无机材料学报 ›› 2023, Vol. 38 ›› Issue (9): 1044-1054.DOI: 10.15541/jim20230049 CSTR: 32189.14.10.15541/jim20230049
所属专题: 【能源环境】钙钛矿(202506); 【能源环境】太阳能电池(202506)
收稿日期:2023-01-31
修回日期:2023-04-28
出版日期:2023-09-20
网络出版日期:2023-06-02
通讯作者:
朱 俊, 教授. E-mail: jzhu@hfut.edu.cn作者简介:张 伦(1992-), 男, 博士研究生. E-mail: zhanglunme@163.com
基金资助:
ZHANG Lun( ), LYU Mei, ZHU Jun(
), LYU Mei, ZHU Jun( )
)
Received:2023-01-31
Revised:2023-04-28
Published:2023-09-20
Online:2023-06-02
Contact:
ZHU Jun, professor. E-mail: jzhu@hfut.edu.cnAbout author:ZHANG Lun (1992-), male, PhD candidate. E-mail: zhanglunme@163.com
Supported by:摘要:
近年来, 有机-无机杂化钙钛矿太阳能电池以其优异的性能和低廉的制造成本受到了广泛关注。然而, 其含有铅元素的毒性以及稳定性阻碍了进一步商业化应用。双钙钛矿材料Cs2AgBiBr6具有稳定性优异、毒性低、载流子寿命长和载流子有效质量小的优势, 是一种颇具潜力的光伏材料, 已被应用于太阳能电池并展现出良好的性能。但是Cs2AgBiBr6钙钛矿太阳能电池的光电转换效率还无法与有机-无机杂化钙钛矿太阳能电池相媲美, 发展仍面临诸多挑战。本文首先介绍了Cs2AgBiBr6的晶体结构及容忍因子等结构参数; 然后介绍了溶液法、反溶剂辅助成膜法、气相法、真空辅助成膜法以及喷涂法等薄膜制备工艺的进展, 评述了各种薄膜制备工艺的优缺点; 接着从元素掺杂、添加剂工程及界面工程(界面能级匹配和界面缺陷钝化)三方面介绍了Cs2AgBiBr6钙钛矿太阳能电池的性能优化策略, 结合近年来的研究进展进行了评述; 最后指出Cs2AgBiBr6钙钛矿太阳能电池面临的挑战, 并从前驱体溶剂工程、带隙工程以及器件降解机理三方面展望了未来研究方向。
