张明慧, 等. 金属-有机骨架材料的改性方法及其光催化制氢应用. 分子催化, 2023, 37(1): 94. |
| [7] |
TIAN B, WU Y Q, LU G X. Metal-free plasmonic boron phosphide/graphitic carbon nitride with core-shell structure photocatalysts for overall water splitting. Applied Catalysis B-Environmental, 2021, 280: 119410.
DOI URL |
| [8] |
KONG C, MIN S X, LU G X. Dye-sensitized NiSx catalyst decorated on graphene for highly efficient reduction of water to hydrogen under visible light irradiation. ACS Catalysis, 2014, 4(8): 2763.
DOI URL |
| [9] | 张志艳, 石琛琛, 张潇, 等. 咔唑基共价有机框架用于光催化析氢. 分子催化, 2023, 37(4): 367. |
| [10] |
JIA M Z, LU G X. 750 nm visible light-driven overall water splitting to H2 and O2 over boron-doped Zn3As2photocatalyst. Applied Catalysis B-Environmental, 2023, 338: 123045.
DOI URL |
| [11] |
ZHANG X Q, LU G X, NING X F, et al. Boron substitution enhanced activity of BxGa1-xAs/GaAs photocatalyst for water splitting. Applied Catalysis B-Environmental, 2021, 300: 120690.
DOI URL |
| [12] | 侯慧霞, 张靖怡, 蔡平龙, 等. 超声驱动制备Au/CdS催化剂及其高效光催化产氢. 分子催化, 2022, 36(2): 129. |
| [13] |
NING X F, LU G X. Photocorrosion inhibition of CdS-based catalysts for photocatalytic overall water splitting. Nanoscale, 2020, 12(3): 1213.
DOI PMID |
| [14] | 王春艳, 武文慧, 史晓敏, 等. 不同形貌ZnS基纳米复合材料的制备及光催化性能. 分子催化, 2021, 35(2): 141. |
| [15] |
DONG J L, ZHANG X Q, LU G X, et al. Generation of enhanced stability of SnO/In(OH)3/InP for photocatalytic water splitting by SnO protection layer. Frontiers in Energy, 2021, 15(3): 710.
DOI |
| [16] |
WU J, YU L B, LIU S S, et al. NiN4/Cr embedded graphene for electrochemical nitrogen fixation. Journal of Inorganic Materials, 2022, 37(10): 1141.
DOI URL |
| [17] | ZHANG X Q, LU GX, WU Y Q, et al. TiO2protection layer and well-matched interfaces enhance the stability of Cu2ZnSnS4/ CdS/TiO2 for visible light driven water splitting. Catalysis Science & Technology, 2021, 11(16): 5505. |
| [18] |
WANG M, LU G X. Improved light harvesting and efficiency for overall water splitting by embedding TiO2 transition layer in GaP/Ga2O3/Ga2Se3multijunction photocatalyst. Solar RRL, 2021, 5(6): 2000619.
DOI URL |
| [19] | 赵茂旭, 张天琦, 段婷婷, 等. 电催化醇选择性氧化为醛酮的研究进展. 分子催化, 2021, 35(6): 583. |
| [20] | 乔劲松, 韩苗苗. 多孔二元过渡金属纳米片阵列电极制备及电催化析氢研究. 分子催化, 2021, 35(5): 449. |
| [21] |
YU F, ZHOU H Q, HUANG Y F, et al. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nature Communications, 2018, 9: 2551.
DOI PMID |
| [22] |
POPCZUN E J, READ C G, ROSKE C W, et al. Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angewandte Chemie International Edition, 2014, 53(21): 5427.
DOI URL |
| [23] |
LIU Q, TIAN J Q, CUI W, et al. Carbon nanotubes decorated with CoP nanocrystals: a highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angewandte Chemie International Edition, 2014, 53(26): 6710.
DOI URL |
| [24] |
CHANG J F, XIAO Y, XIAO M L, et al. Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catalysis, 2015, 5(11): 6874.
DOI URL |
| [25] |
LI X Z, FANG Y Y, LI F, et al. Ultrafine Co2P nanoparticles encapsulated in nitrogen and phosphorus dual-doped porous carbon nanosheet/carbon nanotube hybrids: high-performance bifunctional electrocatalysts for overall water splitting. Journal of Materials Chemistry A, 2016, 4(40): 15501.
DOI URL |
| [26] |
AHNT H S, BARD A J. Assessment of the stability and operability of cobalt phosphide electrocatalyst for hydrogen evolution. Analytical Chemistry, 2017, 89(16): 8574.
DOI PMID |
| [27] |
ZHANG Y, GAO L, HENSEN, E J M, et al. Evaluating the stability of Co2P electrocatalysts in the hydrogen evolution reaction for both acidic and alkaline electrolytes. ACS Energy Letters, 2018, 3(6): 1360.
