无机材料学报 ›› 2020, Vol. 35 ›› Issue (3): 315-323.DOI: 10.15541/jim20190349 CSTR: 32189.14.10.15541/jim20190349
所属专题: 2020年环境材料论文精选(三)有机小分子去除; 【虚拟专辑】污染物吸附水处理(2020~2021)
杜旭东1,唐城元1,杨小丽2,程建波1,贾玉珂1,杨树斌1( )
)
收稿日期:2019-07-15
修回日期:2019-09-12
出版日期:2020-03-20
网络出版日期:2019-10-23
作者简介:杜旭东(1995-), 男, 硕士研究生. E-mail: 2062296740@qq.com
基金资助:
DU Xudong1,TANG Chengyuan1,YANG Xiaoli2,CHENG Jianbo1,JIA Yuke1,YANG Shubin1( )
)
Received:2019-07-15
Revised:2019-09-12
Published:2020-03-20
Online:2019-10-23
About author:DU Xudong (1995-), male, Master candidate. E-mail: 2062296740@qq.com
Supported by:摘要:
实验采用廉价的牡蛎壳制备绿色、高效的生物源碳酸钙(bio-CaCO3)吸附材料, 用于去除污水中的Pb(II)和甲基橙(MO)。通过扫描电子显微镜(SEM), 热重分析(TGA), X射线荧光光谱分析(XRF)等表征方法对材料形貌、组成、结构等进行了分析。采用宏观吸附行为和微观表征研究bio-CaCO3对水体中Pb(II)和MO的吸附过程并阐明机理。研究发现, bio-CaCO3对MO的去除效率约为45% (msorbent/Vsolvent=0.2 g/L, [MO]initial=60 mg/L), SEM分析结果表明bio-CaCO3吸附MO后, 表面形貌发生了明显的变化。bio-CaCO3对Pb(II)的饱和吸附量高达1775 mg/g (pH=5.0, T=298 K), 优于传统的皂土、活性炭等吸附材料。bio-CaCO3吸附Pb(II)的主要吸附机理是CaCO3+Pb(II)→PbCO3, 该过程的ΔH θ=-7.64 kJ/mol, ΔS θ=-17.92 J/(mol·K), ΔG θ=-2.30 kJ/mol(pH=5.0, T=298 K), 吸附Pb(II)后产生大量形貌更加规则的四棱柱结构。研究表明实验制备的bio-CaCO3对Pb(II)和MO均具有良好的吸附性能, 是一种环境友好型高效吸附剂。
中图分类号:
杜旭东, 唐城元, 杨小丽, 程建波, 贾玉珂, 杨树斌. 生物源碳酸钙对污水中Pb(II)和甲基橙吸附行为的研究[J]. 无机材料学报, 2020, 35(3): 315-323.
DU Xudong, TANG Chengyuan, YANG Xiaoli, CHENG Jianbo, JIA Yuke, YANG Shubin. High-efficiency Biogenic Calcium Carbonate for Adsorption of Pb(II) and Methyl Orange from Wastewater[J]. Journal of Inorganic Materials, 2020, 35(3): 315-323.
| Element | Na | Mg | Al | Si | P | S | Cl | K | Ca | Fe | Cu | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass fraction/wt% | 1.10 | 0.28 | 0.04 | 0.11 | 0.09 | 0.21 | 0.29 | 0.02 | 97.43 | 0.07 | 0.03 | 0.33 |
| Oxide | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | Fe2O3 | CuO | SrO |
| Mass fraction/wt% | 1.25 | 0.39 | 0.07 | 0.19 | 0.17 | 0.43 | 0.24 | 0.02 | 96.92 | 0.06 | 0.02 | 0.24 |
表1 牡蛎壳的XRF分析结果
