无机材料学报 ›› 2020, Vol. 35 ›› Issue (3): 309-314.DOI: 10.15541/jim20190370 CSTR: 32189.14.10.15541/jim20190370
收稿日期:2019-07-22
修回日期:2019-08-28
出版日期:2020-03-20
网络出版日期:2019-10-23
作者简介:朱明玉(1995-), 女, 硕士研究生. E-mail: myzhu2018@126.com
基金资助:
ZHU Mingyu,FAN Dezhe,LIU Bei,LIU Shuya,FANG Ming( ),TAN Xiaoli
),TAN Xiaoli
Received:2019-07-22
Revised:2019-08-28
Published:2020-03-20
Online:2019-10-23
About author:ZHU Mingyu(1995-), female, Master candidate. E-mail: myzhu2018@126.com
Supported by:摘要:
Cr(VI)具有非常大的生物毒性, 去除溶液中的Cr(VI)是当前的一个研究热点。本研究制备了C@K2Ti6O13分级纳米材料, 并用不同表征手段对材料的物相和结构等进行表征, 进一步探究了初始pH、吸附时间、离子强度等对C@K2Ti6O13复合纳米结构吸附Cr(VI)的影响。实验结果表明C@K2Ti6O13复合纳米结构对Cr(VI)有较强的吸附能力, 1 h内去除率能够达到50%以上, 其吸附动力学符合准二级动力学模型, 吸附热力学符合Langmuir等温吸附模型, 表明这种分级纳米材料在环境治理方面应用潜力巨大。
中图分类号:
朱明玉, 范德哲, 刘蓓, 刘舒雅, 方明, 谭小丽. C@K2Ti6O13分级纳米材料对Cr(VI)的吸附去除[J]. 无机材料学报, 2020, 35(3): 309-314.
ZHU Mingyu, FAN Dezhe, LIU Bei, LIU Shuya, FANG Ming, TAN Xiaoli. C@K2Ti6O13 Hierarchical Nano Materials: Effective Adsorption Removal of Cr(VI)[J]. Journal of Inorganic Materials, 2020, 35(3): 309-314.
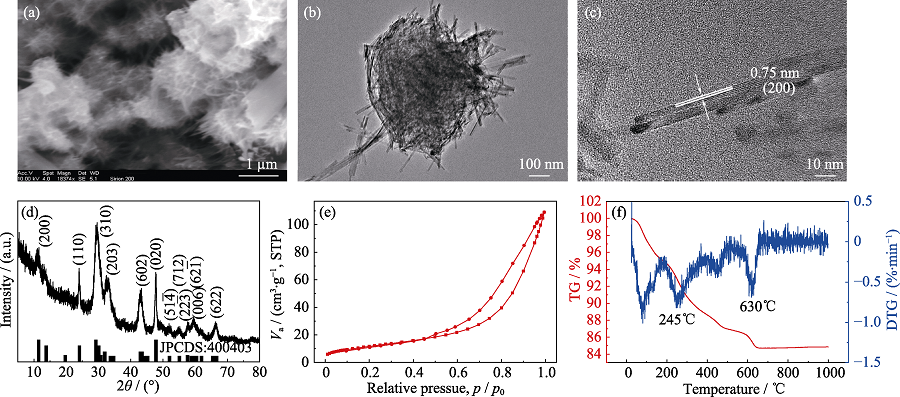
图1 C@K2Ti6O13的(a) SEM照片, (b) TEM照片, (c) HETEM照片, (d) XRD图谱, (e) N2吸附-脱附等温线和(f)热重及其微分曲线
Fig. 1 (a) SEM image, (b) TEM image, (c) HRTEM image, (d) XRD pattern, (e) N2 adsorption-desorption isotherm and (f) TGA- DTG curves of C@K2Ti6O13 hierarchical nanostructures
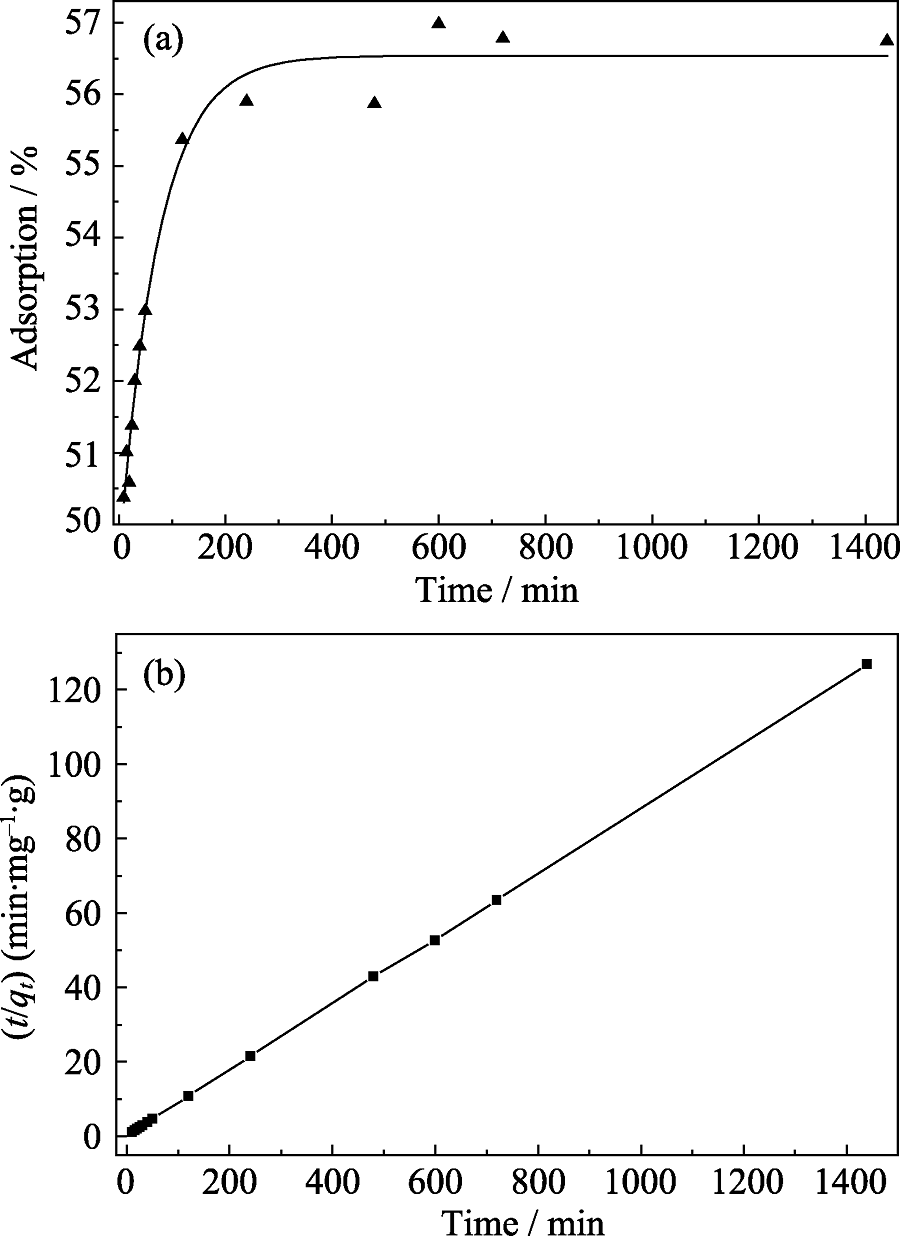
图4 (a) 反应时间对C@K2Ti6O13吸附去除Cr(VI)效果的影响及其(b)准二级动力学模型
Fig. 4 (a) Effect of contact time on Cr(VI) adsorption onto C@K2Ti6O13 HNMs and (b) corresponding fitting curve by the pseudo-second-order kinetic model
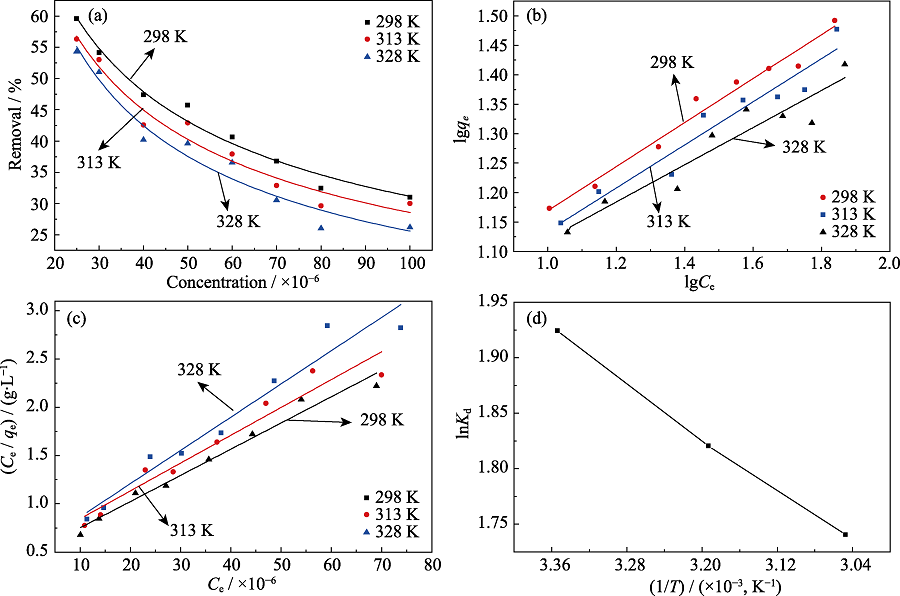
图5 Cr(VI)在C@K2Ti6O13上的(a)吸附等温线, (b)Langmuir模型的拟合, (c)Freundlich模型的拟合和(d)吸附热力学拟合曲线
Fig. 5 (a) Adsorption isotherms, (b) Langmuir isotherm model, (c) Freundlich isotherm model, and (d) plot of lnKd vs 1/T of the Cr(VI) on C@K2Ti6O13
| T/K | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|
| qm | b | R2 | n | Kf | R2 | |
| 298 | 37 | 0.056 | 0.97 | 2.7 | 2.2 | 0.97 |