DOI URL |
| [28] |
HA D H, HAN B H, RISCH M, et al. Activity and stability of cobalt phosphides for hydrogen evolution upon water splitting. Nano Energy, 2016, 29: 37.
DOI URL |
| [29] |
LI D, BAYDOUN H, VERANI C N, et al. Efficient water oxidation using CoMnP nanoparticles. Journal of the American Chemical Society, 2016, 138(12): 4006.
DOI PMID |
| [30] |
WANG F L, ZHOU Y N, LV J Y, et al. Nickel hydroxide armour promoted CoP nanowires for alkaline hydrogen evolution at large current density. International Journal of Hydrogen Energy, 2022, 47(2): 1016.
DOI URL |
| [31] |
SU L, CUI X Z, HE T, et al. Surface reconstruction of cobalt phosphide nanosheets by electrochemical activation for enhanced hydrogen evolution in alkaline solution. Chemical Science, 2019, 10(7): 2019.
DOI PMID |
| [32] |
MAI W S, CUI Q, ZHANG Z Q, et al. CoMoP/NiFe-layered double-hydroxide hierarchical nanosheet arrays standing on Ni foam for efficient overall water splitting. ACS Applied Energy Materials, 2020, 3(8): 8075.
DOI URL |
| [33] |
HOSONO E, FUJIHARA S, HONMA I, et al. Fabrication of morphology and crystal structure controlled nanorod and nanosheet cobalt hydroxide based on the difference of oxygen-solubility between water and methanol, and conversion into Co3O4. Journal of Materials Chemistry, 2005, 15(19): 1938.
DOI URL |
| [34] |
LI Q, WANG YC, ZENG J, et al. Phosphating-induced charge transfer on CoO/CoP interface for alkaline H2evolution. Chinese Chemical Letters, 2021, 32(11): 3355.
DOI URL |
| [35] | CHEN L, WANG Y P, ZHAO X, et al. Trimetallic oxyhydroxides as active sites for large-current-density alkaline oxygen evolution and overall water splitting. Journal of Materials Science & Technology, 2022, 110: 128. |
| [36] |
LIU Y, FENG Q G, LIU W, et al. Boosting interfacial charge transfer for alkaline hydrogen evolution via rational interior Se modification. Nano Energy, 2021, 81: 105641.
DOI URL |
| [37] |
MASIKHWA T M, DANGBEGNON J K, BELLO A, et al. Preparation and electrochemical investigation of the cobalt hydroxide carbonate/activated carbon nanocomposite for supercapacitor applications. Journal of Physics and Chemistry of Solids, 2016, 88: 60.
DOI URL |
| [38] |
HUANG G J, LIANG W L, WU Y L, et al. Co2P/CoP hybrid as a reversible electrocatalyst for hydrogen oxidation/evolution reactions in alkaline medium. Journal of Catalysis, 2020, 390: 23.
DOI URL |
| [39] |
ZHANG H, WU J B, ZHAI C X, et al. From cobalt nitrate carbonate hydroxide hydrate nanowires to porous Co3O4 nanorods for high performance lithium-ion battery electrodes. Nanotechnology, 2008, 19(3): 035711.
DOI URL |
| [40] |
LI Y, LI H X, CAO K Z, et al. Electrospun three dimensional Co/CoP@nitrogen-doped carbon nanofibers network for efficient hydrogen evolution. Energy Storage Materials, 2018, 12: 44.
DOI URL |
| [41] |
LI Y, MALIK M A, O'BRIEN P. Synthesis of single-crystalline CoP nanowires by a one-pot metal-organic route. Journal of the American Chemical Society, 2005, 127(46): 16020.
PMID |
| [42] |
PENG J H, PENG K. Rational design of amorphous NiFe-LDH/ Co3O4-P heterostructure bifunctional electrocatalysts for overall water splitting. Materials Chemistry and Physics, 2023, 297: 127412.
DOI URL |
| [43] |
PAN Y, HU W H, LIU D P, et al. Carbon nanotubes decorated with nickel phosphide nanoparticles as efficient nanohybrid electrocatalysts for the hydrogen evolution reaction. Journal of Materials Chemistry A, 2015, 3(24): 13087.
DOI URL |
| [44] |
YOON H, SONG H J, JU B B, et al. Cobalt phosphide nanoarrays with crystalline-amorphous hybrid phase for hydrogen production in universal-pH. Nano Research, 2020, 13(9): 2469.
DOI |
| [45] |
ZHANG H J, LI X P, HÄHNEL A, et al. Bifunctional heterostructure assembly of NiFe LDH nanosheets on NiCoP nanowires for highly efficient and stable overall water splitting. Advanced Functional Materials, 2018, 28(14): 1706847.