Table 1 XRF results of oyster shell
| Element | Na | Mg | Al | Si | P | S | Cl | K | Ca | Fe | Cu | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass fraction/wt% | 1.10 | 0.28 | 0.04 | 0.11 | 0.09 | 0.21 | 0.29 | 0.02 | 97.43 | 0.07 | 0.03 | 0.33 |
| Oxide | Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | Fe2O3 | CuO | SrO |
| Mass fraction/wt% | 1.25 | 0.39 | 0.07 | 0.19 | 0.17 | 0.43 | 0.24 | 0.02 | 96.92 | 0.06 | 0.02 | 0.24 |
| Adsorbents | BET area/ (m2·g-1) | Pore size/nm | Zeta potential/mV | Size/ nm |
|---|---|---|---|---|
| Oyster shell | 4.32 | 6.53 | -31.0 | 836 |
| Calcined oyster shell | 4.93 | 6.22 | -19.1 | 4156 |
表2 牡蛎壳煅烧前后的物理特性
Table 2 Physical property of oyster and calcined oyster
| Adsorbents | BET area/ (m2·g-1) | Pore size/nm | Zeta potential/mV | Size/ nm |
|---|---|---|---|---|
| Oyster shell | 4.32 | 6.53 | -31.0 | 836 |
| Calcined oyster shell | 4.93 | 6.22 | -19.1 | 4156 |

图2 吸附时间对Pb(II)(A)和MO(B)在bio-CaCO3上吸附的影响
Fig. 2 Effect of adsorption time on the sorption of Pb(II) (A) and MO (B) by bio-CaCO3 T=25 ℃, [Pb(II)]initial =753×10-6, m/V=0.2 g/L, and [NaClO4]=0.01 mol/L, pH=5.0
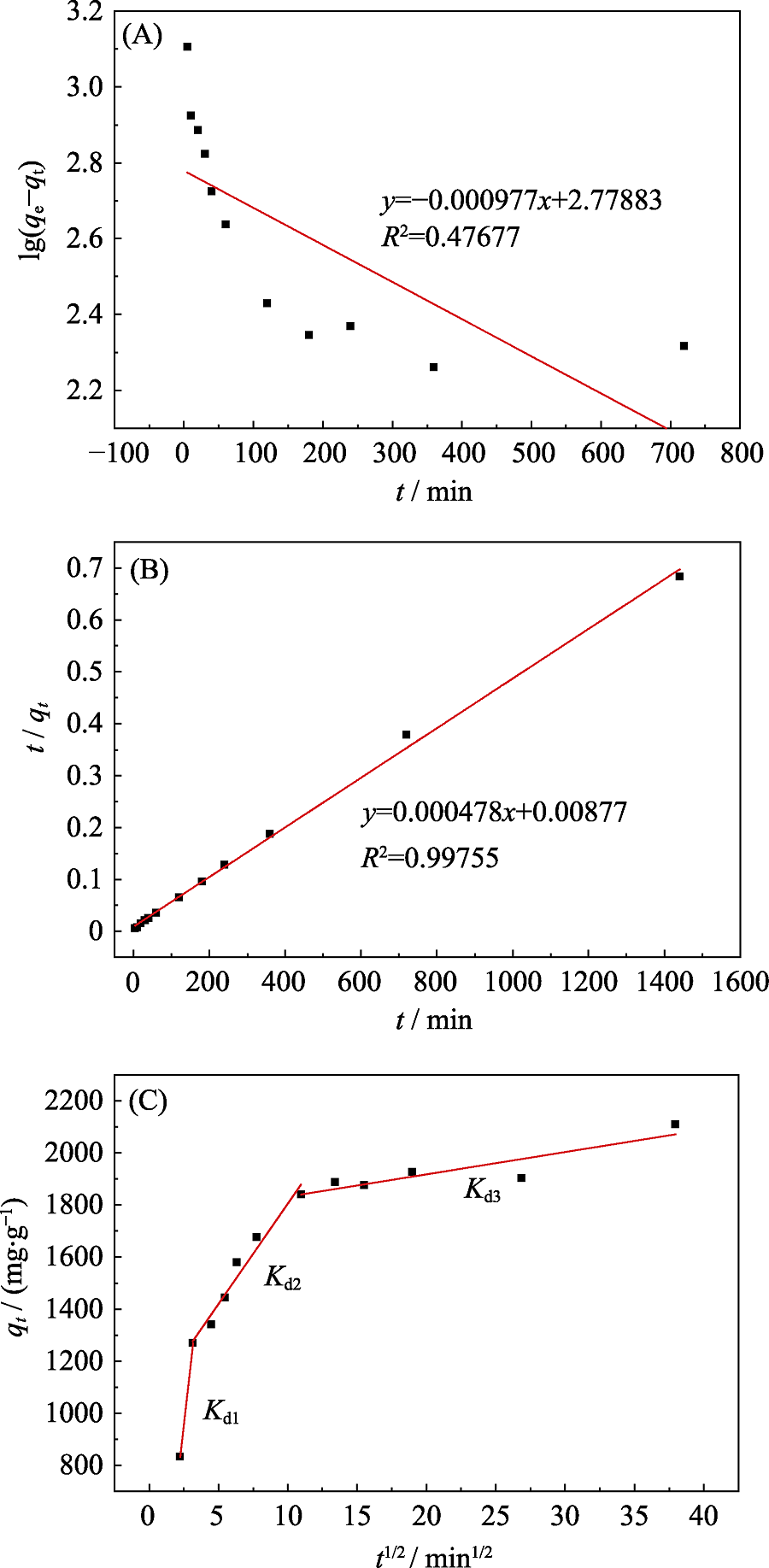
图3 Bio-CaCO3吸附Pb(II)的假一级(A)、假二级(B)和颗粒扩散模型(C)的动力学拟合曲线
Fig. 3 Pseudo-first-order (A), pseudo-second-order (B), and Intraparticle diffusion model (C) fitting for Pb(II) sorption by bio-CaCO3 T=25 ℃, [Pb(II)]initial=753×10-6, msorbent/Vsolvent=0.2 g/L, and [NaClO4]= 0.01 mol/L
| Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|
| R2 | κ1/min-1 | qe/(mg∙g-1) | R2 | κ2/min-1 | qe/(mg∙g-1) |
| 0.477 | 0.00225 | 600.94 | 0.998 | 2.61×10-5 | 2092.05 |
表3 Bio-CaCO3吸附Pb(II)的动力学参数
Table 3 Kinetic parameters of Pb(II) sorption by bio-CaCO3
| Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|
| R2 | κ1/min-1 | qe/(mg∙g-1) | R2 | κ2/min-1 | qe/(mg∙g-1) |
| 0.477 | 0.00225 | 600.94 | 0.998 | 2.61×10-5 | 2092.05 |