中图分类号:
张伦, 吕梅, 朱俊. Cs2AgBiBr6钙钛矿太阳能电池研究进展[J]. 无机材料学报, 2023, 38(9): 1044-1054.
ZHANG Lun, LYU Mei, ZHU Jun. Research Progress of Cs2AgBiBr6 Perovskite Solar Cell[J]. Journal of Inorganic Materials, 2023, 38(9): 1044-1054.
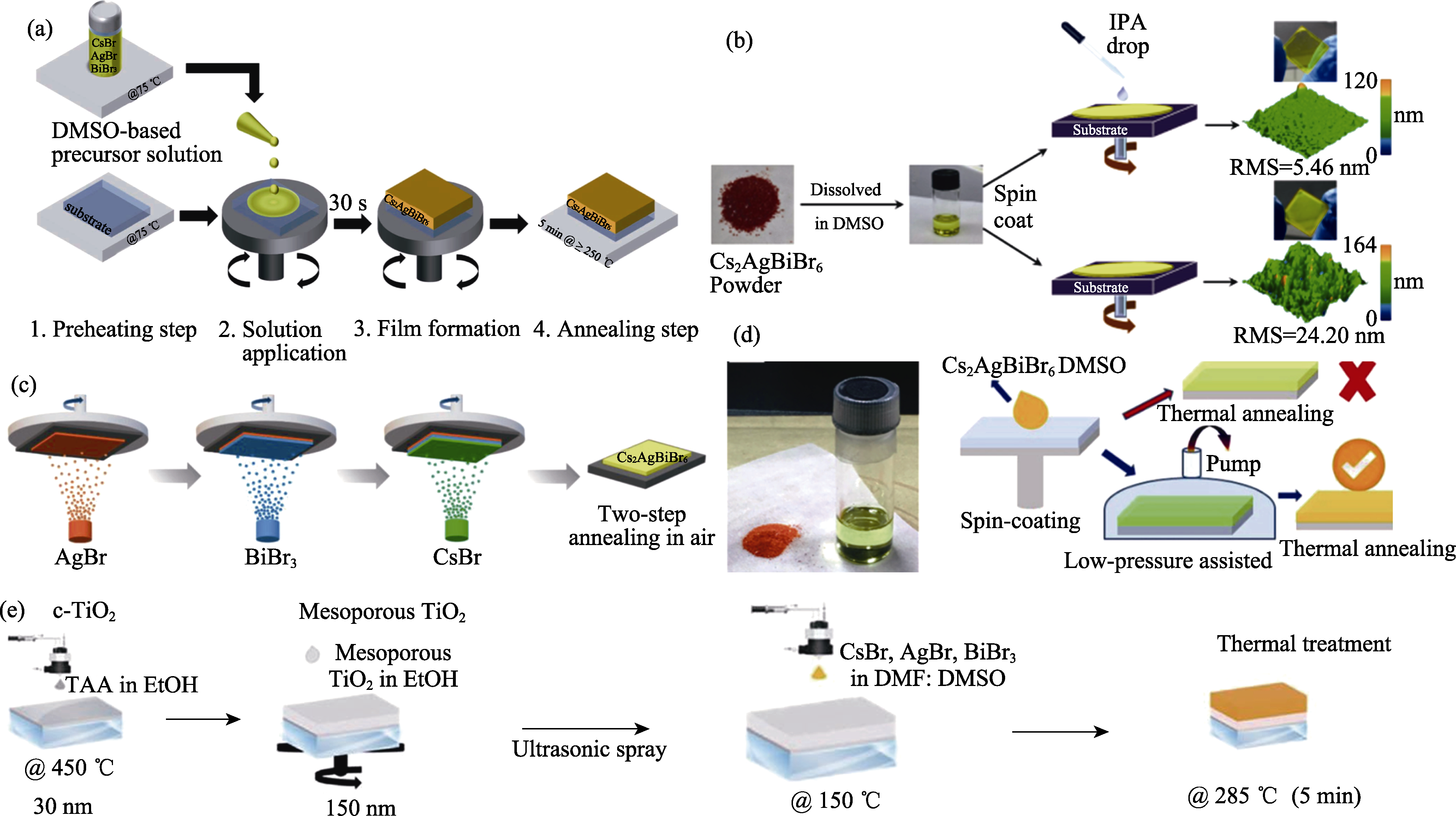
图3 Cs2AgBiBr6薄膜的制备工艺
Fig. 3 Fabrication processes of Cs2AgBiBr6 films (a) Solution processing method[19]; (b) Anti-solvent assisted film forming method[8]; (c) Vapor deposition processing method[35]; (d) Vacuum-assisted film forming method[37]; (e) Spray-coating method[38]
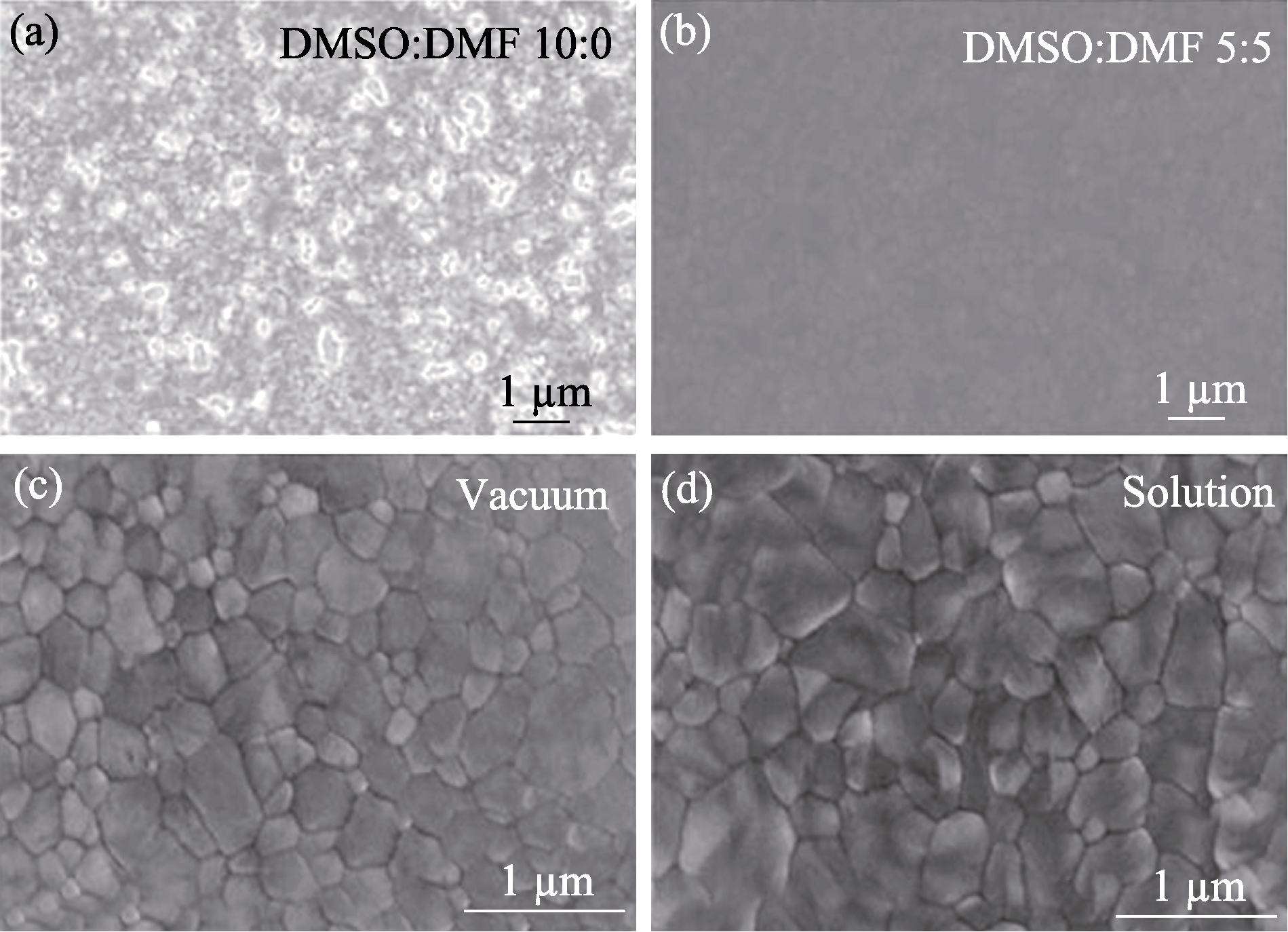
图4 采用(a) DMSO和(b) DMSO+DMF作为前驱溶剂沉积的Cs2AgBiBr6膜的SEM照片[34]; (c)气相法和(d)溶液法制备的Cs2AgBiBr6薄膜的SEM照片[36]
Fig. 4 SEM images of Cs2AgBiBr6 films deposited using (a) DMSO and (b) DMSO+DMF as precursor solvents[34] and prepared by (c) vapor deposition and (d) solution processing[36]
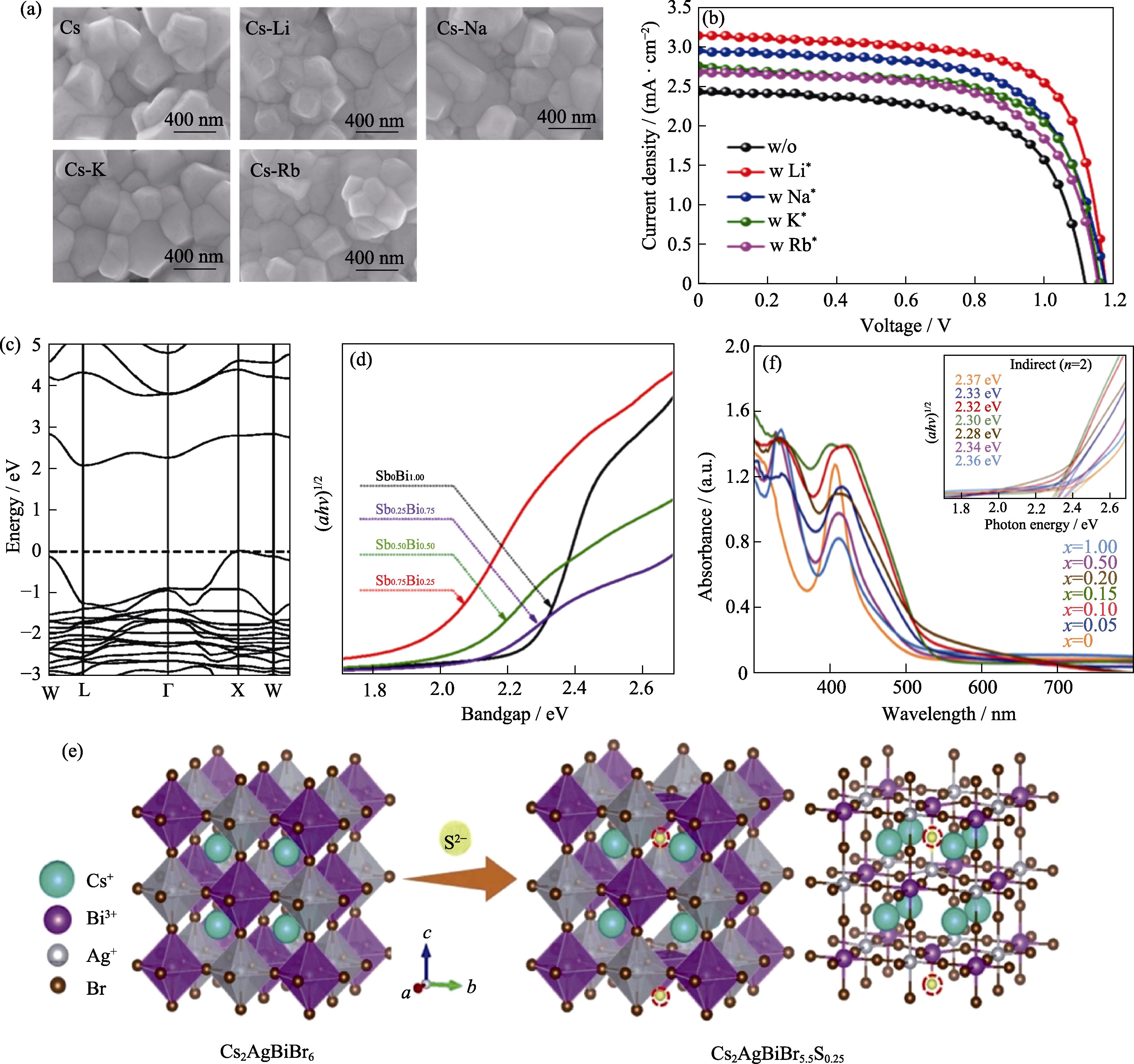
图5 离子掺杂优化Cs2AgBiBr6钙钛矿太阳能电池
