
Journal of Inorganic Materials ›› 2020, Vol. 35 ›› Issue (2): 243-249.DOI: 10.15541/jim20190118
• RESEARCH LETTERS • Previous Articles Next Articles
WU Si1,2,MEI Lei2,HU Kong-Qiu2,CHAI Zhi-Fang2,3,NIE Chang-Ming1( ),SHI Wei-Qun2(
),SHI Wei-Qun2( )
)
Received:2019-03-21
Revised:2019-04-27
Published:2020-02-20
Online:2019-09-20
Supported by:CLC Number:
WU Si,MEI Lei,HU Kong-Qiu,CHAI Zhi-Fang,NIE Chang-Ming,SHI Wei-Qun. pH-dependent Synthesis of Octa-nuclear Uranyl-oxalate Network Mediated by U-shaped Linkers[J]. Journal of Inorganic Materials, 2020, 35(2): 243-249.
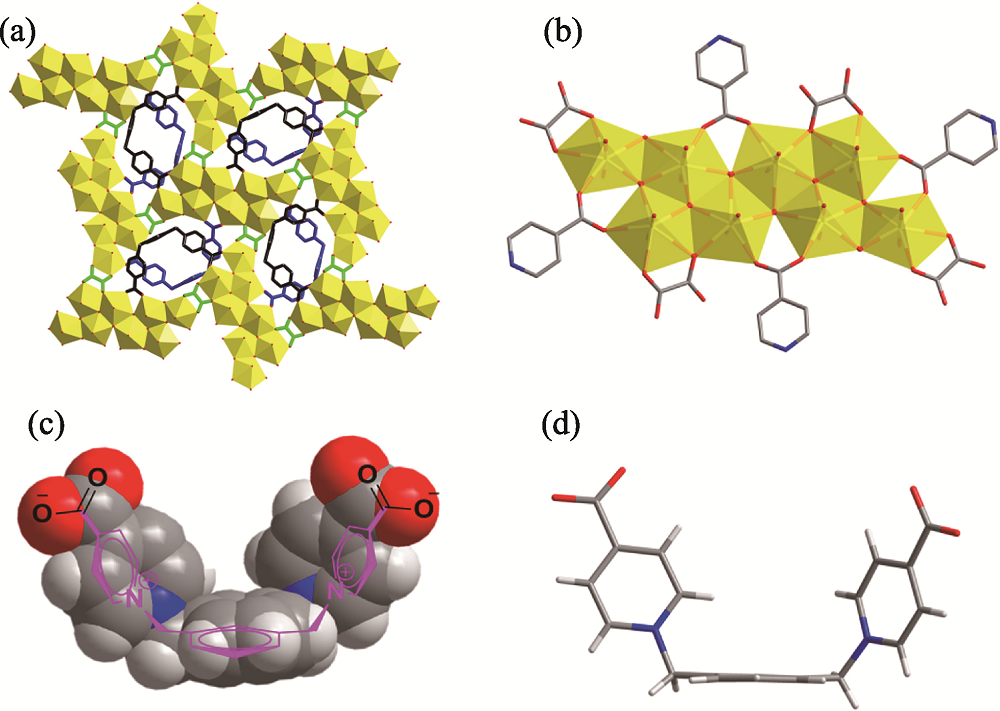
