
Journal of Inorganic Materials ›› 2022, Vol. 37 ›› Issue (5): 481-492.DOI: 10.15541/jim20210502
• REVIEW • Previous Articles Next Articles
WANG Yutong1( ), ZHANG Feifan1, XU Naicai2, WANG Chunxia1, CUI Lishan1, HUANG Guoyong1(
), ZHANG Feifan1, XU Naicai2, WANG Chunxia1, CUI Lishan1, HUANG Guoyong1( )
)
Received:2021-08-13
Revised:2021-10-22
Published:2022-05-20
Online:2021-11-01
Contact:
HUANG Guoyong, professor. E-mail:huanggy@cup.edu.cn
About author:WANG Yutong (1992-), male, PhD candidate. E-mail: 1248736790@qq.com
Supported by:CLC Number:
WANG Yutong, ZHANG Feifan, XU Naicai, WANG Chunxia, CUI Lishan, HUANG Guoyong. Research Progress of LiTi2(PO4)3 Anode for Aqueous Lithium-ion Batteries[J]. Journal of Inorganic Materials, 2022, 37(5): 481-492.
| Type | Operating voltage/V | Safety | Electrolyte | Solvent | Cost |
|---|---|---|---|---|---|
| Organic Li-ion battery | 3.6-4.2 | Low | LiPF6, LiAsF6, etc | EC, DMC, DEC, etc | High |
| Aqeuous Li-ion battery | 1.5-2.0 | High | Li2SO4, LiNO3, etc | H2O | Moderate |
Table 1 Comparison of the characteristics of aqeuous and organic lithium-ion batteries[8]
| Type | Operating voltage/V | Safety | Electrolyte | Solvent | Cost |
|---|---|---|---|---|---|
| Organic Li-ion battery | 3.6-4.2 | Low | LiPF6, LiAsF6, etc | EC, DMC, DEC, etc | High |
| Aqeuous Li-ion battery | 1.5-2.0 | High | Li2SO4, LiNO3, etc | H2O | Moderate |
| Anode material | Specific capacity/ (mAh·g-1) | Potential/ V(vs. Li+/Li) | Potential/ V(vs. NHE) | Features |
|---|---|---|---|---|
| LiTi2(PO4)3 | 138 | 2.5 | -0.5 | Moderate specific capacity, stable framework |
| TiP2O7 | 121 | 2.6 | -0.4 | Low specific capacity, high Li-intercalation potential |
| VO2 | 250 | 2.6 | -0.4 | High specific capacity, poor cycling performance |
| LiV3O8 | 250 | 2.6 | -0.4 | Fragile during cycling |
Table 2 Parameters of some anode materials for aqeuous lithium-ion battery[14]
| Anode material | Specific capacity/ (mAh·g-1) | Potential/ V(vs. Li+/Li) | Potential/ V(vs. NHE) | Features |
|---|---|---|---|---|
| LiTi2(PO4)3 | 138 | 2.5 | -0.5 | Moderate specific capacity, stable framework |
| TiP2O7 | 121 | 2.6 | -0.4 | Low specific capacity, high Li-intercalation potential |
| VO2 | 250 | 2.6 | -0.4 | High specific capacity, poor cycling performance |
| LiV3O8 | 250 | 2.6 | -0.4 | Fragile during cycling |
| Method | Starting materials | Product characteristic | Features | Ref. | ||
|---|---|---|---|---|---|---|
| Li source | Ti source | P source | Morphology | |||
| Solid state | LiH2PO4 | TiO2 | NH4H2PO4 | Irregular particles | Long calcination time, high temperature | [ |
| Sol-Gel | CH3COOLi | Ti(C4H9O)4 | H3PO4 | Particles | Short calcination time, low temperature | [ |
| Hydrothermal synthesis | CH3COOLi | Ti(C4H9O)4 | NH4H2PO4 | Regular particles | Regular particle morphology, great crystallinity | [ |
| Co-precipitation method | LiOH | Ti(C4H9O)4 | H3PO4 | Particles | Requiring precise control | [ |
| Electrospinning | CH3COOLi | Ti(C4H9O)4 | NH4H2PO4 | Fiber | Ideal electrochemical performance, difficult industrialization | [ |
Table 3 Comparison of common synthetic methods of LiTi2(PO4)3
| Method | Starting materials | Product characteristic | Features | Ref. | ||
|---|---|---|---|---|---|---|
| Li source | Ti source | P source | Morphology | |||
| Solid state | LiH2PO4 | TiO2 | NH4H2PO4 | Irregular particles | Long calcination time, high temperature | [ |
