无机材料学报 ›› 2025, Vol. 40 ›› Issue (1): 104-110.DOI: 10.15541/jim20240279 CSTR: 32189.14.10.15541/jim20240279
冯关正1,2,3( ), 杨健1,2, 周渡1,2, 陈啟明1,2,3, 许文涛1,2, 周有福1,2(
), 杨健1,2, 周渡1,2, 陈啟明1,2,3, 许文涛1,2, 周有福1,2( )
)
收稿日期:2024-06-07
修回日期:2024-09-02
出版日期:2025-01-20
网络出版日期:2024-09-02
通讯作者:
周有福, 研究员. E-mail: yfzhou@fjirsm.ac.cn作者简介:冯关正(1998-), 男, 硕士研究生. E-mail: fengguanzheng01@163.com
FENG Guanzheng1,2,3( ), YANG Jian1,2, ZHOU Du1,2, CHEN Qiming1,2,3, XU Wentao1,2, ZHOU Youfu1,2(
), YANG Jian1,2, ZHOU Du1,2, CHEN Qiming1,2,3, XU Wentao1,2, ZHOU Youfu1,2( )
)
Received:2024-06-07
Revised:2024-09-02
Published:2025-01-20
Online:2024-09-02
Contact:
ZHOU Youfu, professor. E-mail: yfzhou@fjirsm.ac.cnAbout author:FENG Guanzheng (1998-), male, Master candidate. E-mail: fengguanzheng01@163.com
Supported by:摘要:
碳热还原氮化法是应用最广泛的制备AlN粉体的方法。该工艺制备的AlN粉体纯度高且烧结活性优良, 但是存在反应温度高、原料难以混合均匀等不足。本研究将水热合成与碳热还原氮化法相结合制备AlN纳米粉体。以硝酸铝为铝源、蔗糖为碳源、尿素为沉淀剂, 在200 ℃水热合成碳和勃姆石(γ-AlOOH)均质复合前驱体。前驱体通过静电吸引形成碳紧密包覆勃姆石的核壳结构(γ-AlOOH@C)。与传统前驱体相比, 水热复合物具有超细颗粒、粒度分布均匀、分散性好、反应活性高、环境友好等优点。在氧化铝相的稳定性方面, 碳壳通过抑制氧化铝的表面积损失, 使γ-Al2O3相比刚玉相(α-Al2O3相)的热稳定性更好, γ-Al2O3在碳热还原过程中能保持较高的反应活性, 该反应始于1300 ℃, 止于1400 ℃(比传统碳热还原法低200 ℃)。本研究通过实验和热力学计算验证了相关机理, 为水热法结合碳热法制备非氧化物纳米陶瓷粉体提供了有效的理论和实验依据。
中图分类号:
冯关正, 杨健, 周渡, 陈啟明, 许文涛, 周有福. 水热-碳热合成AlN纳米粉体的机理[J]. 无机材料学报, 2025, 40(1): 104-110.
FENG Guanzheng, YANG Jian, ZHOU Du, CHEN Qiming, XU Wentao, ZHOU Youfu. Mechanism for Hydrothermal-carbothermal Synthesis of AlN Nanopowders[J]. Journal of Inorganic Materials, 2025, 40(1): 104-110.