| Kd1/(mg∙g-1∙min-1/2) | Kd2/(mg∙g-1∙min-1/2) | Kd3/(mg∙g-1∙min-1/2) | C1 | C2 | C3 | R12 | R22 | R32 |
|---|---|---|---|---|---|---|---|---|
| 470.71 | 77.0 | 8.55 | -220.39 | 1034.38 | 1747.0 | 1.0 | 0.95 | 0.78 |
表4 Bio-CaCO3吸附Pb(II)的颗粒扩散模型参数
Table 4 Intraparticle diffusion model constants and correlation coefficient for Pb(II) sorption by bio-CaCO3
| Kd1/(mg∙g-1∙min-1/2) | Kd2/(mg∙g-1∙min-1/2) | Kd3/(mg∙g-1∙min-1/2) | C1 | C2 | C3 | R12 | R22 | R32 |
|---|---|---|---|---|---|---|---|---|
| 470.71 | 77.0 | 8.55 | -220.39 | 1034.38 | 1747.0 | 1.0 | 0.95 | 0.78 |
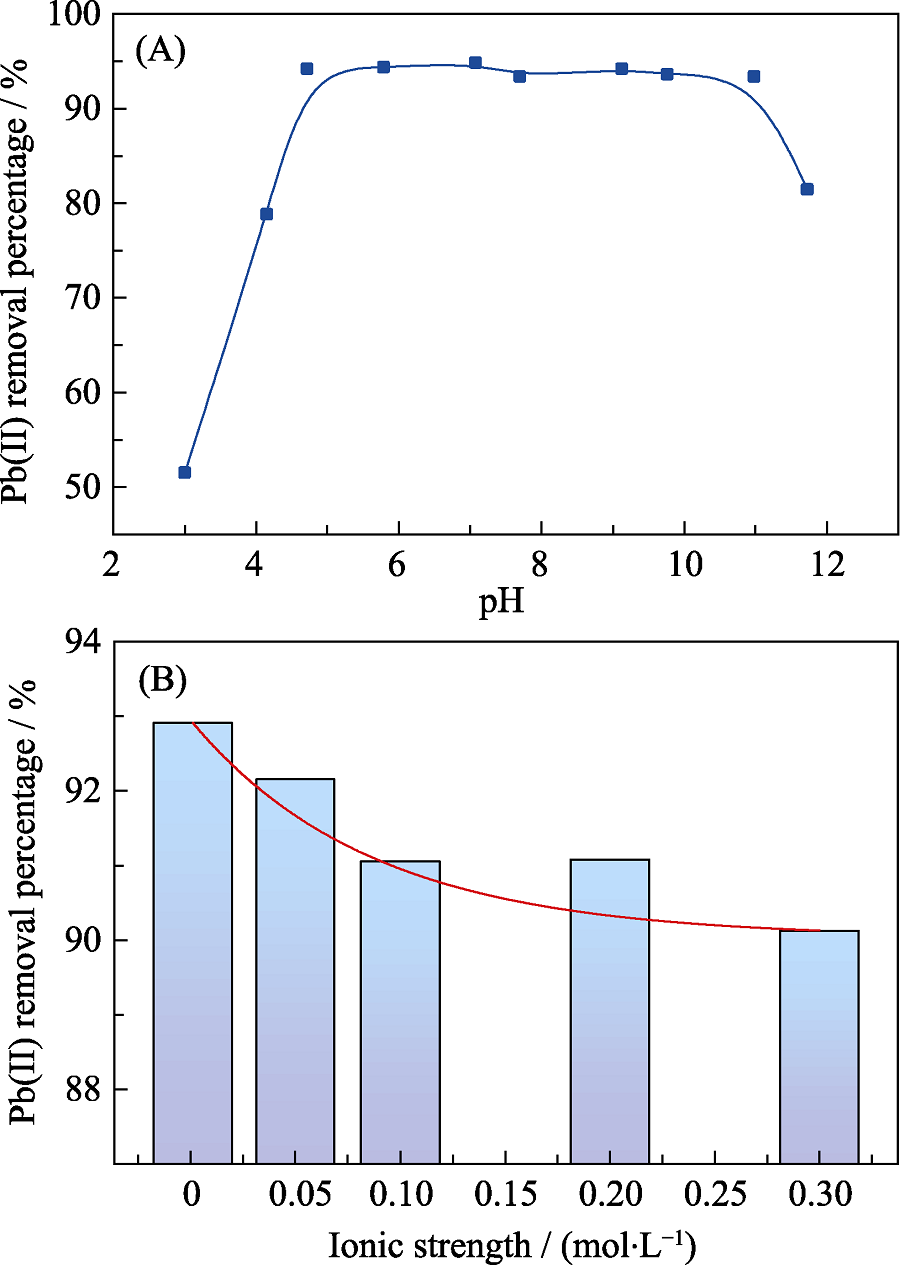
图4 pH (A)和离子强度(B)对bio-CaCO3吸附Pb(II)的影响
Fig. 4 Effects of pH (A) and ionic strength (B) on Pb(II) sorption by bio-CaCO3 T=25 ℃, [Pb(II)]initial=10×10-6, pH=5.0, m/V=0.2 g/L
| T/℃ | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm/ (mg∙g-1) | KL/ (L∙mg-1) | R2 | KF/ (mg∙g-1) | n | R2 | |
| 25 | 1775.33 | 0.041 | 0.992 | 415.3 | 4.1 | 0.898 |
| 35 | 1415.94 | 0.059 | 0.998 | 441.1 | 5.1 | 0.876 |
| 50 | 1237.35 | 0.063 | 0.986 | 421.8 | 5.6 | 0.885 |
表5 Bio-CaCO3吸附Pb(II)的Langmuir和Freundlich模型参数
Table 5 Parameters of Pb(II) sorption by bio-CaCO3 for Langmuir and Freundlich constants models
| T/℃ | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm/ (mg∙g-1) | KL/ (L∙mg-1) | R2 | KF/ (mg∙g-1) | n | R2 | |
| 25 | 1775.33 | 0.041 | 0.992 | 415.3 | 4.1 | 0.898 |
| 35 | 1415.94 | 0.059 | 0.998 | 441.1 | 5.1 | 0.876 |
| 50 | 1237.35 | 0.063 | 0.986 | 421.8 | 5.6 | 0.885 |
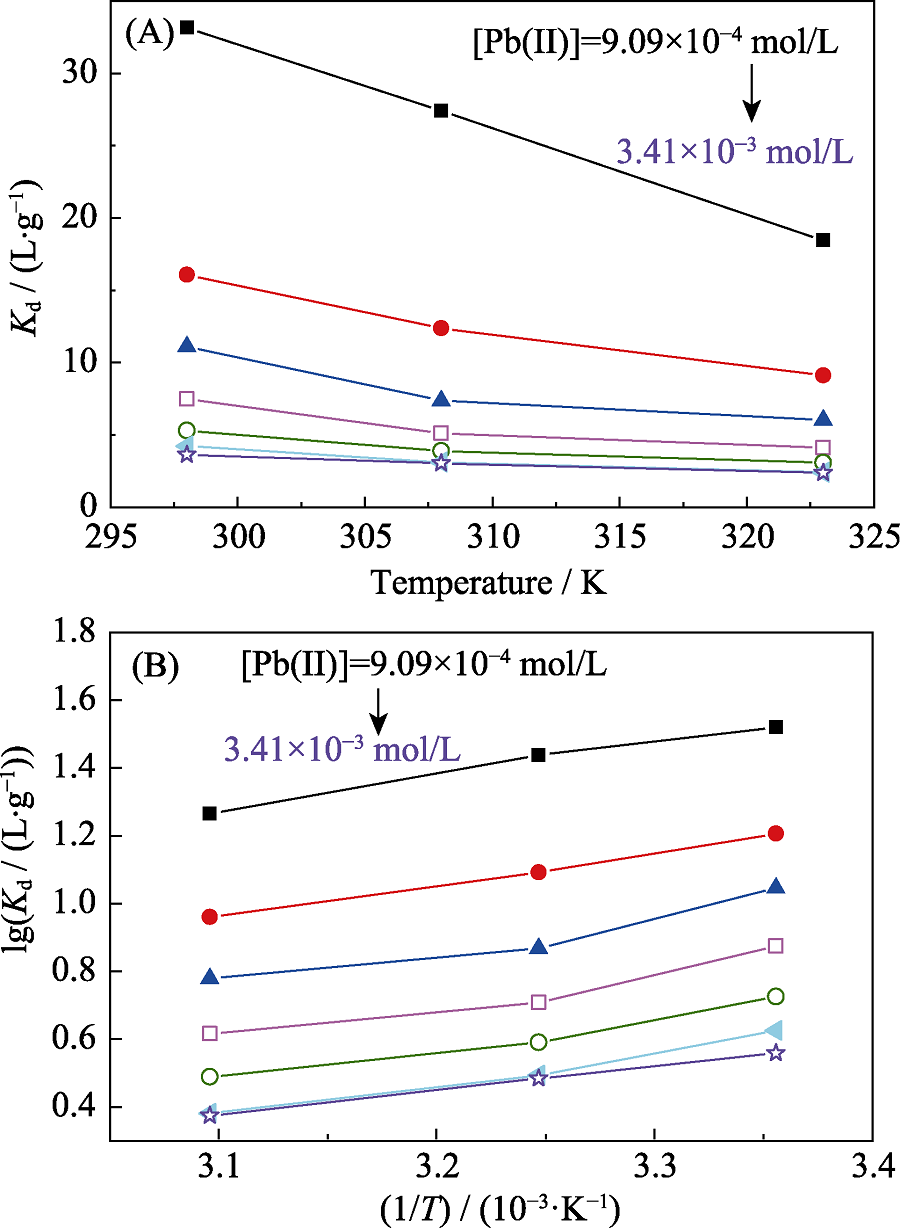
图6 Bio-CaCO3吸附不同浓度Pb(II)的分配系数与温度的变化曲线
Fig. 6 Plots of distribution coefficient against temperature for Pb(II) adsorption with different concentrations by bio-CaCO3 pH=5.0, m/V = 0.2 g/L and [NaClO4]= 0.01 mol/L
| [Pb(II)]initial/ (mol∙L-1) | ΔHθ/ (kJ∙mol-1) | ΔSθ/ (J∙mol-1∙K-1) | ΔGθ/(kJ∙mol-1) | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 323 K | |||
| 9.09×10-4 | -8.24 | -14.92 | -3.79 | -3.64 | -3.42 |