DOI URL |
| [46] |
WANG X B, WANG J L, LIAO J, et al. Surface engineering of superhydrophilic Ni2P@NiFe LDH heterostructure toward efficient water splitting electrocatalysis. Applied Surface Science, 2022, 602: 154287.
DOI URL |
| [47] |
XIAO L, BAO W W, ZHANG J J, et al. Interfacial interaction between NiMoP and NiFe-LDH to regulate the electronic structure toward high-efficiency electrocatalytic oxygen evolution reaction. International Journal of Hydrogen Energy, 2022, 47(15): 9230.
DOI URL |
| [48] |
ELADGHAM E H, RODENE D D, SARKAR R, et al. Electrocatalytic activity of bimetallic Ni-Mo-P nanocrystals for hydrogen evolution reaction. ACS Applied Nano Materials, 2020, 3(8): 8199.
DOI URL |
| [49] |
RYU J, JUNG N, JANG J H, et al. In situ transformation of hydrogen-evolving CoP nanoparticles: toward efficient oxygen evolution catalysts bearing dispersed morphologies with co-oxo/ hydroxo molecular units. ACS Catalysis, 2015, 5(7): 4066.
DOI URL |
| [50] | 鄢维, 李渊. 基于尿素电合成反应的电催化剂研究进展. 分子催化, 2023, 37(2): 187. |
| [1] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [2] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [3] | 瞿牡静, 张淑兰, 朱梦梦, 丁浩杰, 段嘉欣, 代恒龙, 周国红, 李会利. CsPbBr3@MIL-53纳米复合荧光粉的合成、性能及其白光LEDs应用[J]. 无机材料学报, 2024, 39(9): 1035-1043. |
| [4] | 潘建隆, 马官军, 宋乐美, 郇宇, 魏涛. 燃料还原法原位制备高稳定性/催化活性SOFC钴基钙钛矿阳极[J]. 无机材料学报, 2024, 39(8): 911-919. |
| [5] | 苗鑫, 闫世强, 韦金豆, 吴超, 樊文浩, 陈少平. Te基热电器件反常界面层生长行为及界面稳定性研究[J]. 无机材料学报, 2024, 39(8): 903-910. |
| [6] | 陈甜, 罗媛, 朱刘, 郭学益, 杨英. 有机-无机共添加增强柔性钙钛矿太阳能电池机械弯曲及环境稳定性能[J]. 无机材料学报, 2024, 39(5): 477-484. |
| [7] | 张宇晨, 陆知遥, 赫晓东, 宋广平, 朱春城, 郑永挺, 柏跃磊. 硫族MAX相硼化物的物相稳定性和性能预测[J]. 无机材料学报, 2024, 39(2): 225-232. |
| [8] | 王煜, 熊浩, 黄孝坤, 江琳沁, 吴波, 黎健生, 杨爱军. 低剂量异辛酸亚锡调控两步法制备Sn-Pb混合钙钛矿太阳能电池[J]. 无机材料学报, 2024, 39(12): 1339-1347. |
| [9] | 周云凯, 刁亚琪, 王明磊, 张宴会, 王利民. 聚苯胺改性Ti3C2(OH)2抗氧化性的第一性原理计算研究[J]. 无机材料学报, 2024, 39(10): 1151-1158. |
| [10] | 方万丽, 沈黎丽, 李海艳, 陈薪羽, 陈宗琦, 寿春晖, 赵斌, 杨松旺. NiOx介孔层的成膜过程对碳电极钙钛矿太阳能电池性能的影响[J]. 无机材料学报, 2023, 38(9): 1103-1109. |
| [11] | 陈雨, 林埔安, 蔡冰, 张文华. 钙钛矿太阳能电池无机空穴传输材料的研究进展[J]. 无机材料学报, 2023, 38(9): 991-1004. |
| [12] | 胡忠良, 傅赟天, 蒋蒙, 王连军, 江莞. Nb/Mg3SbBi界面层热稳定性研究[J]. 无机材料学报, 2023, 38(8): 931-937. |
| [13] | 刘建, 王凌坤, 许保亮, 赵倩, 王耀萱, 丁艺, 张胜泰, 段涛. 熔盐法低温合成掺钕ZrSiO4陶瓷的物相演变和化学稳定性[J]. 无机材料学报, 2023, 38(8): 910-916. |
| [14] | 肖娅妮, 吕嘉南, 李振明, 刘铭扬, 刘伟, 任志刚, 刘弘景, 杨东旺, 鄢永高. Bi2Te3基热电材料的湿热稳定性研究[J]. 无机材料学报, 2023, 38(7): 800-806. |
| [15] | 汪波, 余健, 李存成, 聂晓蕾, 朱婉婷, 魏平, 赵文俞, 张清杰. Gd/Bi0.5Sb1.5Te3热电磁梯度复合材料的服役稳定性[J]. 无机材料学报, 2023, 38(6): 663-670. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||