Fig. 5 Ion doped Cs2AgBiBr6 perovskite solar cells (a) SEM images of Cs2AgBiBr6, Cs1.99Li0.01AgBiBr6(Cs), Cs1.99Na0.01AgBiBr6(Cs-Li), Cs1.99K0.01AgBiBr6(Cs-Na), and Cs1.99Rb0.01AgBiBr6(Cs-K) films; (b) J-V curves of Cs2AgBiBr6 perovskite solar cells (w/o: Cs2AgBiBr6, w Li+: Cs1.99Li0.01AgBiBr6, w Na+: Cs1.99Na0.01AgBiBr6, w K+: Cs1.99K0.01AgBiBr6, w Rb+: Cs1.99Rb0.01AgBiBr6)[54]; (c) Band structure diagram for Cs2AgBiBr6[57]; (d) Tauc plots of Cs2AgSbxBi1-xBr6 (x=0, 0.25, 0.50, 0.75) films[58]; (e) Crystal structure diagram of Cs2AgBiBr6-2xSx; (f) UV-Vis absorption spectra with inset showing corresponding Tauc plots (right) of Cs2AgBiBr6-2xSx film[60]. Colorful figures are available on website

图6 添加剂工程优化Cs2AgBiBr6薄膜
Fig. 6 Additive engineering optimization of Cs2AgBiBr6 films (a) Schematic illustration of MABr additive assisted Cs2AgBiBr6 crystallization process; (b) SEM images of Cs2AgBiBr6 films prepared (left) without and (right) with MABr[66]; (c) Schematic diagram of the mechanism of additive GuaSCN in the formation process of Cs2AgBiBr6 film; (d) SEM images of Cs2AgBiBr6 films prepared (left) without