| 1.36×10-3 | -7.85 | -16.35 | -2.98 | -2.81 | -2.57 |
| 1.82×10-3 | -8.29 | -19.33 | -2.53 | -2.33 | -2.04 |
| 2.27×10-3 | -8.09 | -20.06 | -2.11 | -1.91 | -1.61 |
| 2.73×10-3 | -7.42 | -18.98 | -1.76 | -1.57 | -1.29 |
| 3.18×10-3 | -7.71 | -20.77 | -1.52 | -1.31 | -1.00 |
| 3.41×10-3 | -5.87 | -15.04 | -1.39 | -1.23 | -1.01 |
| Average | -7.64 | -17.92 | -2.30 | -2.11 | -1.85 |
表6 Bio-CaCO3吸附Pb(II)的热力学参数
Table 6 Thermodynamic parameters for the adsorption of Pb(II) on bio-CaCO3
| [Pb(II)]initial/ (mol∙L-1) | ΔHθ/ (kJ∙mol-1) | ΔSθ/ (J∙mol-1∙K-1) | ΔGθ/(kJ∙mol-1) | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 323 K | |||
| 9.09×10-4 | -8.24 | -14.92 | -3.79 | -3.64 | -3.42 |
| 1.36×10-3 | -7.85 | -16.35 | -2.98 | -2.81 | -2.57 |
| 1.82×10-3 | -8.29 | -19.33 | -2.53 | -2.33 | -2.04 |
| 2.27×10-3 | -8.09 | -20.06 | -2.11 | -1.91 | -1.61 |
| 2.73×10-3 | -7.42 | -18.98 | -1.76 | -1.57 | -1.29 |
| 3.18×10-3 | -7.71 | -20.77 | -1.52 | -1.31 | -1.00 |
| 3.41×10-3 | -5.87 | -15.04 | -1.39 | -1.23 | -1.01 |
| Average | -7.64 | -17.92 | -2.30 | -2.11 | -1.85 |
| Adsorbents | Cs max/(mg·g-1) | pH | T/K | Ref. |
|---|---|---|---|---|
| Activated carbon | 21.80 | 6.0 | 303 | [30] |
| GMZ bentonite | 23.83 | 5.2 | 293 | [31] |
| S3.9%-g-C3N4 | 52.63 | 4.5 | 328 | [32] |
| GO | 937.65 | 4.4 | 298 | [33] |
| r-GO | 92.99 | 4.4 | 298 | [33] |
| Tianjin oyster shell without calcination | 1591 | 5.0 | 298 | [14] |
| Guangzhou calcined oyster shell | 1067 | ~7 | 298 | [13] |
| Rushan calcined oyster shell | 1775 | 5.0 | 298 | This work |
表7 Bio-CaCO3对Pb(II)吸附能力与其他吸附剂的比较
Table 7 Comparison of Pb(II) adsorption capacity of Bio-CaCO3 with other adsorbents
| Adsorbents | Cs max/(mg·g-1) | pH | T/K | Ref. |
|---|---|---|---|---|
| Activated carbon | 21.80 | 6.0 | 303 | [30] |
| GMZ bentonite | 23.83 | 5.2 | 293 | [31] |
| S3.9%-g-C3N4 | 52.63 | 4.5 | 328 | [32] |
| GO | 937.65 | 4.4 | 298 | [33] |
| r-GO | 92.99 | 4.4 | 298 | [33] |