| Sol-Gel | CH3COOLi | Ti(C4H9O)4 | H3PO4 | Particles | Short calcination time, low temperature | [ |
| Hydrothermal synthesis | CH3COOLi | Ti(C4H9O)4 | NH4H2PO4 | Regular particles | Regular particle morphology, great crystallinity | [ |
| Co-precipitation method | LiOH | Ti(C4H9O)4 | H3PO4 | Particles | Requiring precise control | [ |
| Electrospinning | CH3COOLi | Ti(C4H9O)4 | NH4H2PO4 | Fiber | Ideal electrochemical performance, difficult industrialization | [ |
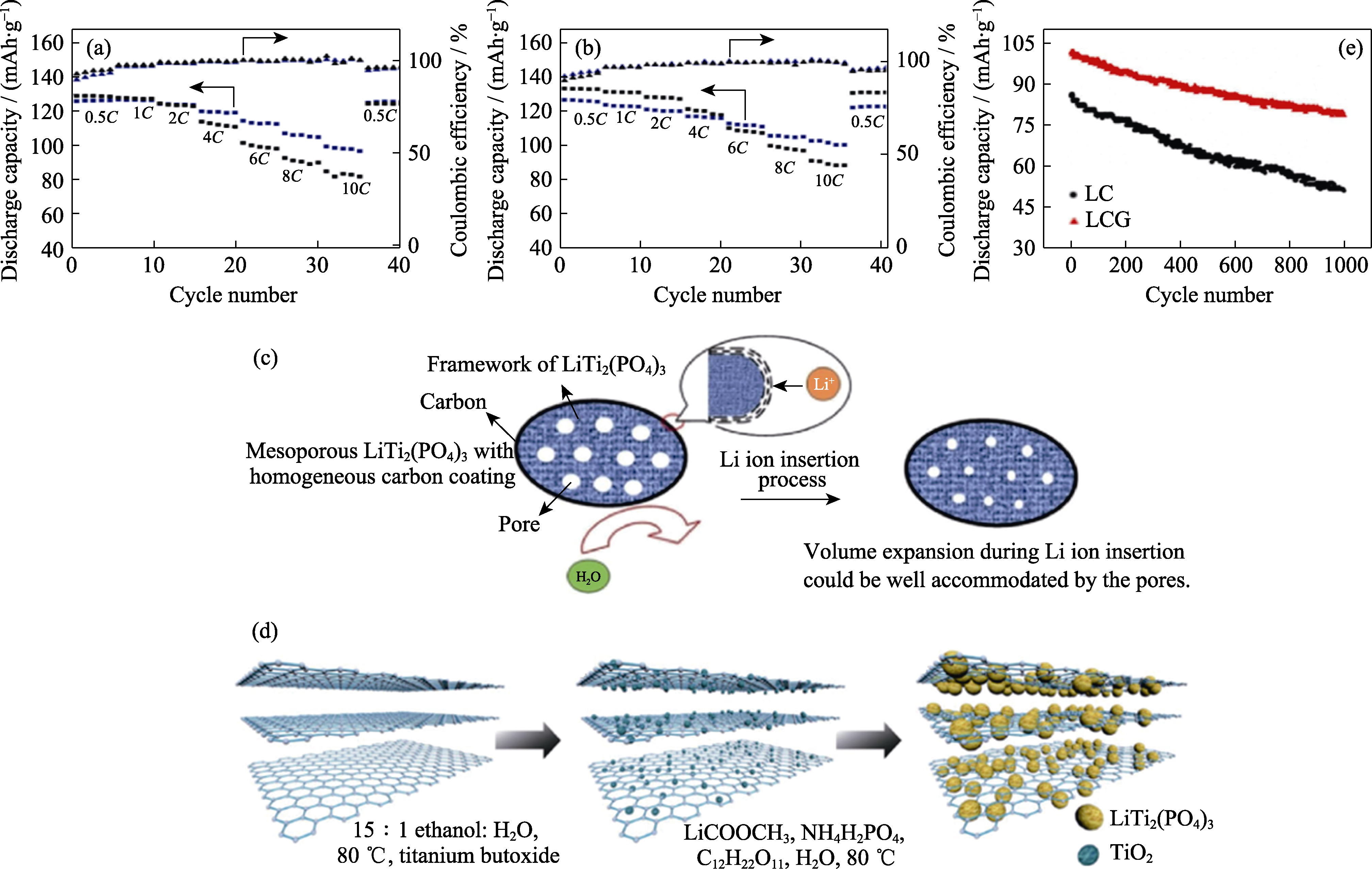
Fig. 5 Comparison chart of rate performance of four coated carbon sources (a, b)[72](Blue and black in (a) indicating polydopamine and phenolic resin; blue and black in (b) indicating polyacrylonitrile and glucose), schematic illustration of the tentative Li+ insertion mechanism in mesoporous LiTi2(PO4)3 with carbon coating layer (c)[75], schematic diagram of the synthesis steps of rGO-LTP (d)[78], and cyclic performance of LC and LCG anodes at 5C for 1000 cycles (e)[80] Colorful figures are available on website
| Calcination parameter | Coating method | Carbon source | Weight percentage of carbon/% | Current density/(mA·g-1) | Specific capacity (cycles)/(mAh·g-1) | Capacity retention/% | Ref. |
|---|---|---|---|---|---|---|---|
| 800 ℃-12 h | In-situ | Citric acid | 6.2 | 138 | 106.1(1)-89(1300) | 84 | [36] |
| 900 ℃-12 h | Ex-situ | Toluene | 12 | 700 | 100(1)-83(200) | 83 | [31] |
| 800 ℃-12 h | Ex-situ | Acetylene Black | 18 | 140 | 106.3(1)-86.5(100) | 81 | [81] |
| 850 ℃-12 h | Ex-situ | Acetylene Black | - | 1400 | 91.3(1)-74.4(100) | 81 | [82] |
| 700 ℃-12 h | In-situ | Pitch | 17.5 | 1380 | 107(1)-75.5(1000) | 70 | [83] |
| 550 ℃-24 h | In-situ | Sucrose | 3.5 | 1400 | 110(1)-104(800) | 94 | [17] |
| 750 ℃-5 h | In-situ | Polyaniline | 5.9 | 276 | 115.2(1)-94.6(1000) | 82 | [84] |
| 750 ℃-5 h | In-situ | Polyacrylonitrile | 5.9 | 690 | 95(1)-82.1(1000) | 86 | [85] |
| 900 ℃-12 h | In-situ | Graphene oxide | 1.79 | ~1380 | 110(1)-100(100) | 91 | [78] |
| 800 ℃-10 h | In-situ | Graphene oxide | - | ~276 | 105(1)-97.86(100) | 93.2 | [77] |
| 700 ℃-5 h | In-situ | Graphene oxide, phenolic resin | 16.2 | ~690 | 101.1(1)-78(1000) | 77.2 | [80] |
| 800 ℃-8 h | Ex-situ | β-Cyclodextrin | 3.13 | ~690 | 120(1)-(200)111.3 | 88.7 | [86] |
Table 4 Comparison of electrochemical performance of different carbon sources and coating methods by Sol-Gel
| Calcination parameter | Coating method | Carbon source | Weight percentage of carbon/% | Current density/(mA·g-1) | Specific capacity (cycles)/(mAh·g-1) | Capacity retention/% | Ref. |
|---|---|---|---|---|---|---|---|
| 800 ℃-12 h | In-situ | Citric acid | 6.2 | 138 | 106.1(1)-89(1300) | 84 | [36] |
| 900 ℃-12 h | Ex-situ | Toluene | 12 | 700 | 100(1)-83(200) | 83 | [31] |
| 800 ℃-12 h | Ex-situ | Acetylene Black | 18 | 140 | 106.3(1)-86.5(100) | 81 | [81] |
| 850 ℃-12 h | Ex-situ | Acetylene Black | - | 1400 | 91.3(1)-74.4(100) | 81 | [82] |
| 700 ℃-12 h | In-situ | Pitch | 17.5 | 1380 | 107(1)-75.5(1000) | 70 | [83] |
| 550 ℃-24 h | In-situ | Sucrose | 3.5 | 1400 | 110(1)-104(800) | 94 | [17] |
| 750 ℃-5 h | In-situ | Polyaniline | 5.9 | 276 | 115.2(1)-94.6(1000) | 82 | [84] |
| 750 ℃-5 h | In-situ | Polyacrylonitrile | 5.9 | 690 | 95(1)-82.1(1000) | 86 | [85] |
| 900 ℃-12 h | In-situ | Graphene oxide | 1.79 | ~1380 | 110(1)-100(100) | 91 | [78] |
| 800 ℃-10 h | In-situ | Graphene oxide | - | ~276 | 105(1)-97.86(100) | 93.2 | [77] |