and (right) with GuaSCN [67]; (e) Schematic illustration of BMPyr+-Br- interaction between ionic liquid BMPyrCl and Cs2AgBiBr6 perovskite; (f) SEM images of Cs2AgBiBr6 films prepared (left) without and (right) with BMPyrCl[70]. Colorful figures are available on website
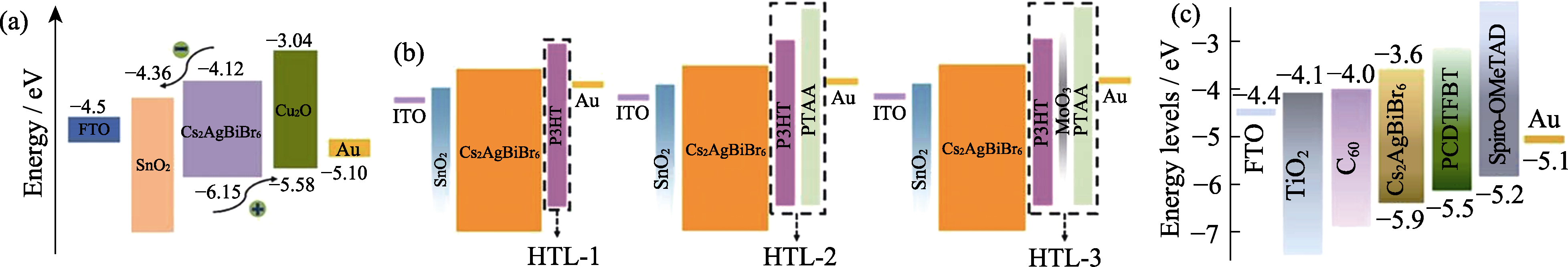
图7 Cs2AgBiBr6钙钛矿太阳能电池界面能级匹配示意图
Fig. 7 Schematic diagrams of the interface energy level alignments in Cs2AgBiBr6 solar cells (a) Cu2O[71] and (b) HTL-1, HTL-2 or HTL-3[72] as hole transport layers; (c) C60/TiO2 as electron transport layers[73]
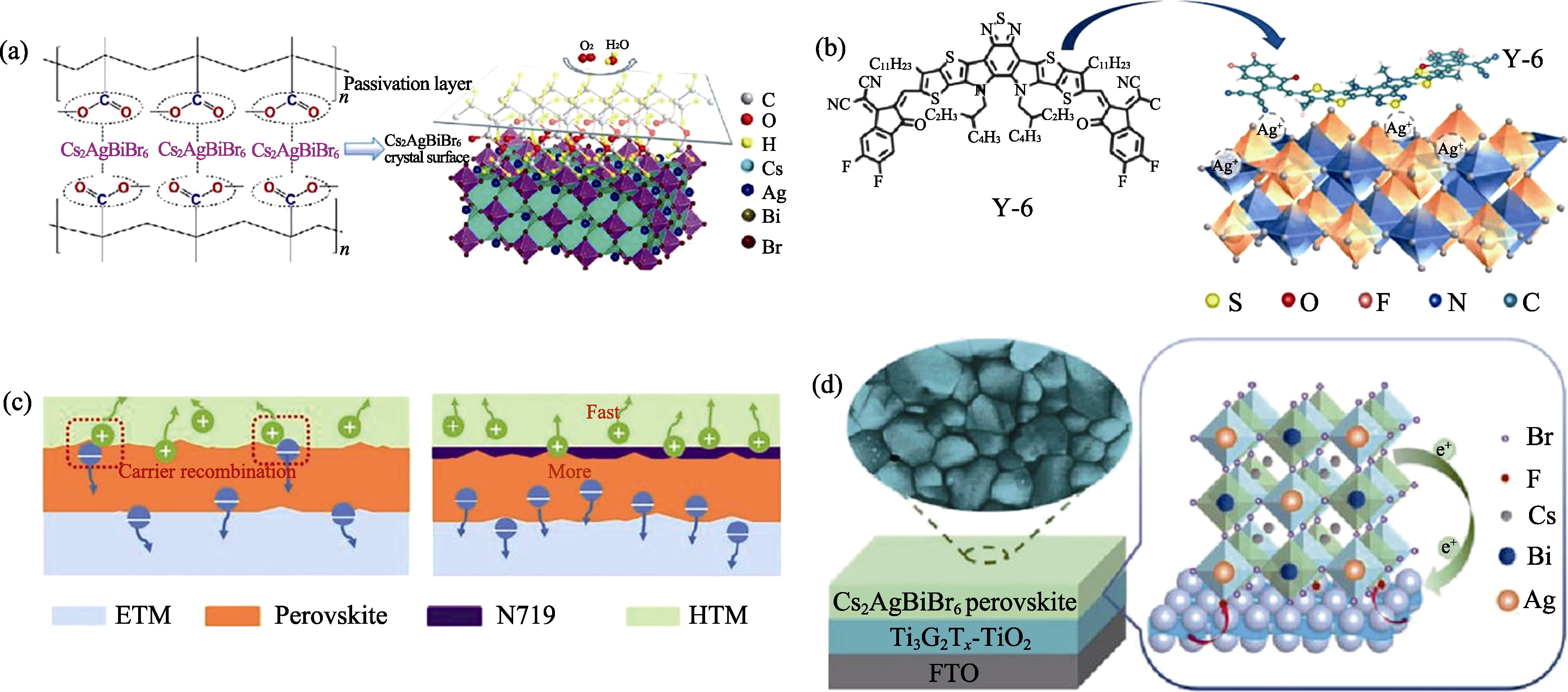
图8 Cs2AgBiBr6钙钛矿太阳能电池界面缺陷钝化示意图
Fig. 8 Schematic diagrams of interface defect passivation in Cs2AgBiBr6 solar cells (a) PMMA[74], (b) Y-6[75] and (c) N719[76] passivating Cs2AgBiBr6/HTL interfaces; (d) MXene passivating Cs2AgBiBr6/ETL interface[77]. Colorful figures are available on website
| [1] |
KOMIJA A, TESHIMA K, SHIRAI Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. Journal of the American Chemical Society, 2009, 131(17): 6050.