Fig. 1 Octa-nuclear uranyl-oxalate network reinforced by U-shaped zwitterionic dicarboxylate linkers (a) two-dimensional coordination network; (b) octa-nuclear uranyl (U8) motif, [(UO2)8O4(μ3-OH)2(μ2-OH)2]4+; (c) U-shaped linker in a space- filling mode overlapped with its molecular structure; (d) U-shaped linker in a stick mode Color codes: uranyl polyhedra in yellow; U-shaped linkers in dark or blue
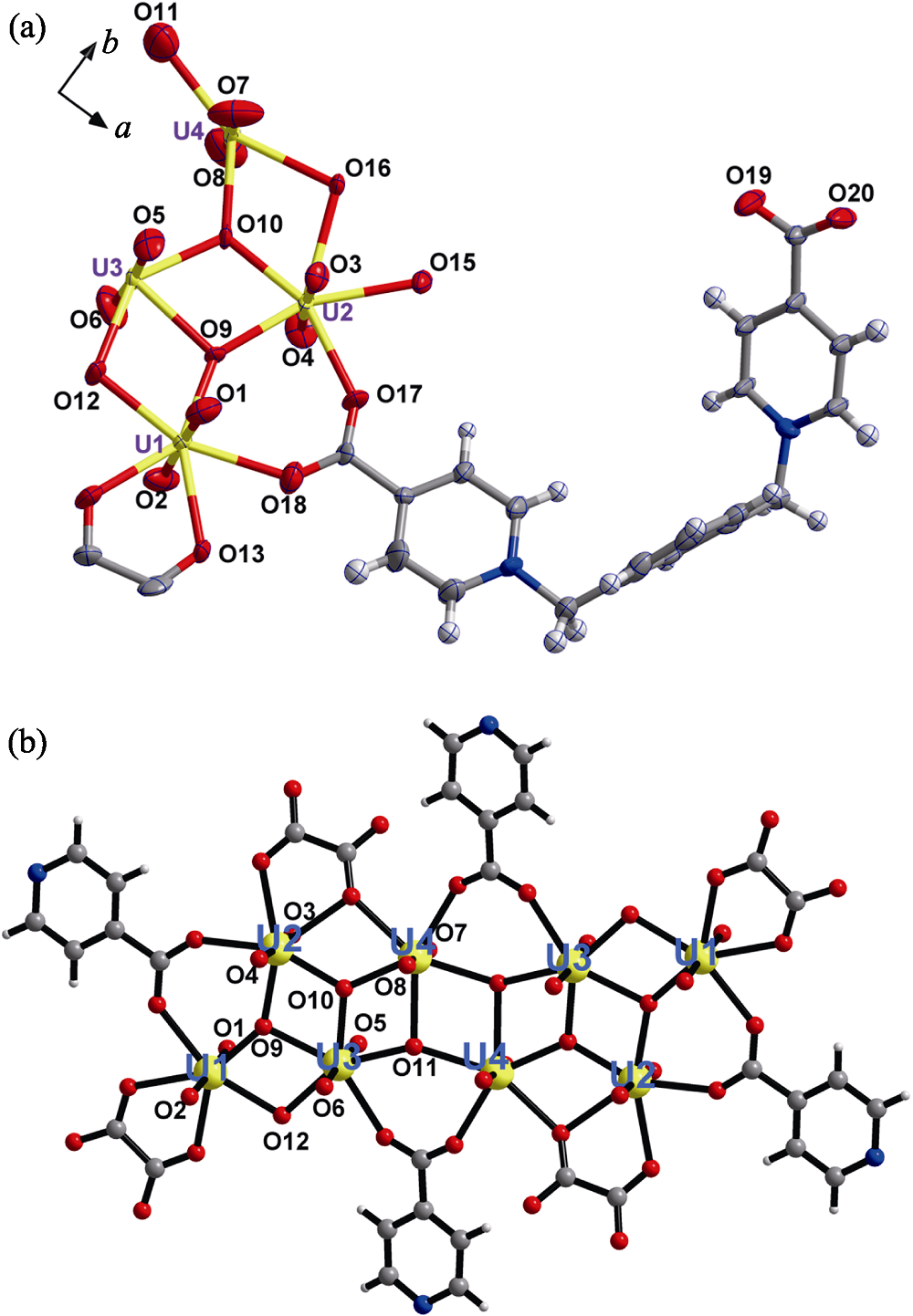
Fig. 2 Crystal structure of compound 1 (a) ORTEP view of compound 1 with the 30% probability level for thermal ellipsoids; (b) octa-nuclear uranyl (U8) motif in compound 1 showing detailed coordination spheres of