| 700 ℃-5 h | In-situ | Graphene oxide, phenolic resin | 16.2 | ~690 | 101.1(1)-78(1000) | 77.2 | [80] |
| 800 ℃-8 h | Ex-situ | β-Cyclodextrin | 3.13 | ~690 | 120(1)-(200)111.3 | 88.7 | [86] |
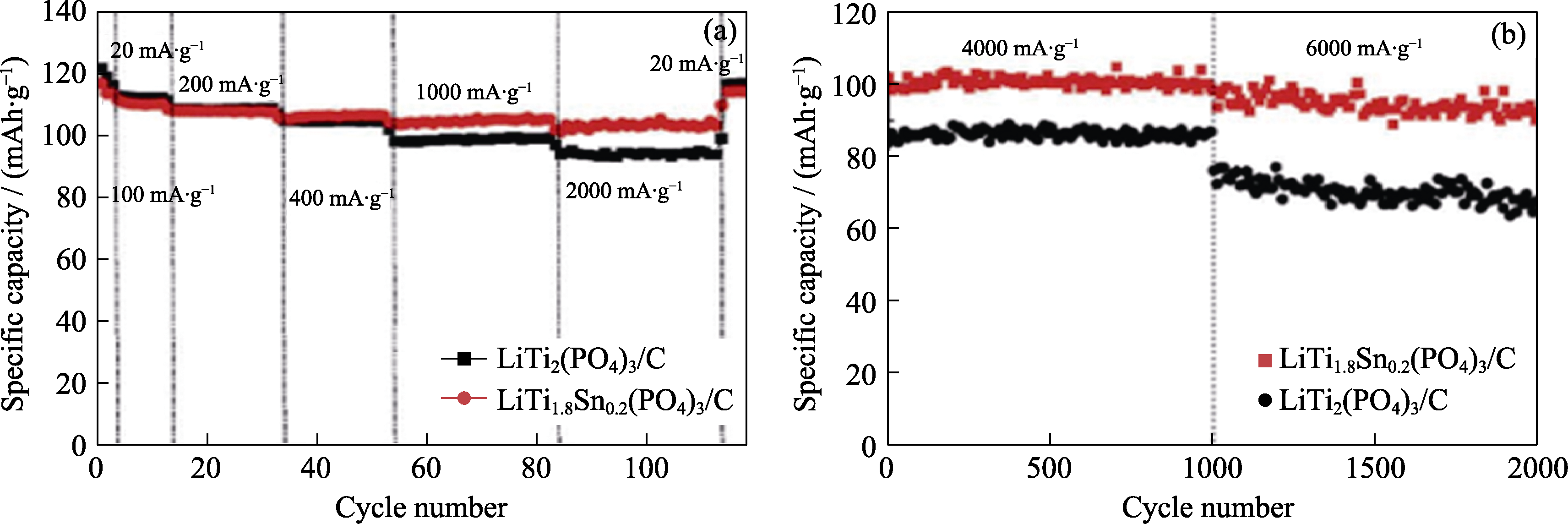
Fig. 6 Discharge capacity for successive cycling at different current densities (a), long-term cycling behavior at current densities of 4 and 6 A·g-1 (b) of LiTi2(PO4)3/C and LiTi1.8Sn0.2(PO4)3/C[24]
| [1] | WENG Y, XU S, HUANG G, et al. Synthesis. Synthesis and performance of Li[(Ni1/3Co1/3Mn1/3)(1-x)Mgx]O2 prepared from spent lithium ion batteries. Jounral of Hazard Materials, 2013, 246- 247:163-172. |
| [2] | KIM T, SONG W, SON D Y,et al. Lithium-ion batteries: outlook on present, future, and hybridized technologies. Journal of Materials Chemistry A, 2019,7(7):2942-2964. |
| [3] | ARMAND M, TARASCON J M. Building better batteries. Nature, 2008,451:652-657. |
| [4] | ZHANG H, ZHAO H, KHAN M A,et al. Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. Journal of Materials Chemistry A, 2018,6(42):20564-20620. |
| [5] | GOODENOUGH J B, PARK K S. The Li-ion rechargeable battery: a perspective. Journal of the American Chemical Society, 2013,135(4):1167-1176. |
| [6] | SUO L, L H. The past, present and future of lithium ion batteries. Physics, 2020,49(1):17-23. |
| [7] | LI W, DAHN J R, WAINWRIGHT D S. Rechargeable lithium batteries with aqueous electrolytes. Science, 1994,264(5162):1115-1118. |
| [8] | ZHOU D. A New Anode Material of Na2V6O16 Nanowires for Aqueous Rechargeable Lithium Battery. Changsha: Central South University, Master Dissertation, 2013. |
| [9] | LI W, MCKINNON W R, R D J. Lithium intercalation from aqueous solutions. Journal of Electrochemical Society, 1994,141:2310-2316. |
| [10] | TANG W, ZHU Y, HOU Y,et al. Aqueous rechargeable lithium batteries as an energy storage system of superfast charging. Energy & Environmental Science, 2013,6(7):2093-2104. |
| [11] | DEMIR-CAKAN R, PALACIN M R, CROGUENNEC L. Rechargeable aqueous electrolyte batteries: from univalent to multivalent cation chemistry. Journal of Materials Chemistry A, 2019,7(36):20519-20539. |
| [12] | LUO J Y, CUI W J, HE P,et al. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nature Chemistry, 2010,2(9):760-765. |
| [13] | LIU Z, HUANG Y, HUANG Y,et al. Voltage issue of aqueous rechargeable metal-ion batteries. Chemical Society Review, 2020,49(1):180-232. |
| [14] | LIU W, WANG B, LI L. Recent progress in electrode materials for aqueous lithium-ion batteries. Energy Storage Science and Technology, 2014,3(1):9-20. |
| [15] | AATIQ A, MENETRIER M, CROGUENNEC L,et al. On the structure of Li3Ti2(PO4)3. Journal of Materials Chemistry, 2002,12(10):2971-2978. |
| [16] | GIAROLA M, SANSON A, TIETZ F,et al. Structure and vibrational dynamics of nasicon-type LiTi2(PO4)3. Journal of Physical Chemistry C, 2017,121(7):3697-3706. |
| [17] | EL-SHINAWI H, JANEK J. Low-temperature synthesis of macroporous LiTi2(PO4)3/C with superior lithium storage properties. RSC Advances, 2015,5(19):14887-14891. |
| [18] | GUTIERREZ A, BENEDEK N A, MANTHIRAM A. Crystal- chemical guide for understanding redox energy variations of M 2+/ 3+ couples in polyanion cathodes for lithium-ion batteries . Chemistry of Materials, 2013,25(20):4010-4016. |
| [19] | DELMAS C, NADIRI A, SOUBEYROUX L J. The nasicon-type titatium phosphates ATi2(PO4)3(A=Li, Na) as electrode materials. Solid State Ionics, 1988, 28-30:419-423. |