DOI PMID |
| [2] | NREL. Best research-cell efficiency chart. [2023-04-05]. https://www.nrel.gov/pv/cell-efficiency.html. |
| [3] |
ZHAO J, WEI L, JIA C, et al. Metallic tin substitution of organic lead perovskite films for efficient solar cells. Journal of Materials Chemistry A, 2018, 6(41): 20224.
DOI URL |
| [4] |
LIU D, YIN Y X, LIU F J, et al. Thickness-dependent highly sensitive photodetection behavior of lead-free all-inorganic CsSnBr3 nanoplates. Rare Metals, 2022, 41(5): 1753.
DOI |
| [5] |
HU W, HE X, FANG Z, et al. Bulk heterojunction gifts bismuth- based lead-free perovskite solar cells with record efficiency. Nano Energy, 2020, 68: 104362.
DOI URL |
| [6] | JIA Q, LI C, TIAN W, et al. Large-grained all-inorganic bismuth- based perovskites with narrow band gap via Lewis acid-base adduct approach. ACS Applied Materials & Interfaces, 2020, 12(39): 43876. |
| [7] |
MA Z, SHI Z, YANG D, et al. Electrically-driven violet light-emitting devices based on highly stable lead-free perovskite Cs3Sb2Br9 quantum dots. ACS Energy Letters, 2020, 5(2): 385.
DOI URL |
| [8] |
GAO W, RAN W, XI J, et al. High-quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2% efficiency. ChemPhysChem, 2018, 19(14): 1696.
DOI URL |
| [9] | 顾津宇, 齐朋伟, 彭扬, 等. 无机非铅钙钛矿太阳能电池研究进展. 物理化学学报, 2017, 33(7): 1379. |
| [10] |
JIANG X, LI H, ZHOU Q, et al. One-step synthesis of SnI2·(DMSO)x adducts for high-performance tin perovskite solar cells. Journal of the American Chemical Society, 2021, 143(29): 10970.
DOI URL |
| [11] |
ZHOU J, HAO M, ZHANG Y, et al. Chemo-thermal surface dedoping for high-performance tin perovskite solar cells. Matter, 2022, 5(2): 683.
DOI URL |
| [12] |
YU B B, CHEN Z, ZHU Y, et al. Heterogeneous 2D/3D tin-halides perovskite solar cells with certified conversion efficiency breaking 14%. Advanced Materials, 2021, 33(36): 2102055.
DOI URL |
| [13] |
SLAVNEY A H, HU T, LINDENBERG A M, et al. A bismuth- halide double perovskite with long carrier recombination lifetime for photovoltaic applications. Journal of the American Chemical Society, 2016, 138(7): 2138.
DOI URL |
| [14] |
PAN W, WU H, LUO J, et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit. Nature Photonics, 2017, 11(11): 726.
DOI URL |
| [15] |
FENG H J, DENG W, YANG K, et al. Double perovskite Cs2BBiX6 (B=Ag, Cu; X=Br, Cl)/TiO2 heterojunction: an efficient Pb-free perovskite interface for charge extraction. The Journal of Physical Chemistry C, 2017, 121(8): 4471.
DOI URL |
| [16] |
LI Y J, WU T, SUN L, et al. Lead-free and stable antimony- silver-halide double perovskite (CH3NH3)2AgSbI6. RSC Advances, 2017, 7(56): 35175.
DOI URL |
| [17] |
IGBARI F, WANG Z K, LIAO L S. Progress of lead-free halide double perovskites. Advanced Energy Materials, 2019, 9(12): 1803150.
DOI URL |
| [18] |
BARTEL C J, CLARY J M, SUTTON C, et al. Inorganic halide double perovskites with optoelectronic properties modulated by sublattice mixing. Journal of the American Chemical Society, 2020, 142(11): 5135.
DOI PMID |
| [19] |
NING W, GAO F. Structural and functional diversity in lead-free halide perovskite materials. Advanced Materials, 2019, 31(22): 1900326.
DOI URL |
| [20] |
PECUNIA V, OCCHIPINTI L G, Chakraborty A, et al. Lead-free halide perovskite photovoltaics: challenges, open questions, and opportunities. APL Materials, 2020, 8(10): 100901.
DOI URL |
| [21] |
GREUL E, PETRUS M L, BINEK A, et al. Highly stable, phase pure Cs2ABiBr6 double perovskite thin films for optoelectronic applications. Journal of Materials Chemistry A, 2017, 5(37): 19972.
DOI URL |
| [22] |
ZHANG Z, SUN Q, LU Y, et al. Hydrogenated Cs2AgBiBr6 for significantly improved efficiency of lead-free inorganic double perovskite solar cell. Nature Communications, 2022, 13: 3397.
DOI |
| [23] |
ZHAO Y, MA F, QU Z, et al. Inactive (PbI2)2RbCl stabilizes perovskite films for efficient solar cells. Science, 2022, 377(6605): 531.