| Tianjin oyster shell without calcination | 1591 | 5.0 | 298 | [14] |
| Guangzhou calcined oyster shell | 1067 | ~7 | 298 | [13] |
| Rushan calcined oyster shell | 1775 | 5.0 | 298 | This work |
| [1] | YANG S B, HU J, CHEN C L , et al. Mutual effects of Pb(II), and humic acid adsorption on multiwalled carbon nanotubes/polyacrylamide composites from aqueous solutions. Environmental Science & Technology, 2011,45(8):3621-3627. |
| [2] | LIU X L, MA R, WANG X X , et al. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a review. Environmental Pollution, 2019,252:62-73. |
| [3] | REDDY K R, HASSAN M, GOMES V G . Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Applied Catalysis a-General, 2015,489:1-16. |
| [4] | ZHU Y, MURALI S, CAI W , et al. Graphene and graphene oxide: synthesis, properties, and applications. Advanced Materials, 2010,22(35):3906-3924. |
| [5] | DENG Q, LU L X, ZHANG R R . Adsorption property of oyster shell powder to Cu 2+ . Guangzhou Chemical Industry, 2016,44(23):63-65. |
| [6] | XU CONG-BIN, YANG WEN-JIE, SUN HONG-LIANG , et al. Performance and mechanism of Pb(II) removal by expanded graphite loaded with Zero-valent iron. Journal of Inorganic Materials, 2018,33(01):41-47. |
| [7] | SUN Q, QI Q, ZHANG J , et al. Structure and adsorption property of magnetic ZnFe2O4-halloysite composite material. Journal of Inorganic Materials, 2018,33(4):390-396. |
| [8] | WANG N, PANG H W, YU S J , et al. Investigation of adsorption mechanism of layered double hydroxides and their composites on radioactive uranium: a review. Acta Chimica Sinica, 2019,77(2):143-152. |
| [9] | YANG S, HAN C, WANG X , et al. Characteristics of cesium ion sorption from aqueous solution on bentonite- and carbon nanotube- based composites. Journal of Hazardous Materials, 2014,274:46-52. |
| [10] | AHMAD M, RAJAPAKSHA A U, LIM J E , et al. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere, 2014,99:19-33. |