| [20] | WANG H, HUANG K, ZENG Y,et al. Electrochemical properties of TiP2O7 and LiTi2(PO4)3 as anode material for lithium ion battery with aqueous solution electrolyte. Electrochimica Acta, 2007,52(9):3280-3285. |
| [21] | JIANG Z, LI Y, HAN C,et al. K doping on Li site enables LiTi2(PO4)3/C excellent lithium storage performance. Solid State Ionics, 2019,341:115036. |
| [22] | YU S, TEMPEL H, SCHIERHOLZ R,et al. LiTi2(PO4)3/C anode material with a spindle-like morphology for batteries with high rate capability and improved cycle life. ChemElectroChem, 2016,3(7):1157-1169. |
| [23] | SUN J, SUN Y, GAI L,et al. Carbon-coated mesoporous LiTi2(PO4)3 nanocrystals with superior performance for lithium-ion batteries. Electrochimica Acta, 2016,200:66-74. |
| [24] | LIU L, SONG T, HAN H,et al. Electrospun Sn-doped LiTi2(PO4)3/C nanofibers for ultra-fast charging and discharging. Journal of Materials Chemistry A, 2015,3(19):10395-10402. |
| [25] | WANG G X, BRADHURST D H, DOU S X,et al. LiTi2(PO4)3 with NASICON-type structure as lithium-storage materials. Journal of Power Sources, 2003,124(1):231-236. |
| [26] | LI W,R. D J. Lithium-ion cells with aqueous electrolytes. Journal of Electrochemical Society, 1995,142:1742-1746. |
| [27] | KOHLER J, MAKIHARA H, UEGAITO H,et al. LiV3O8: characterization as anode material for an aqueous rechargeable Li-ion battery system. Electrochim. Acta, 2000,46:59-65. |
| [28] | ZHENG W. Solid-state Synthesis and Surface Modification of LiFePO4 and LiTi2(PO4)3 for Lithium Ion Electrode Materials. Zhengjiang: Zhengjiang University,Doctoral Dissertation, 2010. |
| [29] | FENG C, LI L, TANG J,et al. Synthesis and electrochemical performance of a new type of anode material LiTi2(PO4)3. Power Technology, 2015,39(2):242-244. |
| [30] | LI W, LI Y, CAO M,et al. Synthesis and electrochemical performance of alginic acid-based carbon-coated Li3V2(PO4)3 composite by rheological phase method. Acta Phys-ChimSin, 2017,33(11):2261-2267. |
| [31] | LUO J Y, XIA Y Y. Aqueous lithium-ion battery LiTi2(PO4)3/LiMn2O4 with high power and energy densities as well as superior cycling stability. Advanced Functional Materials, 2007,17(18):3877-3884. |
| [32] | TANG Z K, XUE Y F, TEOBALDI G,et al. The oxygen vacancy in Li-ion battery cathode materials. Nanoscale Horizons, 2020,5(11):1453-1466. |
| [33] | LUO J Y, CHEN L J, ZHAO Y J,et al. The effect of oxygen vacancies on the structure and electrochemistry of LiTi2(PO4)3 for lithium-ion batteries: a combined experimental and theoretical study. Journal of Power Sources, 2009,194(2):1075-1080. |
| [34] | CHENG C. Study of Anode Materials for Aqueous Rechargeable Lithium-ion Batteries. Changsha: Xiangtan University, Master Dissertation, 2010. |
| [35] | MARIAPPAN C R, GALVEN C, CROSNIER-LOPEZ M P,et al. Synthesis of nanostructured LiTi2(PO4)3 powder by a Pechini-type polymerizable complex method. Journal of Solid State Chemistry, 2006,179(2):450-456. |
| [36] | WESSELLS C, HUGGINS R A, CUI Y. Recent results on aqueous electrolyte cells. Journal of Power Sources, 2011,196(5):2884-2888. |
| [37] | ZHOU X L, YAN Z G, LI S Y,et al. Single crystalline LiTi2(PO4)3 nanowires by porous template with improved electrochemical performance. Materials Today Energy, 2018,7:113-121. |
| [38] | ZHOU X. Lithium Titanium Phosphate and Carbon/copper Composite Electrode Materials: Controlled Preparation, Structural Study and Electrochemical Performance. Beijing: Beijing University of Technology, Doctoral Dissertation, 2014. |