DOI URL |
| [24] |
JEONG S, SEO S, YANG H, et al. Cyclohexylammonium-based 2D/3D perovskite heterojunction with funnel-like energy band alignment for efficient solar cells (23.91%). Advanced Energy Materials, 2021, 11(42): 2102236.
DOI URL |
| [25] |
MIN H, KIM M, LEE S, et al. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science, 2019, 366(6466): 749.
DOI URL |
| [26] |
XIAO K, LIN R, HAN Q, et al. All-perovskite tandem solar cells with 24.2% certified efficiency and area over 1 cm2 using surface- anchoring zwitterionic antioxidant. Nature Energy, 2020, 5(1): 870.
DOI |
| [27] |
TRESS W, SIRTL M T. Cs2AgBiBr6 double perovskites as lead-free alternatives for perovskite solar cells. Solar RRL, 2022, 6(2): 2100770.
DOI URL |
| [28] |
SAVORY C N, WALSH A, SCANLON D O, et al. Can Pb-free halide double perovskites support high-efficiency solar cells. ACS Energy Letters, 2016, 1(5): 949.
DOI URL |
| [29] |
YADAV S C, SRIVASTAVA A, MANJUNATH V, et al. Properties, performance and multidimensional applications of stable lead-free Cs2AgBiBr6 double perovskite. Materials Today Physics, 2022, 26: 100731.
DOI URL |
| [30] |
KUNG P K, LI M H, LIN P Y, et al. Lead-free double perovskites for perovskite solar cells. Solar RRL, 2020, 4(2): 1900306.
DOI URL |
| [31] |
LI C, LU X, DING W, et al. Formability of ABX3 (X=F, Cl, Br, I) halide perovskites. Acta Crystallographica Section B: Structural Science, 2008, 64(6): 702.
DOI URL |
| [32] |
BARTEL C J, SUTTON C, GOLDSMITH B P, et al. New tolerance factor to predict the stability of perovskite oxides and halides. Science Advances, 2019, 5(2): eaav0693.
DOI URL |
| [33] |
SU J, MOU T, WEN J, et al. First-principles study on the structure, electronic, and optical properties of Cs2AgBiBr6-xClx mixed-halide double perovskites. The Journal of Physical Chemistry C, 2020, 124(9): 5371.
DOI URL |
| [34] |
ZHAO D, WANG B, LIANG C, et al. Facile deposition of high-quality Cs2AgBiBr6 films for efficient double perovskite solar cells. Science China Materials, 2020, 63(8): 1518.
DOI |
| [35] |
WANG M, ZENG P, BAI S, et al. High-quality sequential- vapor-deposited Cs2AgBiBr6 thin films for lead-free perovskite solar cells. Solar RRL, 2018, 2(12): 1800217.
DOI URL |
| [36] |
IGBARI F, WANG R, WANG Z K, et al. Composition stoichiometry of Cs2AgBiBr6 films for highly efficient lead-free perovskite solar cells. Nano Letters, 2019, 19(3): 2066.
DOI URL |
| [37] |
WU C, ZHANG Q, LIU Y, et al. The dawn of lead-free perovskite solar cell: highly stable double perovskite Cs2AgBiBr6 film. Advanced Science, 2018, 5(3): 1700759.
DOI URL |
| [38] |
DAEM N, DEWALQUE J, LANG F, et al. Spray-coated lead-free Cs2AgBiBr6 double perovskite solar cells with high open-circuit voltage. Solar RRL, 2021, 5(9): 2100422.
DOI URL |
| [39] |
REN Y, DUAN B, XU Y, et al. New insight into solvent engineering technology from evolution of intermediates via one-step spin-coating approach. Science China Materials, 2017, 60(17): 392.
DOI URL |
| [40] |
TODOROV T, MITZI D B. Direct liquid coating of chalcopyrite light-asorbing layers for photovoltaic devices. European Journal of Inorganic Chemistry, 2010, 2010(1): 17.
DOI URL |
| [41] | YANG J, BAO C, NING W, et al. Stable, high-sensitivity and fast-response photodetectors based on lead-free Cs2AgBiBr6 double perovskite films. Advanced Optical Materials, 2019, 7(13): 1801732. |
| [42] |
DUAN J, YANG Y, TANG J, et al. MACl enhanced electron extraction in all-inorganic Cs2AgBiBr6perovskite photovoltaics. Chemical Communications, 2023, 59(9): 1173.
DOI URL |
| [43] |
PANTALER M, CHO K T, QUELOZ V I E, et al. Hysteresis-free lead-free double-perovskite solar cells by interface engineering. ACS Energy Letters, 2018, 3(8): 1781.
DOI URL |
| [44] |
NING W, WANG F, WU B, et al. Long electron-hole diffusion length in high-quality lead-free double perovskite films. Advanced Materials, 2018, 30(20): 1706246.
DOI URL |
| [45] |
THANH N T K, MACLEAN N, MAHIDDINE S. Mechanisms of nucleation and growth of nanoparticles in solution. Chemical Reviews, 2014, 114(15): 7610.
DOI PMID |
| [46] |
DUNLAP-SHOHL W A, ZHOU Y, PADTURE N P, et al. Synthetic approaches for halide perovskite thin films. Chemical Reviews, 2018, 119(5): 3193.
DOI URL |
| [47] |
JUNG M, JI S G, KIM G, et al. Perovskite precursor solution chemistry: from fundamentals to photovoltaic applications. Chemical Society Reviews, 2019, 48(7): 2011.