| 313 | 37 | 0.052 | 0.95 | 2.7 | 2.2 | 0.93 |
| 328 | 29 | 0.066 | 0.94 | 3.2 | 2.2 | 0.89 |
表1 C@K2Ti6O13复合结构对Cr(VI)吸附的Freundlich和Langmuir等温吸附模型拟合相关参数
Table 1 Parameters simulated by Langmuir and Freundlich models of C@K2Ti6O13
| T/K | Langmuir model | Freundlich model | ||||
|---|---|---|---|---|---|---|
| qm | b | R2 | n | Kf | R2 | |
| 298 | 37 | 0.056 | 0.97 | 2.7 | 2.2 | 0.97 |
| 313 | 37 | 0.052 | 0.95 | 2.7 | 2.2 | 0.93 |
| 328 | 29 | 0.066 | 0.94 | 3.2 | 2.2 | 0.89 |
| ΔHθ /(kJ·mol-1) | ΔSθ /(kJ·K-1·mol-1) | ΔGθ/(kJ·mol-1) | ||
|---|---|---|---|---|
| 298 K | 313 K | 323 K | ||
| 0.075 | 0.0124 | -4.77 | -4.74 | -4.75 |
表2 对Cr(VI)吸附的热力学参数
Table 2 Thermodynamic parameters for Cr(VI) adsorption
| ΔHθ /(kJ·mol-1) | ΔSθ /(kJ·K-1·mol-1) | ΔGθ/(kJ·mol-1) | ||
|---|---|---|---|---|
| 298 K | 313 K | 323 K | ||
| 0.075 | 0.0124 | -4.77 | -4.74 | -4.75 |
| [1] | GLADYSZ-PLASKA AGNIESZKA, MAJDAN MAREK, PIKUS STANISLAW , et al. Simultaneous adsorption of chromium(VI) and phenol on natural red clay modified by HDTMA. Chemical Engineering Journal, 2012,179:140-150. |
| [2] | MELITA LARISA, POPESCU MARIA . Removal of Cr(VI) from industrial water effluents and surface waters using activated composite membranes. Journal of Membrane Science, 2008,312(1/2):157-162. |
| [3] | LI XIAO-FAN, SHI SHAO-YUAN, CAO HONG-BIN , et al. Comparative study of chromium(VI) removal from simulated industrial wastewater with ion exchange resins. Russian Journal of Physical Chemistry A, 2018,92(6):1229-1236. |
| [4] | CHUANG SHENGMING, YA VINH, FENG CHIAOLIN , et al. Electrochemical Cr(VI) reduction using a sacrificial Fe anode: impacts of solution chemistry and stoichiometry. Separation and Purification Technology, 2018,191:167-172. |
| [5] | HONG HAN-LIE, JIANG WEI-TEH, ZHANG XIAO-LING , et al. Adsorption of Cr(VI) on STAC-modified rectorite. Applied Clay Science, 2008,42(1/2):292-299. |