| [39] | ZHOU D, LI J, CHEN C,et al. A hydrothermal synthesis of Ru-doped LiMn1.5Ni0.5O4 cathode materials for enhanced electrochemical performance. RSC Advances, 2021,11(21):12549-12558. |
| [40] | SONG Y, XIE B, SONG S,et al. Regeneration of LiFePO4 from spent lithium-ion batteries via a facile process featuring acid leaching and hydrothermal synthesis. Green Chemistry, 2021,23(11):3963-3971. |
| [41] | WANG J, QIN X, GUO J,et al. A porous hierarchical micro/nano LiNi0.5Mn1.5O4 cathode material for Li-ion batteries synthesized by a urea-assisted hydrothermal method. Dalton Transactions, 2018,47(21):7333-7343. |
| [42] | QIN X, ZHOU M, ZONG B,et al. Urea-assisted hydrothermal synthesis of a hollow hierarchical LiNi0.5Mn1.5O4 cathode material with tunable morphology characteristics. RSC Advances, 2018,8(53):30087-30097. |
| [43] | YUE Y, PANG W. Hydrothermal synthesis and characterization of LiTi2(PO4)3. Journal of Materials Science Letters, 1990,9:1392. |
| [44] | LIANG Y, HISAMO T, SUMI S,et al. Direct fabrication of thin-film LiTi2(PO4)3 electrodes using the hydrothermal method. Solid State Ionics, 2016,296:7-12. |
| [45] | LI M, LIU L, ZHANG N,et al. Mesoporous LiTi2(PO4)3/C composite with trace amount of carbon as high-performance electrode materials for lithium ion batteries. Journal of Alloys and Compounds, 2018,749:1019-1027. |
| [46] | HOU P, ZHANG H, ZI Z,et al. Core-shell and concentration- gradient cathodes prepared via co-precipitation reaction for advanced lithium-ion batteries. Journal of Materials Chemistry A, 2017,5(9):4254-4279. |
| [47] | LI H, LI Z, CUI Y,et al. Long-cycled Li2ZnTi3O8/TiO2 composite anode material synthesized via a one-pot co-precipitation method for lithium ion batteries. New Journal of Chemistry, 2017,41(3):975-981. |
| [48] | 杨勇. 固态电化学. 北京: 化学工业出版社, 2017. |
| [49] | 盖利刚, 孙家香, 姜海辉. 一种碳包覆介孔磷酸钛锂的制备方法: 中国. ZL201510957301.8. 2015. 12. 18. |
| [50] | OGHBAEI M, MIRZAEE O. Microwave versus conventional sintering: a review of fundamentals, advantages and applications. Journal of Alloys and Compounds, 2010,494(1/2):175-189. |
| [51] | RIQUET G, MARINEL S, BREARD Y,et al. Direct and hybrid microwave solid state synthesis of CaCu3Ti4O12 ceramic: microstructures and dielectric properties. Ceramics International, 2018,44(13):15228-15235. |
| [52] | ZHANG M, GARCIA-ARAEZ N, HECTOR A L. Understanding and development of olivine LiCoPO4 cathode materials for lithium- ion batteries. Journal of Materials Chemistry A, 2018,6(30):14483-14517. |
| [53] | LUDWIG J, NORDLUND D, DOEFF M M,et al. Synthesis and characterization of metastable, 20 nm-sized Pna21-LiCoPO4 nanospheres. Journal of Solid State Chemistry, 2017,248:9-17. |
| [54] | GUO X, JIA X, HU H,et al. Synthesis of LiTi2(PO4)3 ultrafine powder by Sol-Gel and microwave heating method. Materials Reports, 2007,21(11A):68-71. |
| [55] | HU J, HUANG W, YANG L,et al. Structure and performance of the LiFePO4 cathode material: from the bulk to the surface. Nanoscale, 2020,12(28):15036-15044. |
| [56] | YANG C, LEE D J, KIM H,et al. Synthesis of nano-sized urchin-shaped LiFePO4 for lithium ion batteries. RSC Advances, 2019,9(24):13714-13721. |
| [57] | XIANG J, ZHANG P, LV S,et al. Spinel LiMn2O4 nanoparticles fabricated by the flexible soft template/Pichini method as cathode materials for aqueous lithium-ion capacitors with high energy and power density. RSC Advances, 2021,11(25):14891-14898. |