DOI PMID |
| [48] | DING B, GAO L, LIANG L, et al. Facile and scalable fabrication of highly efficient lead iodide perovskite thin-film solar cells in air using gas pump method. ACS Applied Materials & Interfaces, 2016, 8(31): 20067. |
| [49] |
BISHOP J E, READ C D, SMITH J A, et al. Fully spray-coated triple-cation perovskite solar cells. Scientific Reports, 2020, 10(1): 6610.
DOI PMID |
| [50] |
TURREN-CRUZ S H, HAGFELDT A, SALIBA M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science, 2018, 362(6413): 449.
DOI URL |
| [51] |
UCHIDA R, BINET S, ARORA N, et al. Insights about the absence of Rb cation from the 3D perovskite lattice: effect on the structural, morphological, and photophysical properties and photovoltaic performance. Small, 2018, 14(36): 1802033.
DOI URL |
| [52] | YI C, LUO J, MELONI S, et al. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy & Environmental Science, 2016, 9(2): 656. |
| [53] |
ZHANG Z, WU C, WANG D, et al. Improvement of Cs2AgBiBr6 double perovskite solar cell by rubidium doping. Organic Electronics, 2019, 74: 204.
DOI URL |
| [54] | LI J, DUAN J, DU J, et al. Alkali metal ion-regulated lead-free, all-inorganic double perovskites for HTM-free, carbon-based solar cells. ACS Applied Materials & Interfaces, 2020, 12(42): 47408. |
| [55] |
MCCLURE E T, BALL M R, WINDL W, et al. Cs2AgBiX6 (X = Br, Cl): new visible light absorbing, lead-free halide perovskite semiconductors. Chemistry of Materials, 2016, 28(5): 1348.
DOI URL |
| [56] |
FILIP M R, HILLMAN S, HAGHIGHIRAD A A, et al. Band gaps of the lead-free halide double perovskites Cs2BiAgCl6and Cs2BiAgBr6 from theory and experiment. The Journal of Physical Chemistry Letters, 2016, 7(13): 2579.
DOI URL |
| [57] |
SEBASTIÁ-LUNA P, CALBO J, ALBIACH-SEBASTIÁN N, et al. Tuning the optical absorption of Sn-, Ge-, and Zn-substituted Cs2AgBiBr6 double perovskites: structural and electronic effects. Chemistry of Materials, 2021, 33(20): 8028.
DOI URL |
| [58] |
LIU Y, ZHANG L, WANG M, et al. Bandgap-tunable double-perovskite thin films by solution processing. Materials Today, 2019, 28: 25.
DOI |
| [59] |
PANTALER M, OLTHOF S, MEERHOLZ K, et al. Bismuth-antimony mixed double perovskites Cs2AgBi1-xSbxBr6 in solar cells. MRS Advances, 2019, 4(64): 3545.
DOI URL |
| [60] |
PAI N, LU J, WANG M, et al. Enhancement of the intrinsic light harvesting capacity of Cs2AgBiBr6 double perovskite via modification with sulphide. Journal of Materials Chemistry A, 2020, 8(4): 2008.
DOI URL |
| [61] |
LYU M, LEEA D K, PARK N G. Effect of alkaline earth metal chloride additives BCl2 (B = Mg, Ca, Sr and Ba) on photovoltaic performance of FAPbI3 based perovskite solar cells. Nanoscale Horiz, 2020, 5(9): 1332.
DOI URL |
| [62] |
LYU M, PARK N G. Effect of additives AX (A=FA, MA, Cs, Rb, NH4, X=Cl, Br, I) in FAPbI3 on photovoltaic parameters of perovskite solar cells. Solar RRL, 2020, 4(10): 2000331.
DOI URL |
| [63] |
FENG J, ZHU X, YANG Z, et al. Record efficiency stable flexible perovskite solar cell using effective additive assistant strategy. Advanced Materials, 2018, 30(35): 1801418.
DOI URL |
| [64] |
LI T, PAN Y, WANG Z, et al. Additive engineering for highly efficient organic-inorganic halide perovskite solar cells: recent advances and perspectives. Journal of Materials Chemistry A, 2017, 5(25): 12602.
DOI URL |
| [65] |
MOORE D T, SAI H, TAN K W, et al. Crystallization kinetics of organic-inorganic trihalide perovskites and the role of the lead anion in crystal growth. Journal of the American Chemical Society, 2015, 137(6): 2350.
DOI PMID |
| [66] |
WU H, WANG Y, LIU A, et al. Methylammonium bromide assisted crystallization for enhanced lead-free double perovskite photovoltaic performance. Advanced Functional Materials, 2022, 32(14): 2109402.
DOI URL |
| [67] |
YANG X, XIE A, XIANG H, et al. First investigation of additive engineering for highly efficient Cs2AgBiBr6-based lead-free inorganic perovskite solar cells. Applied Physics Reviews, 2021, 8: 041402.
DOI URL |
| [68] |
YANG A, ZHANG L, XU Y, et al. VOC over 1.2 V for Cs2AgBiBr6 solar cells based on formamidinium acetate additive. Journal of Materials Science: Materials in Electronics, 2022, 33: 18758.
DOI |
| [69] |
ZHANG L, XU Y, NIU P J, et al. Regulating the film crystallization kinetics with thiourea additive in Cs2AgBiBr6 solar cells. Journal of Physics D: Applied Physics, 2023, 56(7): 075501.
DOI |
| [70] |
LI J, MENG X, WU Z, et al. Pinning bromide ion with ionic liquid in lead-free Cs2AgBiBr6 double perovskite solar cells. Advanced Functional Materials, 2022, 32(25): 2112991.