| Phase | Surface energy/ (J·m-2) | Ref. | Method |
|---|---|---|---|
| α-Al2O3 | 2.04 | [ | MD simulation |
| 2.64 | [ | High-temperature calorimetry | |
| 2.57 | [ | Static lattice calculation | |
| 2.03 | [ | MD simulation | |
| 4.89 | [ | Ab initio calculation | |
| γ-Al2O3 | 0.79 | [ | MD simulation |
| 1.66 | [ | High-temperature calorimetry | |
| 1.53 | [ | High-temperature calorimetry |
Table 1 Compilation of measured and calculated surface energies in literature
| Phase | Surface energy/ (J·m-2) | Ref. | Method |
|---|---|---|---|
| α-Al2O3 | 2.04 | [ | MD simulation |
| 2.64 | [ | High-temperature calorimetry | |
| 2.57 | [ | Static lattice calculation | |
| 2.03 | [ | MD simulation | |
| 4.89 | [ | Ab initio calculation | |
| γ-Al2O3 | 0.79 | [ | MD simulation |
| 1.66 | [ | High-temperature calorimetry | |
| 1.53 | [ | High-temperature calorimetry |
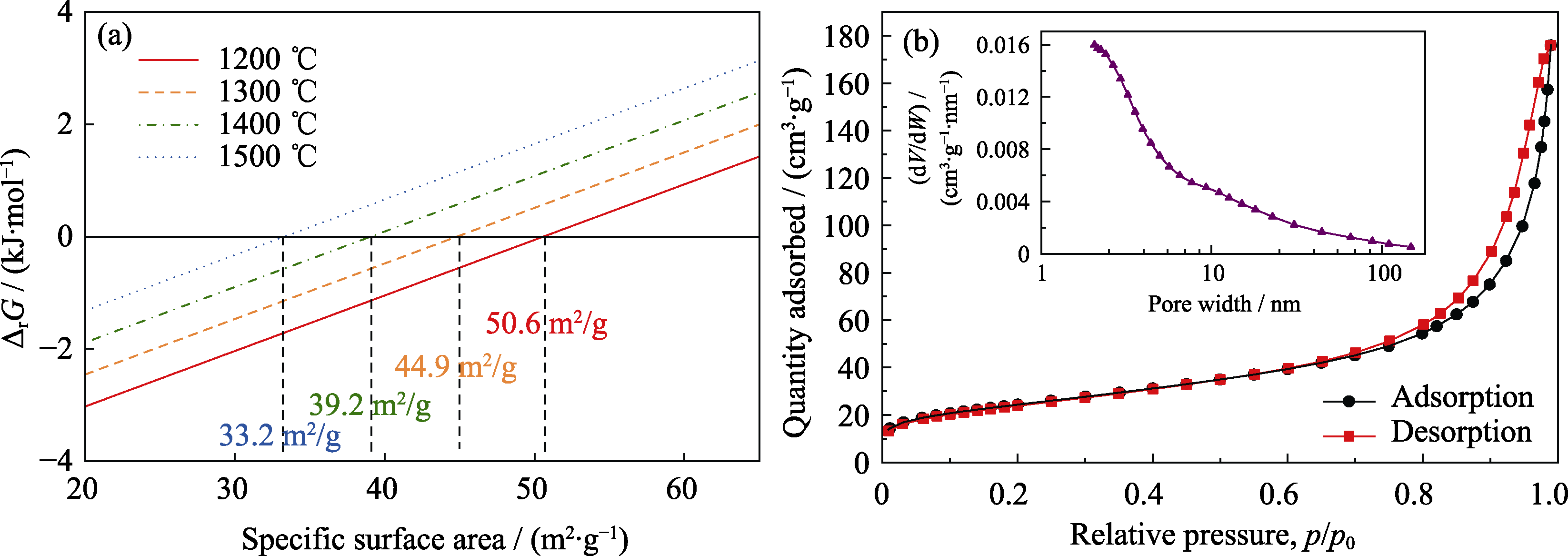
Fig. 5 (a) Gibbs free energy of the transformation from γ-Al2O3 to α-Al2O3 at different temperatures calculated as a function of specific surface area, and (b) N2 adsorption-desorption isotherm of γ-Al2O3 with inset showing pore size distribution determined by application of BJH method to the isotherm
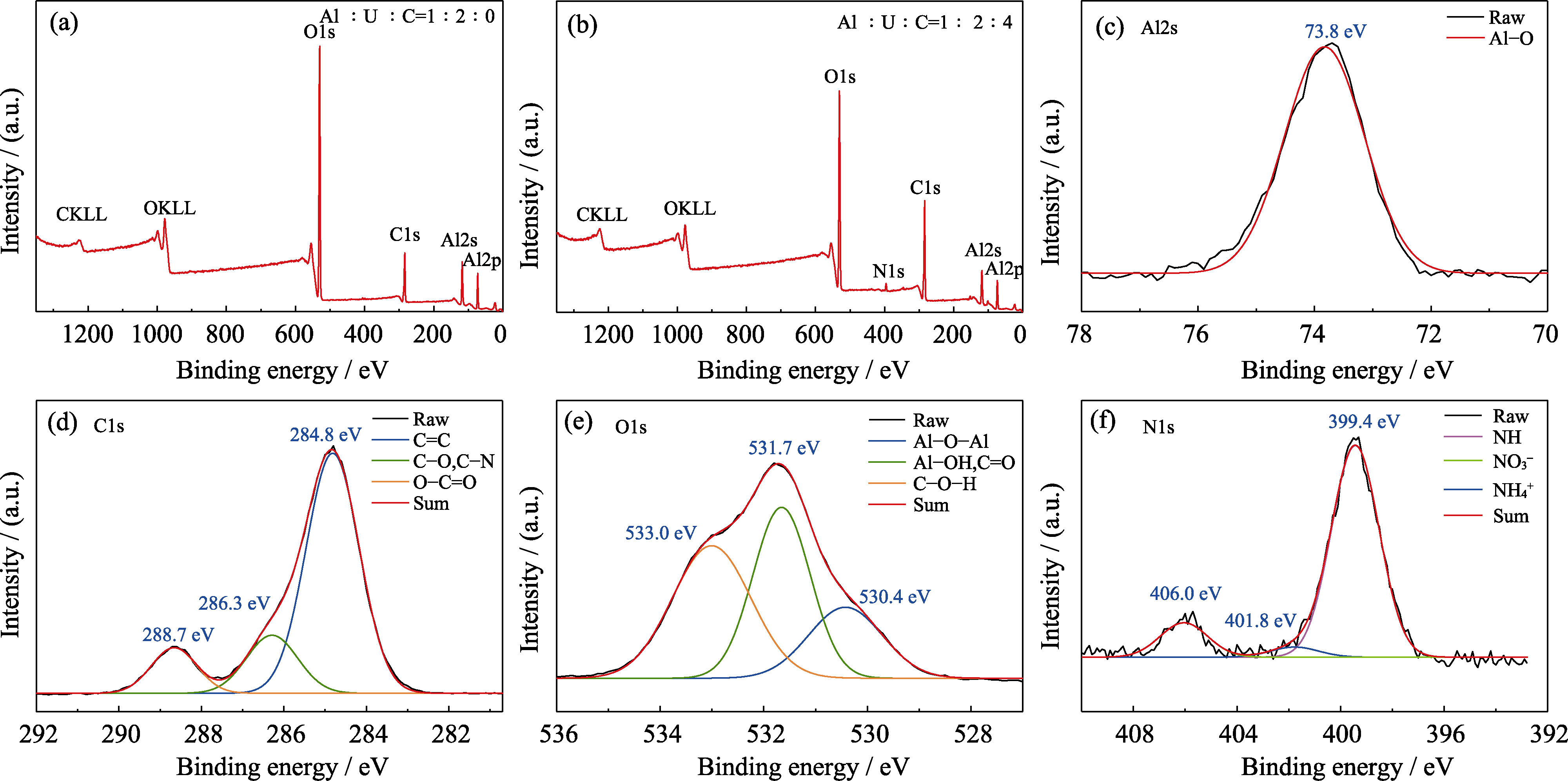
Fig. S3 (a, b) Survey spectra of the precursors at (a) Al : U : C=1 : 2 : 0 and (b) Al : U : C=1 : 2 : 4; (c-f) XPS spectra of (c) Al2s, (d) C1s, (e) O1s, and (f) N1s for the precursors at Al : U : C=1 : 2 : 4
| [1] | YIM W M, PAFF R J. Thermal expansion of AlN, sapphire, and silicon. Journal of Applied Physics, 1974, 45(3): 1456. |