| [58] | JO J, NAM S, HAN S,et al. One-pot pyro synthesis of a nanosized-LiMn2O4/C cathode with enhanced lithium storage properties. RSC Advances, 2019,9(42):24030-24038. |
| [59] | QI W, SHAPTER J G, WU Q,et al. Nanostructured anode materials for lithium-ion batteries: principle, recent progress and future perspectives. Journal of Materials Chemistry A, 2017,5(37):19521-19540. |
| [60] | TIAN L, YU H, ZHANG W,et al. The star material of lithium ion batteries, LiFePO4: basic properties, optimize moderation and future prospects. Materials Reports, 2019,33(11):3561-3579. |
| [61] | DENG W, WANG X, LIU C,et al. Touching the theoretical capacity: synthesizing cubic LiTi2(PO4)3/C nanocomposites for high-performance lithium-ion battery. Nanoscale, 2018,10(14):6282-6287. |
| [62] | WU Y, CHONG S, LIU Y,et al. High electrochemical performance of nanocrystallized carbon-coated LiFePO4 modified by tris (pentafluorophenyl) borane as a cathode material for lithium-ion batteries. RSC Advances, 2018,8(51):28978-28986. |
| [63] | WANG Y, WANG X, JIANG A,et al. A versatile nitrogen-doped carbon coating strategy to improve the electrochemical performance of LiFePO4 cathodes for lithium-ion batteries. Journal of Alloys and Compounds, 2019,810:151889. |
| [64] | PARK G D, HONG J H, JUNG D S,et al. Unique structured microspheres with multishells comprising graphitic carbon-coated Fe3O4 hollow nanopowders as anode materials for high-performance Li-ion batteries. Journal of Materials Chemistry A, 2019,7(26):15766-15773. |
| [65] | KU D J, LEE J H, LEE S J,et al. Effects of carbon coating on LiNi0.5Mn1.5O4 cathode material for lithium ion batteries using an atmospheric microwave plasma torch. Surface and Coatings Technology, 2019,376:25-30. |
| [66] | SUN W, LIU J, LIU X,et al. Bimolecular-induced hierarchical nanoporous LiTi2(PO4)3/C with superior high-rate and cycling performance. Chemical Communications, 2017,53(62):8703-8706. |
| [67] | TAN Y, XUE B. Research progress on lithium titanate as anode material in lithium-ion battery. Journal of Inorganic Materials, 2018,33(5):475-482. |
| [68] | LI H, ZHOU H. Enhancing the performances of Li-ion batteries by carbon-coating: present and future. Chemical Communications, 2012,48(9):1201-1217. |
| [69] | YE J, LI C, RAO M,et al. Effects of different carbon solutions on electrochemical performance of LiTi2(PO4)3/C composite anode material. Power Technology, 2020,44(3):322-325. |
| [70] | LUO S, TIAN Y, TANG Z,et al. Effect of the structure of pyrolytic carbon on the performance of LiFePO4/C composite cathode material. Rare Metal Materials and Engineering, 2009,38:13-15. |
| [71] | CHEN Y, HE H, LIU L,et al. Thermal decomposition of glucose and sucrose by kinetics analysis. The Chinese Joumal of Process Engineering, 2010,10(4):720-725. |
| [72] | ZHANG C, WEN Y, ZHANG P,et al. Effect of organic carbon source on performance of LiTi2(PO4)3/C composite electrodes in aqueous solutions. Chemical Journal of Chinese Universities, 2020,41(6):1352-1361. |
| [73] | LIN L, CONG Z, CAO J,et al. Multifunctional Fe3O4@Polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano, 2014,8(4):3876-3883. |
| [74] | HE Z, JIANG Y, MENG W,et al. Advanced LiTi2(PO4)3@N-doped carbon anode for aqueous lithium ion batteries. Electrochimica Acta, 2016,222:1491-1500. |