| [11] | WANG X X, CHEN L, WANG L , et al. Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Science China Chemistry, 2019,62(8):933-967. |
| [12] | YANG S, OKADA N, NAGATSU M . The highly effective removal of Cs(+) by low turbidity chitosan-grafted magnetic bentonite. Journal of Hazardous Materials, 2016,301:8-16. |
| [13] | ZHOU X L, LIU W Z, ZHANG J , et al. Biogenic calcium carbonate with hierarchical organic inorganic composite structure enhancing the removal of Pb(II) from wastewater. ACS Applied Materials & Interfaces, 2017,9(41):35785-35793. |
| [14] | DU Y, LIAN F, ZHU L . Biosorption of divalent Pb, Cd and Zn on aragonite and calcite mollusk shells. Environmental Pollution, 2011,159(7):1763-1768. |
| [15] | CHANDRASIRI C, YEHDEGO T, PEETHAMPARAN S . Synthesis and characterization of bio-cement from conch shell waste. Construction and Building Materials, 2019,212:775-786. |
| [16] | CHEN X L, ZHANG X Y, WANG Y , et al. Synergistic fire safety improvement between oyster shell powder and ammonium polyphosphate in TPU composites. Polymers for Advanced Technologies, 2019,30(7):1564-1575. |
| [17] | CHEN X L, ZHANG X Y, WANG W D , et al. Fire-safe agent integrated with oyster shell and melamine polyphosphate for thermoplastic polyurethane. Polymers for Advanced Technologies, 2019,30(7):1576-1588. |
| [18] | DICKINSON G H, IVANINA A V, MATOO O B , et al. Interactive effects of salinity and elevated CO2 levels on juvenile eastern oysters, Crassostrea virginica. Journal of Experimental Biology, 2012,215(1):29-43. |
| [19] | WU Q, CHEN J, CLARK M , et al. Adsorption of copper to different biogenic oyster shell structures. Applied Surface Science, 2014,311:264-272. |
| [20] | YEN H Y, LI J Y . Process optimization for Ni(II) removal from wastewater by calcined oyster shell powders using Taguchi method. Journal of Environmental Management, 2015,161:344-349. |