| [2] | SLACK G A, TANZILLI R A, POHL R O, et al. The intrinsic thermal conductivity of AIN. Journal of Physics and Chemistry of Solids, 1987, 48(7): 641. |
| [3] | SELVADURAY G, SHEET L. Aluminium nitride: review of synthesis methods. Materials Science & Technology, 1993, 9(6): 463. |
| [4] | RUTKOWSKI P J, KATA D. Thermal properties of AlN polycrystals obtained by pulse plasma sintering method. Journal of Advanced Ceramics, 2013, 2(2): 180. |
| [5] | SHEPPARD L M. Aluminum nitride: a versatile but challenging material. American Ceramic Society Bulletin, 1990, 69(11): 1801. |
| [6] | BAIK Y, DREW R A L. Aluminum nitride: processing and applications. Key Engineering Materials, 1996, 122: 553. |
| [7] | LEE H M, BHARATHI K, KIM D K. Processing and characterization of aluminum nitride ceramics for high thermal conductivity. Advanced Engineering Materials, 2014, 16(6): 655. |
| [8] | CHIKAMI H, FUKUSHIMA J, HAYASHI Y, et al. Kinetics of microwave synthesis of AlN by carbothermal-reduction-nitridation at low temperature. Journal of the American Ceramic Society, 2018, 101(11): 4905. |
| [9] | WANG Y M, QIAO L, ZHENG J W, et al. Preparation of AlN with low agglomeration using polyethylene glycol and emulsifier to disperse the ultrafine raw powders. Ceramics International, 2023, 49(1): 1390. |
| [10] | KOMEYA K, MATSUKAZE N, MEGURO T. Synthesis of AlN by direct nitridation of Al alloys. Journal of the Ceramic Society of Japan, 1993, 101(1180): 1319. |