| [75] | SUN D, TANG Y, HE K,et al. Long-lived aqueous rechargeable lithium batteries using mesoporous LiTi2( PO4)3@Canode. Scientific Reports, 2015,5:17452. |
| [76] | XU T, ZHAO M, SU Z,et al. Nanostructured LiTi2(PO4)3 anode with superior lithium and sodium storage capability aqueous electrolytes. Journal of Power Sources, 2021,481:229110. |
| [77] | ROH H K, KIM H K, ROH K C,et al. LiTi2(PO4)3/reduced graphene oxide nanocomposite with enhanced electrochemical performance for lithium-ion batteries. RSC Advances, 2014,4(60):31672-31677. |
| [78] | LIM C H, KANNAN A G, LEE H W,et al. A high power density electrode with ultralow carbon via direct growth of particles on graphene sheets. Journal of Materials Chemistry A, 2013,1(20):6183-6190. |
| [79] | WANG H, YANG Y, LIANG Y,et al. LiMn1-xFexPO4 nanorods grown on graphene sheets for ultrahigh-rate-performance lithium ion batteries. Angewandte Chemie International Edition, 2011,50(32):7364-7368. |
| [80] | ZHOU Z, LUO W, HUANG H,et al. LiTi2(PO4)3@carbon/ graphene hybrid as superior anode materials for aqueous lithium ion batteries. Ceramics International, 2017,43(1):99-105. |
| [81] | ZHOU M, LIU L, YI L,et al. Synthesis of LiTi2(PO4)3-acetylene black nanocomposites for lithium ion batteries by the polyvinyl alcohol assisted Sol-Gel method and ball-milling. Journal of Power Sources, 2013,234:292-301. |
| [82] | LIU L, ZHOU M, WANG G,et al. Synthesis and characterization of LiTi2(PO4)3/C nanocomposite as lithium intercalation electrode materials. Electrochimica Acta, 2012,70:136-141. |
| [83] | WENG G M, SIMON TAM L Y, LU Y C. High-performance LiTi2(PO4)3 anodes for high-areal-capacity flexible aqueous lithium-ion batteries. Journal of Materials Chemistry A, 2017,5(23):11764-11771. |
| [84] | HE Z, JIANG Y, ZHU J,et al. N-doped carbon coated LiTi2(PO4)3 as superior anode using PANi as carbon and nitrogen bi-sources for aqueous lithium ion battery. Electrochimica Acta, 2018,279:279-288. |
| [85] | ZHOU Z, XIANG A, XIA M,et al. Advanced LiTi2(PO4)3 anode with high performance for aqueous rechargeable lithium battery. Ceramics International, 2018,44(17):21599-21606. |
| [86] | YE J M, LI C M. Synthesis of LiTi2(PO4)3@carbon anode material with superior performance using beta-cyclodextrin as carbon sources. Ionics, 2020,26(6):2845-2853. |
| [87] | BOUNAR N, BENABBAS A, ROPA P,et al. Synthesis and ionic conductivity of nasicon-structured LiTi2xSnx(PO4)3 anode material for lithium-ion batteries. Advances in Materials and Processing Technologies, 2017,3(3):241-249. |
| [88] | HE Z, JIANG Y, ZHU J,et al. Boosting the performance of LiTi2(PO4)3/C anode for aqueous lithium ion battery by Sn doping on Ti sites. Journal of Alloys and Compounds, 2018,731:32-38. |
| [89] | LIU N, HE Z, ZHANG X,et al. Synthesis and electrochemical properties of Na-doped LiTi2(PO4)3@carbon composite as anode for aqueous lithium ion batteries. Ceramics International, 2017,43(14):11481-11487. |
| [90] | WANG H, ZHANG H, CHENG Y,et al. Rational design and synthesis of LiTi2(PO4)3-xFx anode materials for high-performance aqueous lithium ion batteries. Journal of Materials Chemistry A, 2017,5(2):593-599. |
| [91] | 张华民, 王怀清, 冯凯, 等. 一种阴离子掺杂的磷酸钛锂负极材料及其制备和应用. ZL201610490240.3. 2016. 6. 29. |
| [92] | LUO H, TANG Y, XIANG Z,et al. Cl-doping strategy to boost the lithium storage performance of lithium titanium phosphate. Frontiers in Chemistry, 2020,8:349. |