DOI URL |
| [71] | XIAO B, TAN Y, YI Z, et al. Band matching strategy for all-inorganic Cs2AgBiBr6 double perovskite solar cells with high photovoltage. ACS Applied Materials & Interfaces, 2021, 13(31): 37027. |
| [72] |
ZHANG Z, WU C, WANG D, et al. Efficient nonlead double perovskite solar cell with multiple hole transport layers. ACS Applied Energy Materials, 2020, 3(10): 9594.
DOI URL |
| [73] | LUO T, ZHANG Y, CHANG X, et al. Dual interfacial engineering for efficient Cs2AgBiBr6 based solar cells. Journal of Energy Chemistry, 2021, 53: 373. |
| [74] |
LI J, YAN F, YANG P, et al. Suppressing interfacial shunt loss via functional polymer for performance improvement of lead-free Cs2AgBiBr6 double perovskite solar cells. Solar RRL, 2021, 6(4): 2100791.
DOI URL |
| [75] |
LI B, WU X, ZHANG S, et al. Efficient and stable Cs2AgBiBr6 double perovskite solar cells through in-situ surface modulation. Chemical Engineering Journal, 2022, 446: 137144.
DOI URL |
| [76] |
YANG X, CHEN Y, LIU P, et al. Simultaneous power conversion efficiency and stability enhancement of Cs2AgBiBr6 lead-free inorganic perovskite solar cell through adopting a multifunctional dye interlayer. Advanced Functional Materials, 2020, 30(23): 2001557.
DOI URL |
| [77] |
LI Z, WANG P, MA C, et al. Single-layered MXene nanosheets doping TiO2 for efficient and stable double perovskite solar cells. Journal of the American Chemical Society, 2021, 143(6): 2593.
DOI URL |
| [78] |
WANG B, LI N, YANG L, et al. Chlorophyll derivative-sensitized TiO2 electron transport layer for record efficiency of Cs2AgBiBr6 double perovskite solar cells. Journal of the American Chemical Society, 2021, 143(5): 2207.
DOI URL |
| [1] | 朱文杰, 唐璐, 陆继长, 刘江平, 罗永明. 钙钛矿型氧化物催化氧化挥发性有机化合物的研究进展[J]. 无机材料学报, 2025, 40(7): 735-746. |
| [2] | 胡智超, 杨鸿宇, 杨鸿程, 孙成礼, 杨俊, 李恩竹. P-V-L键理论在微波介质陶瓷性能调控中的应用[J]. 无机材料学报, 2025, 40(6): 609-626. |
| [3] | 吴琼, 沈炳林, 张茂华, 姚方周, 邢志鹏, 王轲. 铅基织构压电陶瓷研究进展[J]. 无机材料学报, 2025, 40(6): 563-574. |
| [4] | 张碧辉, 刘小强, 陈湘明. Ruddlesden-Popper结构杂化非常规铁电体的研究进展[J]. 无机材料学报, 2025, 40(6): 587-608. |
| [5] | 吴杰, 杨帅, 王明文, 李景雷, 李纯纯, 李飞. 铅基织构压电陶瓷的发展历程、现状与挑战[J]. 无机材料学报, 2025, 40(6): 575-586. |
| [6] | 姜昆, 李乐天, 郑木鹏, 胡永明, 潘勤学, 吴超峰, 王轲. PZT陶瓷的低温烧结研究进展[J]. 无机材料学报, 2025, 40(6): 627-638. |
| [7] | 倪晓萌, 许方贤, 刘静静, 张帅, 郭华飞, 袁宁一. 甲脒亚磺酸添加剂提升Sb2(S,Se)3薄膜质量及其光伏性能[J]. 无机材料学报, 2025, 40(4): 372-378. |
| [8] | 田睿智, 兰正义, 殷杰, 郝南京, 陈航榕, 马明. 基于微流控技术的纳米无机生物材料制备: 原理及其研究进展[J]. 无机材料学报, 2025, 40(4): 337-347. |
| [9] | 张继国, 吴田, 赵旭, 杨钒, 夏天, 孙士恩. 钠离子电池正极材料循环稳定性提升策略及产业化进程[J]. 无机材料学报, 2025, 40(4): 348-362. |
| [10] | 殷杰, 耿佳毅, 王康龙, 陈忠明, 刘学建, 黄政仁. SiC陶瓷的3D打印成形与致密化新进展[J]. 无机材料学报, 2025, 40(3): 245-255. |
| [11] | 谌广昌, 段小明, 朱金荣, 龚情, 蔡德龙, 李宇航, 杨东雷, 陈彪, 李新民, 邓旭东, 余瑾, 刘博雅, 何培刚, 贾德昌, 周玉. 直升机特定结构先进陶瓷材料研究进展与应用展望[J]. 无机材料学报, 2025, 40(3): 225-244. |
| [12] | 范晓波, 祖梅, 杨向飞, 宋策, 陈晨, 王子, 罗文华, 程海峰. 质子调控型电化学离子突触研究进展[J]. 无机材料学报, 2025, 40(3): 256-270. |
| [13] | 海热古·吐逊, 郭乐, 丁嘉仪, 周嘉琪, 张学良, 努尔尼沙·阿力甫. 上转换荧光探针辅助的光学成像技术在肿瘤显影中的应用研究进展[J]. 无机材料学报, 2025, 40(2): 145-158. |
| [14] | 孙树娟, 郑南南, 潘昊坤, 马猛, 陈俊, 黄秀兵. 单原子催化剂制备方法的研究进展[J]. 无机材料学报, 2025, 40(2): 113-127. |
| [15] | 陶桂龙, 支国伟, 罗添友, 欧阳佩东, 衣新燕, 李国强. 空腔型薄膜体声波滤波器的关键技术进展[J]. 无机材料学报, 2025, 40(2): 128-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||