all uranyl centers Color codes: uranium atoms in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms pale gray
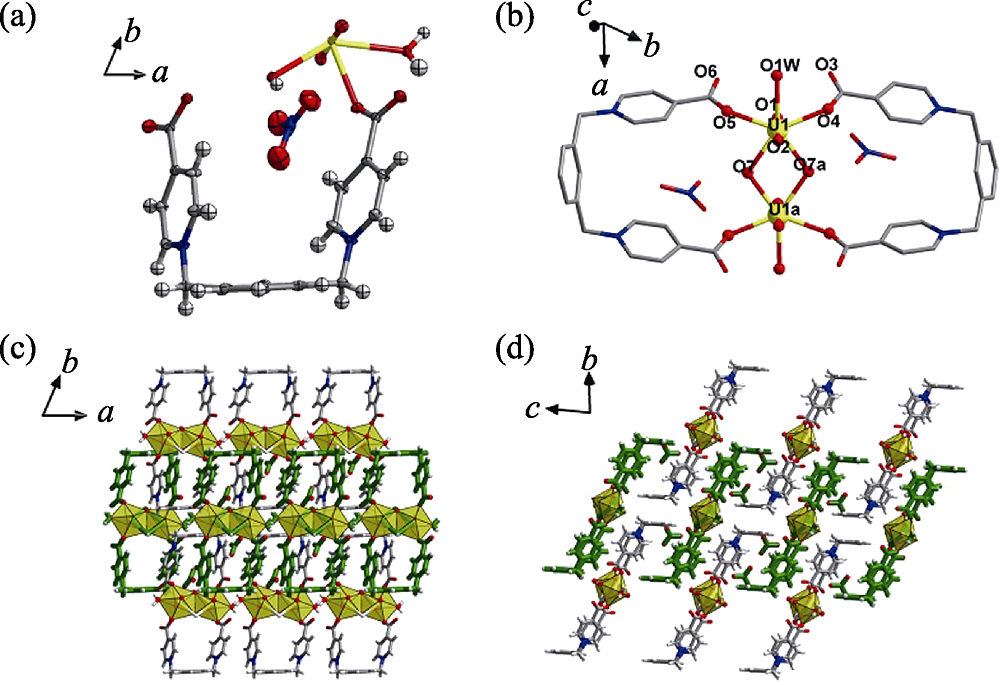
Fig. 3 Crystal structure of compound 2 (a) ORTEP view of compound 2 with the 30% probability level for thermal ellipsoids; (b) coordination environment of each uranyl center for dimeric uranyl motif; (c-d) crystal lattice stacking for compound 2 viewed for c axis (c) and a axis (d) Color codes: uranium atoms in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms pale gray; the U-shaped linkers in green
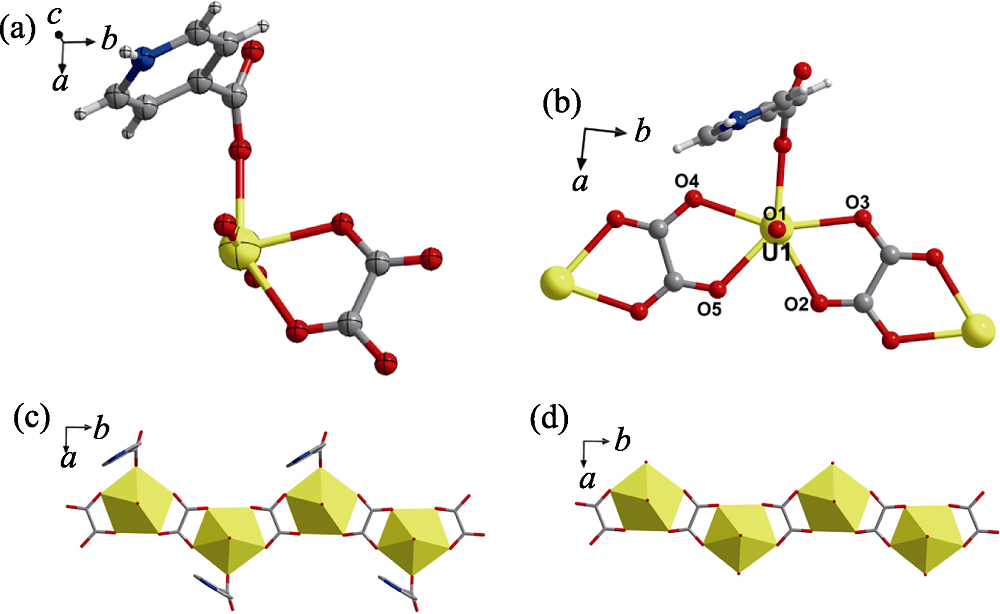