| [6] | WANG JIAN, LIANG YU, JIN QING-QING , et al. Simultaneous removal of graphene oxide and chromium(VI) on the rare earth doped titanium dioxide coated carbon sphere composites. ACS Sustainable Chemistry & Engineering, 2017,5(6):5550-5561. |
| [7] | MOHANTY KAUSTUBHA, JHA MOUSAM, MEIKAP B C , et al. Removal of chromium(VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. Chemical Engineering Science, 2005,60:3049-3059. |
| [8] | LAZARIDIS N K, PANDI T A, MATIS K A . Chromium(VI) removal from aqueous solutions by Mg-Al-CO3 hydrotalcite: sorption-desorption kinetic and equilibrium studies. Industrial & Engineering Chemistry Research, 2004,43:2209-2215. |
| [9] | YANG ZHI-HUI, WANG BING, CHAI LI-YUAN , et al. Removal of Cr,(Ⅲ) and Cr(VI) from aqueous solution by adsorption on sugarcane pulp residue. Journal of Central South University of Technology, 2009,16(1):101-107. |
| [10] | LIU XIAO-YUAN, LIU BAO-DAN, JIANG YA-NAN , et al. In-situ synthesis of perovskite SrTiO3 nanostructures with modified morphology and tunable optical absorption property. Journal of Inorganic Materials, 2019,34(1):65-71. |
| [11] | TAN XIAO-LI, LIU GE, MEI HUI-YANG , et al. Fabrication of GO/Na2Ti3O7 composite and its efficient removal of 60Co(II) from radioactive wastewater. Science China-Chemistry, 2019,49(1):145-154. |
| [12] | MARIANA HINOJOSA-REYES, CAMPOSECO-SOLIS ROBERTO, RUIZ FACUNDO . H2Ti3O7 titanate nanotubes for highly effective adsorption of basic fuchsin dye for water purification. Microporous and Mesoporous Materials, 2019,276:183-191. |
| [13] | HUANG JI-QUAN, CAO YONG-GE, LIU ZHU-GUANG , et al. Efficient removal of heavy metal ions from water system by titanate nanoflowers. Chemical Engineering Journal, 2012,180:75-80. |
| [14] | LIU WEN, SUN WEI-LING, HAN YUN-FEI , et al. Adsorption of Cu(II) and Cd(II) on titanate nanomaterials synthesized via hydrothermal method under different NaOH concentrations: role of sodium content. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 2014,452:138-147. |