| [11] | KIMURA I, ICHIYA K, ISHII M, et al. Synthesis of fine AlN powder by a floating nitridation technique using an N2/NH3 gas mixture. Journal of Materials Science Letters, 1989, 8(3): 303. |
| [12] | WEI Z L, LI K, GE B Z, et al. Synthesis of nearly spherical AlN particles by an in-situ nitriding combustion route. Journal of Advanced Ceramics, 2021, 10(2): 291. |
| [13] | YAMAKAWA T, TATAMI J, WAKIHARA T, et al. Synthesis of AlN nanopowder from γ-Al2O3 by reduction-nitridation in a mixture of NH3-C3H8. Journal of the American Ceramic Society, 2006, 89(1): 171. |
| [14] | ZHANG Q H, GAO L. Synthesis of nanocrystalline aluminum nitride by nitridation of δ-Al2O3 nanoparticles in flowing ammonia. Journal of the American Ceramic Society, 2006, 89(2): 415. |
| [15] | KIM J K, JUNG W S. Nitridation of δ-alumina to aluminum nitride under a flow of ammonia and its mechanism. Journal of the Ceramic Society of Japan, 2011, 119(1389): 351. |
| [16] | JUNG W S. Synthesis of aluminum nitride powder from δ-alumina nanopowders under a mixed gas flow of nitrogen and hydrogen. Ceramics International, 2012, 38(1): 871. |
| [17] | YOSHIMURA M, BYRAPPA K. Hydrothermal processing of materials: past, present and future. Journal of Materials Science, 2008, 43(7): 2085. |
| [18] | WANG Q, LI H, CHEN L Q, et al. Monodispersed hard carbon spherules with uniform nanopores. Carbon, 2001, 39(14): 2211. |
| [19] |
GONG Y T, XIE L, LI H R, et al. Sustainable and scalable production of monodisperse and highly uniform colloidal carbonaceous spheres using sodium polyacrylate as the dispersant. Chemical Communications, 2014, 50(84): 12633.