Fig. 4 Crystal structure of compound 3 (a) ORTEP view of compound 3 with the 30% probability level for thermal ellipsoids; (b) coordination environments of uranyl center; (c-d) the extended structure based on one-dimensional oxalate-bridging monomeric uranyl chain with (c) or without (d) terminal isonicotinate ligands Color codes: uranium atoms or polyhedras in yellow; oxygen atoms in red; carbon atoms in dark gray; nitrogen atoms in blue; hydrogen atoms in pale gray
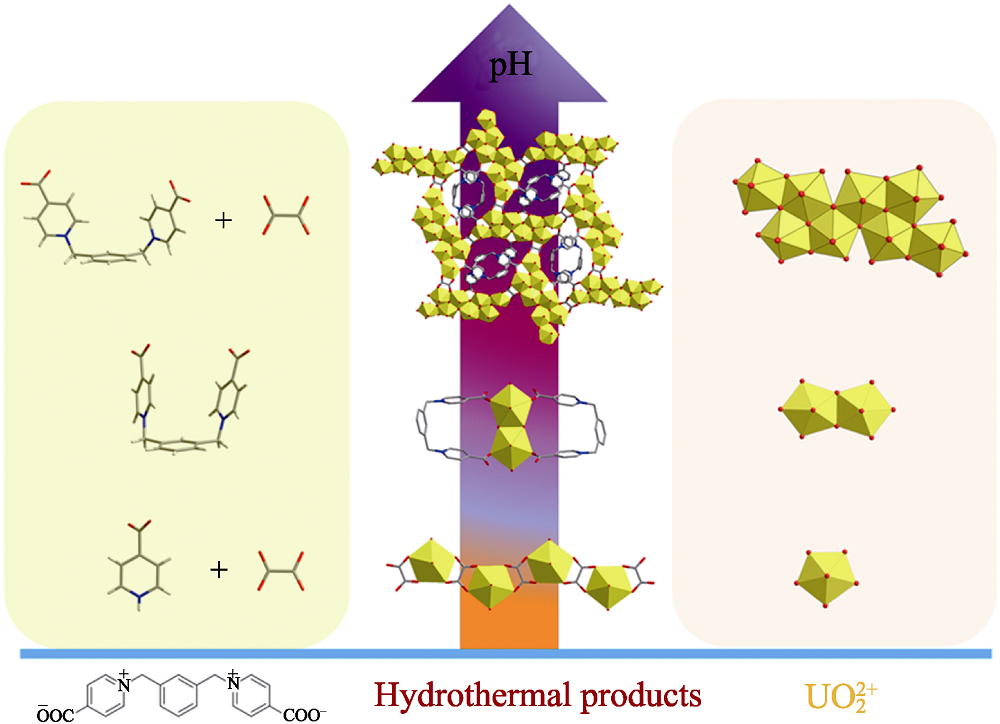
Fig. 5 pH-dependent regulation of hydrothermal reactions of m-Xyl-BPy4CA linkers and uranyl Color codes: uranium polyhedras in yellow; oxygen atoms in red; carbon atoms in gray; nitrogen atoms in blue