| [21] | YANG S B, DU X D . Enhanced dispersion of carbon nanotubes in water by plasma induced graft poly(N,N-dimethylacrylamide) and its application in humic acid capture. Journal of Molecular Liquids, 2019,277:380-387. |
| [22] | NIU Z W, WEI X Y, QIANG S R , et al. Spectroscopic studies on U(VI) incorporation into CaCO3: effects of aging time and U(VI) concentration. Chemosphere, 2019,220:1100-1107. |
| [23] | YANG S, ZHAO D, ZHANG H , et al. Impact of environmental conditions on the sorption behavior of Pb(II) in Na-bentonite suspensions. Journal of Hazardous Materials, 2010,183(1/2/3):632-640. |
| [24] | WANG XIANG-XUE, YU SHU-JUN, XIANG-KE W . Removal of radionuclides by metal-organic framework-based materials. Journal of Inorganic Materials, 2019,34(1):17-26. |
| [25] | TRAVLOU N A, KYZAS G Z, LAZARIDIS N K , et al. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir, 2013,29(5):1657-1668. |
| [26] | TAHIR S S, RAUF N . Thermodynamic studies of Ni(II) adsorption onto bentonite from aqueous solution. The Journal of Chemical Thermodynamics, 2003,35(12):2003-2009. |
| [27] | SHEN J, SCHAFER A . Removal of fluoride and uranium by nanofiltration and reverse osmosis: a review. Chemosphere, 2014,117:679-691. |
| [28] | LIU Z, SHEN Q, ZHANG Q , et al. The removal of lead ions of the aqueous solution by calcite with cotton morphology. Journal of Materials Science, 2014,49(15):5334-5344. |
| [29] | CHEN W, LU Z, XIAO B , et al. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. Journal of Cleaner Production, 2019,211:1250-1258. |
| [30] | RAO M M, RAMANA D K, SESHAIAH K , et al. Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. Journal of Hazardous Materials, 2009,166(2):1006-1013. |
| [31] | XU D, TAN X L, CHEN C L , et al. Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: effect of pH, ionic strength, foreign ions and temperature. Applied Clay Science, 2008,41(1):37-46. |