| [15] | ZHU MING-YU, CAI YA-WEN, LIU SHU-YA , et al. K2Ti6O13 hybridized graphene oxide: effective enhancement in photodegradation of RhB and photoreduction of U(VI). Environmental Pollution, 2019,248:448-455. |
| [16] | ZHU HONG-SHAN, TAN XIAO-LI, TAN LI-QIANG , et al. Magnetic porous polymers prepared via high internal phase emulsions for efficient removal of Pb2+ and Cd2+. ACS Sustainable Chemistry & Engineering, 2018,6(4):5206-5213. |
| [17] | LIU GE, MEI HUI-YANG, TAN XIAO-LI , et al. Enhancement of Rb+ and Cs+ removal in 3D carbon aerogel-supported Na2Ti3O7. Journal of Molecular Liquids, 2018,262:476-483. |
| [18] | TAN XIAO-LI, FANG MING, TAN LI-QIANG , et al. Core-shell hierarchical C@Na2Ti3O7·9H2O nanostructures for the efficient removal of radionuclides. Environmental Science: Nano, 2018,5(5):1140-1149. |
| [19] | LIU SHOU-XIN, SUN JIAN, HUANG ZHAN-HUA . Carbon spheres/activated carbon composite materials with high Cr(VI) adsorption capacity prepared by a hydrothermal method. Journal of Hazardous Materials, 2010,173(1/2/3):377-383. |
| [20] | WU JIN, ZHU HONG-SHAN, LIU GE , et al.Fabrication of core- shell CMNP@PmPD nanocomposite for efficient As(V) adsorption and reduction. ACS Sustainable Chemistry & Engineering,, 2017,5(5):4399-4407. |
| [21] | WANG JIAN, WANG XIANG-XUE, ZHAO GUI-XIA , et al.Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium(VI) from aqueous solutions. Chemical Engineering Journal, 2018,334:569-578. |
| [22] | CAI YA-WEN, WANG XIN, FENG JING-HUA , et al.Fully phosphorylated 3D graphene oxide foam for the significantly enhanced U(VI) sequestration. Environmental Pollution, 2019,249:434-442. |
| [23] | SEHATI S, ENTEZARI M H . Ultrasound facilitates the synthesis of potassium hexatitanate. Ultrasonics Sonochemistry, 2016,32:348-356. |
| [24] | WANG JIAN, ZHU MING-YU, CHEN ZHONG-SHAN , et al. Polyacrylamide modified molybdenum disulfide composites for efficient removal of graphene oxide from aqueous solutions. Chemical Engineering Journal, 2019,361:651-659. |