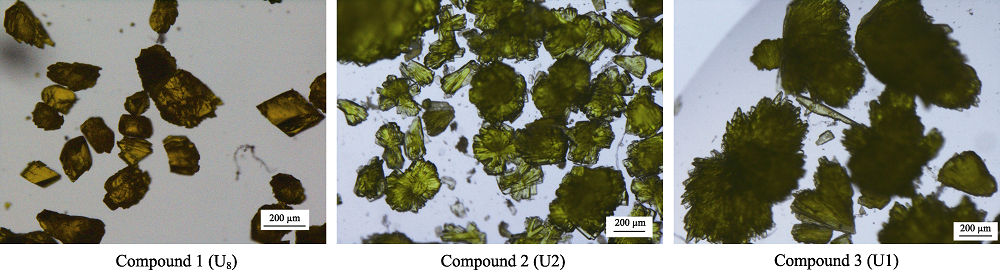
Fig. S1 Different optical morphologies of 1 with octa-nuclear uranyl (U8) motifs, 2 with binuclear uranyl (U2) motifs and 3 with monomeric uranyl (U1) motifs
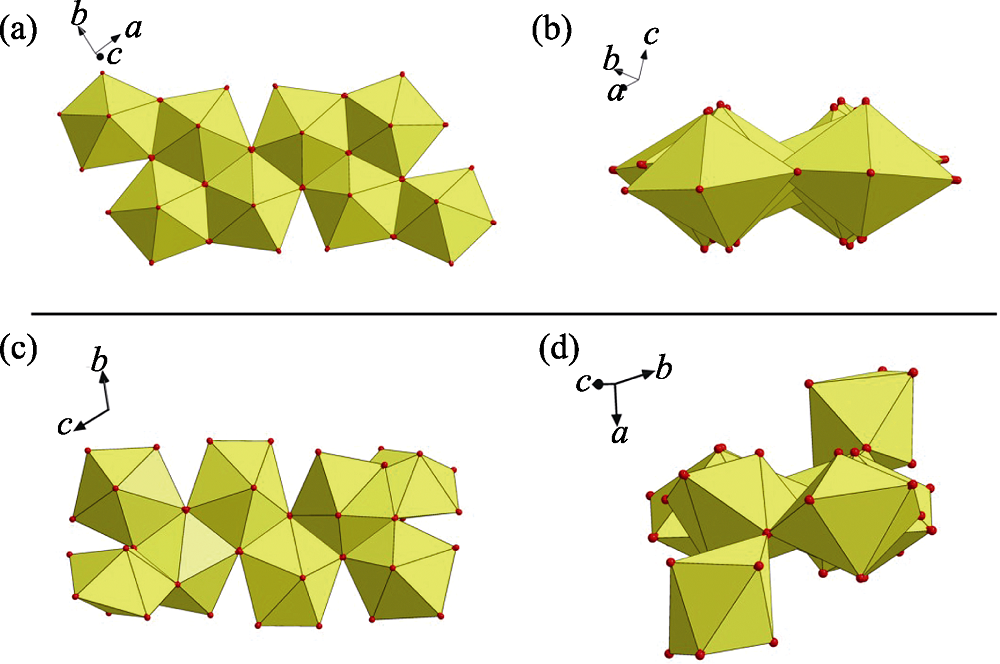
Fig. S5 (a) A nearly planar geometry of U8 motif found in this work; (b) a non-planar U8 motif with cation-cation interactions (CCIs) reported by Loiseau, et al[1]
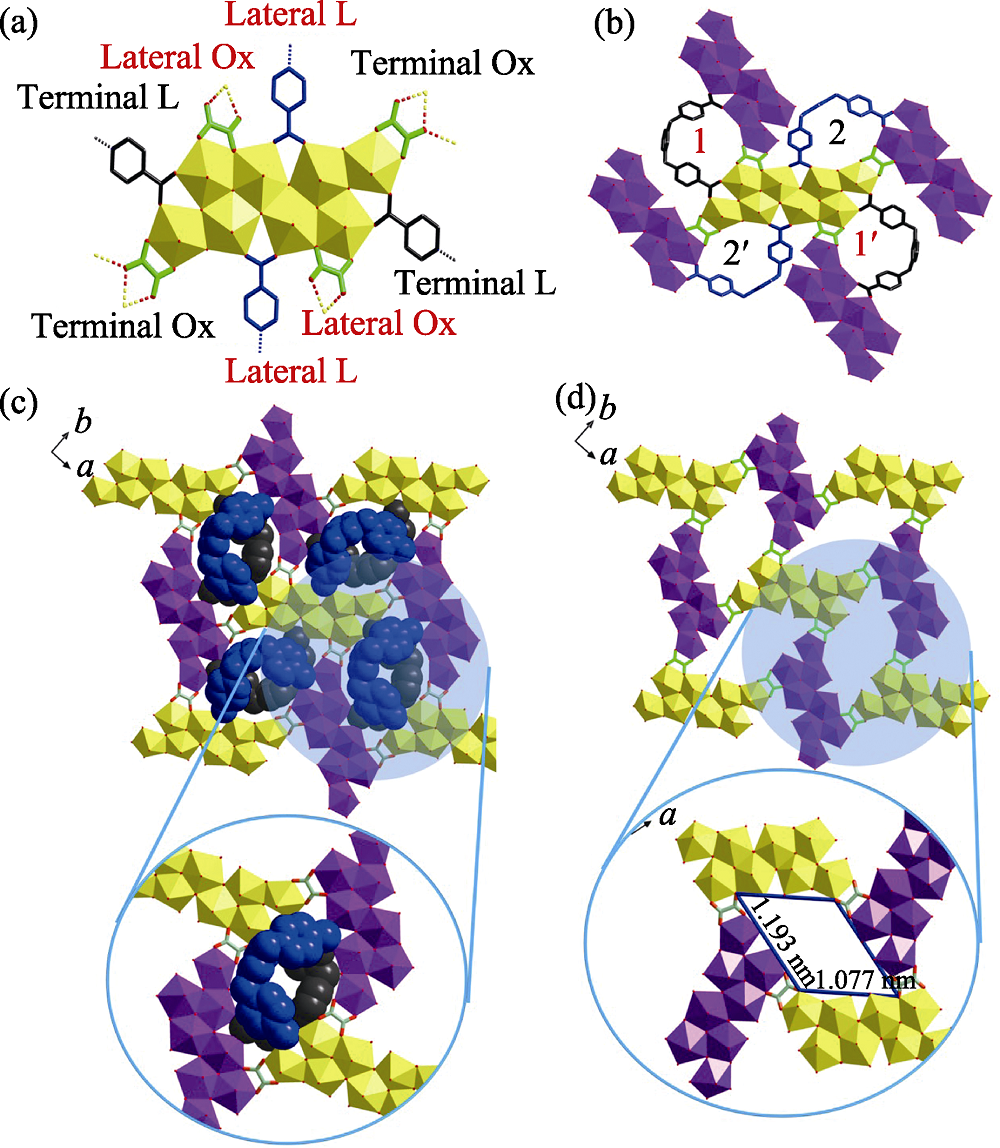
Fig. S6 (a-b) Eight-connected U8 motif with four oxalate (Ox) and four m-Xyl-BPy4CA (L) moieties extends from four directions through oxalate