| [93] | JIANG Z, LI Y H, HAN C,et al. Endowing LiTi2(PO4)3/C with excellent electrochemical performances through rational crystal doping. Ceramics International, 2019,45(17):23406-23410. |
| [1] | ZHU Wenjie, TANG Lu, LU Jichang, LIU Jiangping, LUO Yongming. Research Progress on Catalytic Oxidation of Volatile Organic Compounds by Perovskite Oxides [J]. Journal of Inorganic Materials, 2025, 40(7): 735-746. |
| [2] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [3] | WU Qiong, SHEN Binglin, ZHANG Maohua, YAO Fangzhou, XING Zhipeng, WANG Ke. Research Progress on Lead-based Textured Piezoelectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 563-574. |
| [4] | ZHANG Bihui, LIU Xiaoqiang, CHEN Xiangming. Recent Progress of Hybrid Improper Ferroelectrics with Ruddlesden-Popper Structure [J]. Journal of Inorganic Materials, 2025, 40(6): 587-608. |
| [5] | WU Jie, YANG Shuai, WANG Mingwen, LI Jinglei, LI Chunchun, LI Fei. Textured PT-based Piezoelectric Ceramics: Development, Status and Challenge [J]. Journal of Inorganic Materials, 2025, 40(6): 575-586. |
| [6] | JIANG Kun, LI Letian, ZHENG Mupeng, HU Yongming, PAN Qinxue, WU Chaofeng, WANG Ke. Research Progress on Low-temperature Sintering of PZT Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 627-638. |
| [7] | TIAN Ruizhi, LAN Zhengyi, YIN Jie, HAO Nanjing, CHEN Hangrong, MA Ming. Microfluidic Technology Based Synthesis of Inorganic Nano-biomaterials: Principles and Progress [J]. Journal of Inorganic Materials, 2025, 40(4): 337-347. |
| [8] | ZHANG Jiguo, WU Tian, ZHAO Xu, YANG Fan, XIA Tian, SUN Shien. Improvement of Cycling Stability of Cathode Materials and Industrialization Process for Sodium-ion Batteries [J]. Journal of Inorganic Materials, 2025, 40(4): 348-362. |
| [9] | YIN Jie, GENG Jiayi, WANG Kanglong, CHEN Zhongming, LIU Xuejian, HUANG Zhengren. Recent Advances in 3D Printing and Densification of SiC Ceramics [J]. Journal of Inorganic Materials, 2025, 40(3): 245-255. |
| [10] | CHEN Guangchang, DUAN Xiaoming, ZHU Jinrong, GONG Qing, CAI Delong, LI Yuhang, YANG Donglei, CHEN Biao, LI Xinmin, DENG Xudong, YU Jin, LIU Boya, HE Peigang, JIA Dechang, ZHOU Yu. Advanced Ceramic Materials in Helicopter Special Structures: Research Progress and Application Prospect [J]. Journal of Inorganic Materials, 2025, 40(3): 225-244. |
| [11] | FAN Xiaobo, ZU Mei, YANG Xiangfei, SONG Ce, CHEN Chen, WANG Zi, LUO Wenhua, CHENG Haifeng. Research Progress on Proton-regulated Electrochemical Ionic Synapses [J]. Journal of Inorganic Materials, 2025, 40(3): 256-270. |
| [12] | HAIREGU Tuxun, GUO Le, DING Jiayi, ZHOU Jiaqi, ZHANG Xueliang, NUERNISHA Alifu. Research Progress of Optical Bioimaging Technology Assisted by Upconversion Fluorescence Probes in Tumor Imaging [J]. Journal of Inorganic Materials, 2025, 40(2): 145-158. |
| [13] | SUN Shujuan, ZHENG Nannan, PAN Haokun, MA Meng, CHEN Jun, HUANG Xiubing. Research Progress on Preparation Methods of Single-atom Catalysts [J]. Journal of Inorganic Materials, 2025, 40(2): 113-127. |
| [14] | TAO Guilong, ZHI Guowei, LUO Tianyou, OUYANG Peidong, YI Xinyan, LI Guoqiang. Progress on Key Technologies of Cavity-structured Thin Film Bulk Acoustic Wave Filter [J]. Journal of Inorganic Materials, 2025, 40(2): 128-144. |
| [15] | ZHOU Fan, TIAN Zhilin, LI Bin. Research Progress on Carbide Ultra-high Temperature Ceramic Anti-ablation Coatings for Thermal Protection System [J]. Journal of Inorganic Materials, 2025, 40(1): 1-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||