DOI PMID |
| [20] | CHEN W, LI D, TIAN L, et al. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chemistry, 2018, 20(19): 4438. |
| [21] | XIANG M, ZHOU Y, XU W, et al. Hydrothermal-carbothermal synthesis of highly sinterable AlN nanopowders. Journal of the American Ceramic Society, 2017, 100(6): 2482. |
| [22] | XIANG M, ZHOU Y F, XU W T, et al. Transparent AlN ceramics sintered from nanopowders produced by the wet chemical method. Journal of the Ceramic Society of Japan, 2018, 126(4): 241. |
| [23] | YANG J, CONG Y, LING J R, et al. Preparation of transparent AlON from powders synthesized by novel CRN method. Journal of the European Ceramic Society, 2022, 42(3): 935. |
| [24] | FANKHÄNEL J, SILBERNAGL D, GHASEM Z K M, et al. Mechanical properties of boehmite evaluated by atomic force microscopy experiments and molecular dynamic finite element simulations. Journal of Nanomaterials, 2016, 2016(1): 5017213. |
| [25] | WU J, XU W, DONG T, et al. Self-assembly of graphene reinforced ZrO2 composites with deformation-sensing performance. Ceramics International, 2022, 48(21): 32131. |
| [26] | ANSI V A, SREELAKSHMI P, RAVEENDRAN P, et al. Table sugar derived carbon dot—a promising green reducing agent. Materials Research Bulletin, 2021, 139: 111284. |
| [27] | YANG J, WANG L H, JIANG X X, et al. AlN nanoparticles prepared through a gelation-polymerization process. Ceramics International, 2020, 46(11): 17486. |
| [28] | HE Q, QIN M L, HUANG M, et al. Mechanism and kinetics of combustion-carbothermal synthesis of AlN nanopowders. Ceramics International, 2017, 43(12): 8755. |
| [29] | MAO X X, XU Y G, MAO X J, et al. Synthesis of fine AlN powders by foamed precursor-assisted carbothermal reduction- nitridation method. Journal of Inorganic Materials, 2019, 34(10): 1123. |
| [30] | CARSTENS S, MEYER R, ENKE D. Towards macroporous α-Al2O3—routes, possibilities and limitations. Materials, 2020, 13(7): 1787. |
| [31] | CESTEROS Y, SALAGRE P, MEDINA F, et al. Several factors affecting faster rates of gibbsite formation. Chemistry of Materials, 1999, 11(1): 123. |
| [32] | MCHALE J M, NAVROTSKY A, PERROTTA A J. Effects of increased surface area and chemisorbed H2O on the relative stability of nanocrystalline γ-Al2O3 and α-Al2O3. The Journal of Physical Chemistry B, 1997, 101(4): 603. |
| [33] | MCHALE J M, AUROUX A, PERROTTA A J, et al. Surface energies and thermodynamic phase stability in nanocrystalline aluminas. Science, 1997, 277(5327): 788. |