| [25] | PANG HONG-WEI, DIAO ZHUO-FAN, WANG XIANG-XUE , et al. Adsorptive and reductive removal of U(VI) by dictyophora indusiate-derived biochar supported sulfide NZVI from wastewater. Chemical Engineering Journal, 2019,366:368-377. |
| [26] | FANG MING, TAN XIAO-LI . Review on the mechanism of metal surface plasmon resonance enhanced photocatalysis of semiconductor nanomaterials. Journal of Nantong University (Natural Science Edition), 2019,18(2):1-13. |
| [27] | WANG JIAN, WANG PENG-YI, WANG HUI-HUI , et al.Preparation of molybdenum disulfide coated Mg/Al layered double hydroxide composites for efficient removal of chromium(VI). ACS Sustainable Chemistry & Engineering, 2017,5(8):7165-7174. |
| [1] | 魏建文, 张丽娟, 耿琳琳, 李誉, 廖雷, 王敦球. 以ZSM-5/MCM-48为载体制备新型高容量CO2吸附剂的性能及机理研究[J]. 无机材料学报, 2025, 40(7): 833-839. |
| [2] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [3] | 梁锐辉, 钟鑫, 洪督, 黄利平, 牛亚然, 郑学斌. Yb2O3改性硅黏结层的环境障涂层体系耐高温水氧腐蚀行为研究[J]. 无机材料学报, 2025, 40(4): 425-432. |
| [4] | 洪培萍, 梁龙, 吴炼, 马颖康, 庞浩. ZIF-67结构调控及其对盐酸金霉素的吸附性能研究[J]. 无机材料学报, 2025, 40(4): 388-396. |
| [5] | 李捷, 罗志新, 崔阳, 张广珩, 孙鲁超, 王京阳. 大气等离子喷涂Y3Al5O12/Al2O3陶瓷涂层的CMAS腐蚀抗力[J]. 无机材料学报, 2024, 39(6): 671-680. |
| [6] | 方光武, 谢浩元, 张华军, 高希光, 宋迎东. CMC-EBC损伤耦合机理及一体化设计研究进展[J]. 无机材料学报, 2024, 39(6): 647-661. |
| [7] | 陈甜, 罗媛, 朱刘, 郭学益, 杨英. 有机-无机共添加增强柔性钙钛矿太阳能电池机械弯曲及环境稳定性能[J]. 无机材料学报, 2024, 39(5): 477-484. |
| [8] | 吴光宇, 舒松, 张洪伟, 李建军. 接枝内酯基活性炭增强苯乙烯吸附性能研究[J]. 无机材料学报, 2024, 39(4): 390-398. |
| [9] | 谢天, 宋二红. 弹性应变对C、H、O在过渡金属氧化物表面吸附的影响[J]. 无机材料学报, 2024, 39(11): 1292-1300. |
| [10] | 晁少飞, 薛艳辉, 吴琼, 伍复发, MUHAMMAD Sufyan Javed, 张伟. MXene异质结Ti-O-H-O电子快速通道促进高效率储钾[J]. 无机材料学报, 2024, 39(11): 1212-1220. |
| [11] | 陶顺衍, 杨加胜, 邵芳, 吴应辰, 赵华玉, 董绍明, 张翔宇, 熊瑛. 航机CMC热端部件用热喷涂涂层的机遇与挑战[J]. 无机材料学报, 2024, 39(10): 1077-1083. |
| [12] | 范栋, 钟鑫, 王亚文, 张振忠, 牛亚然, 李其连, 张乐, 郑学斌. 富铝CMAS对稀土硅酸盐环境障涂层的腐蚀行为与机制研究[J]. 无机材料学报, 2023, 38(5): 544-552. |
| [13] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [14] | 郭春霞, 陈伟东, 闫淑芳, 赵学平, 杨傲, 马文. 埃洛石纳米管负载锆氧化物吸附水中砷的研究[J]. 无机材料学报, 2023, 38(5): 529-536. |
| [15] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||