| [32] | LI X, XING J L, ZHANG C L , et al. Adsorption of lead on sulfur- doped graphitic carbon nitride nanosheets: experimental and theoretical calculation study. ACS Sustainable Chemistry & Engineering, 2018,6(8):10606-10615. |
| [33] | ZHANG J, XIE X, LIANG C , et al. Characteristics and mechanism of Pb(II) adsorption/desorption on GO/r-GO under sulfide-reducing conditions. Journal of Industrial And Engineering Chemistry, 2019,73:233-240. |
| [1] | 魏建文, 张丽娟, 耿琳琳, 李誉, 廖雷, 王敦球. 以ZSM-5/MCM-48为载体制备新型高容量CO2吸附剂的性能及机理研究[J]. 无机材料学报, 2025, 40(7): 833-839. |
| [2] | 江宗玉, 黄红花, 清江, 王红宁, 姚超, 陈若愚. 铝离子掺杂MIL-101(Cr)的制备及其VOCs吸附性能研究[J]. 无机材料学报, 2025, 40(7): 747-753. |
| [3] | 洪培萍, 梁龙, 吴炼, 马颖康, 庞浩. ZIF-67结构调控及其对盐酸金霉素的吸附性能研究[J]. 无机材料学报, 2025, 40(4): 388-396. |
| [4] | 吴光宇, 舒松, 张洪伟, 李建军. 接枝内酯基活性炭增强苯乙烯吸附性能研究[J]. 无机材料学报, 2024, 39(4): 390-398. |
| [5] | 谢天, 宋二红. 弹性应变对C、H、O在过渡金属氧化物表面吸附的影响[J]. 无机材料学报, 2024, 39(11): 1292-1300. |
| [6] | 晁少飞, 薛艳辉, 吴琼, 伍复发, MUHAMMAD Sufyan Javed, 张伟. MXene异质结Ti-O-H-O电子快速通道促进高效率储钾[J]. 无机材料学报, 2024, 39(11): 1212-1220. |
| [7] | 伍林, 胡明蕾, 王丽萍, 黄少萌, 周湘远. TiHAP@g-C3N4异质结的制备及光催化降解甲基橙[J]. 无机材料学报, 2023, 38(5): 503-510. |
| [8] | 马晓森, 张丽晨, 刘砚超, 汪全华, 郑家军, 李瑞丰. 13X@SiO2合成及其甲苯吸附性能[J]. 无机材料学报, 2023, 38(5): 537-543. |
| [9] | 郭春霞, 陈伟东, 闫淑芳, 赵学平, 杨傲, 马文. 埃洛石纳米管负载锆氧化物吸附水中砷的研究[J]. 无机材料学报, 2023, 38(5): 529-536. |
| [10] | 王世怡, 冯爱虎, 李晓燕, 于云. Fe3O4负载Ti3C2Tx对Pb(II)的吸附性能研究[J]. 无机材料学报, 2023, 38(5): 521-528. |
| [11] | 于业帆, 徐玲, 倪忠斌, 施冬健, 陈明清. 普鲁士蓝/生物炭材料的制备及其氨氮吸附机理[J]. 无机材料学报, 2023, 38(2): 205-212. |
| [12] | 凌洁, 周安宁, 王文珍, 贾忻宇, 马梦丹. Cu/Mg比对Cu/Mg-MOF-74的CO2吸附性能的影响[J]. 无机材料学报, 2023, 38(12): 1379-1386. |
| [13] | 汤亚, 孙盛睿, 樊佳, 杨庆峰, 董满江, 寇佳慧, 刘阳桥. 粉煤灰衍生水合硅酸钙PEI改性及吸附去除Cu(II)与催化降解有机污染物[J]. 无机材料学报, 2023, 38(11): 1281-1291. |
| [14] | 戴洁燕, 冯爱虎, 米乐, 于洋, 崔苑苑, 于云. NaY沸石分子吸附涂层对典型空间污染物的吸附机制研究[J]. 无机材料学报, 2023, 38(10): 1237-1244. |
| [15] | 王红宁, 黄丽, 清江, 马腾洲, 黄维秋, 陈若愚. 有机-无机氧化硅空心球的合成及VOCs吸附应用[J]. 无机材料学报, 2022, 37(9): 991-1000. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||