| [34] | BLONSKI S, GAROFALINI S H. Molecular dynamics simulations of α-alumina and γ-alumina surfaces. Surface Science, 1993, 295(1/2): 263. |
| [35] | TASKER P W. Surfaces of magnesia and alumina. Advances in Ceramics, 1984, 10: 176. |
| [36] | MACKRODT W C, DAVEY R J, BLACK S N, et al. The morphology of α-Al2O3 and α-Fe2O3: the importance of surface relaxation. Journal of Crystal Growth, 1987, 80(2): 441. |
| [37] | CAUSÀ M, DOVESI R, PISANI C, et al.Ab initio characterization of the (0001) and (101̄0) crystal faces of α-alumina. Surface Science, 1989, 215(1/2): 259. |
| [38] | CASTRO R H R, USHAKOV S V, GENGEMBRE L, et al. Surface energy and thermodynamic stability of γ-alumina: effect of dopants and water. Chemistry of Materials, 2006, 18(7): 1867. |
| [39] | TSUGE A, INOUE H, KASORI M, et al. Raw material effect on AIN powder synthesis from Al2O3 carbothermal reduction. Journal of Materials Science, 1990, 25(5): 2359. |
| [1] | 余升阳, 苏海军, 姜浩, 余明辉, 姚佳彤, 杨培鑫. 激光增材制造超高温氧化物陶瓷孔隙缺陷形成及抑制研究进展[J]. 无机材料学报, 2025, 40(9): 944-956. |
| [2] | 李福平, 褚家宝, 仇海波, 党薇, 李晨曦, 赵康, 汤玉斐. SiO2纤维气凝胶的压缩回弹机理[J]. 无机材料学报, 2025, 40(9): 981-988. |
| [3] | 刘江平, 管鑫, 唐振杰, 朱文杰, 罗永明. 含氮挥发性有机化合物催化氧化的研究进展[J]. 无机材料学报, 2025, 40(9): 933-943. |
| [4] | 李荣辉, 钱骏. 一步醇热法制备纳米CeO2-ZrO2固溶体及其除砷性能[J]. 无机材料学报, 2025, 40(9): 989-996. |
| [5] | 魏建文, 张丽娟, 耿琳琳, 李誉, 廖雷, 王敦球. 以ZSM-5/MCM-48为载体制备新型高容量CO2吸附剂的性能及机理研究[J]. 无机材料学报, 2025, 40(7): 833-839. |
| [6] | 余艺平, 肖鹏, 赵长浩, 徐梦迪, 姚立冬, 李伟, 王松. 耐高温层状Ta/Ta0.5Hf0.5C金属陶瓷的高频等离子体风洞烧蚀行为研究[J]. 无机材料学报, 2025, 40(7): 790-798. |
| [7] | 孙晶, 李翔, 毛小建, 章健, 王士维. 月桂酸改性剂对氮化铝粉体抗水解性能的影响[J]. 无机材料学报, 2025, 40(7): 826-832. |
| [8] | 郭子玉, 朱云洲, 王力, 陈健, 李红, 黄政仁. Zn2+催化剂对酚醛树脂/乙二醇制备多孔碳微观孔结构的影响[J]. 无机材料学报, 2025, 40(5): 466-472. |
| [9] | 李紫薇, 弓伟露, 崔海峰, 叶丽, 韩伟健, 赵彤. 前驱体法制备(Zr, Hf, Nb, Ta, W)C-SiC复相陶瓷及性能研究[J]. 无机材料学报, 2025, 40(3): 271-280. |
| [10] | 袁龙, 贾如, 袁梦, 张健, 段羽, 孟祥东. X射线诱导光致变色材料的机理与应用[J]. 无机材料学报, 2025, 40(10): 1097-1110. |
| [11] | 王文婷, 徐敬军, 马科, 李美栓, 李兴超, 李同起. 原位反应/热压合成Ti2AlC-20TiB2复合材料在1000~1300 ℃空气中的高温氧化行为[J]. 无机材料学报, 2025, 40(1): 31-38. |
| [12] | 黄洁, 汪刘应, 王滨, 刘顾, 王伟超, 葛超群. 基于微纳结构设计的电磁性能调控研究进展[J]. 无机材料学报, 2024, 39(8): 853-870. |
| [13] | 何宗倍, 陈放, 刘佃光, 李统业, 曾强. 模拟核芯FCM燃料的振荡烧结行为研究[J]. 无机材料学报, 2024, 39(5): 501-508. |
| [14] | 程博, 安晓航, 李定华, 杨荣杰. ATH/ADP配比对EVA阻燃性能及机理转变的影响[J]. 无机材料学报, 2024, 39(5): 509-516. |
| [15] | 李秋实, 殷广明, 吕伟超, 王怀尧, 李婧琳, 杨红光, 关芳芳. Na+/g-C3N4材料的制备及光催化降解亚甲基蓝机理[J]. 无机材料学报, 2024, 39(10): 1143-1150. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||