ligands (a), which thus connecting four adjacent ones with each oxalate ligand going together with a U-shaped bidentate m-Xyl-BPy4CA linker (b); (c) U8-based uranyl-oxalate 2D network (enlarged diagram: a minimum rhombic loop); (d) U8-based uranyl-oxalate 2D network with all the cross-linking m-Xyl-BPy4CA linkers omitted for clarity (enlarged diagram: a minimum rhombic loop in size of 1.193 nm× 1.077 nm)
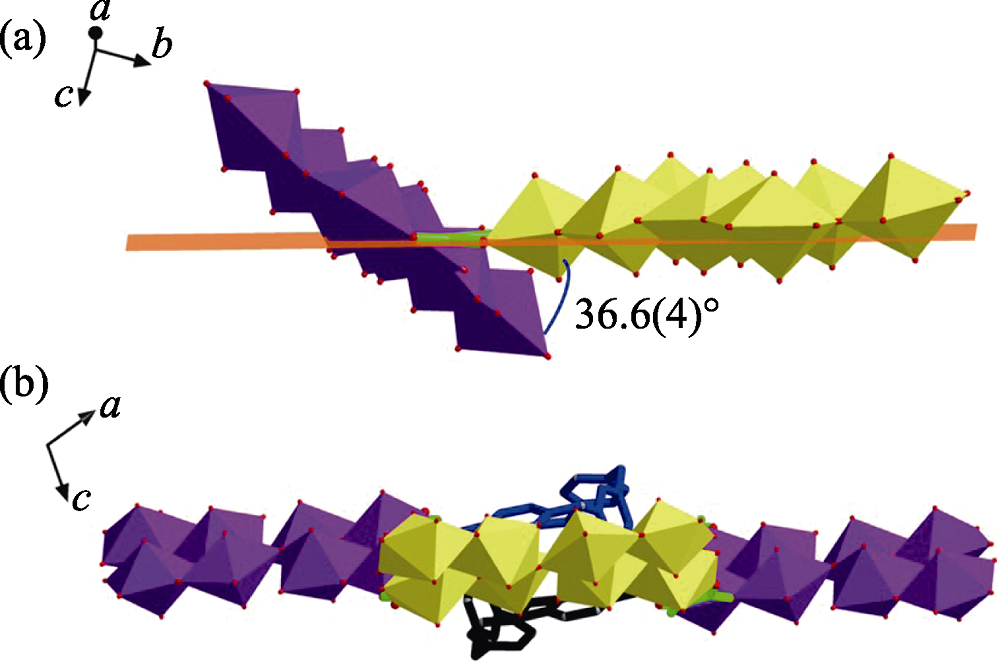
Fig. S7 Each U8 motif displays a different overall orientation from that of its adjacent U8 with an angle of inclination of 36.6(4)° (a), resulting in a distortion of the rhombic loop (b)
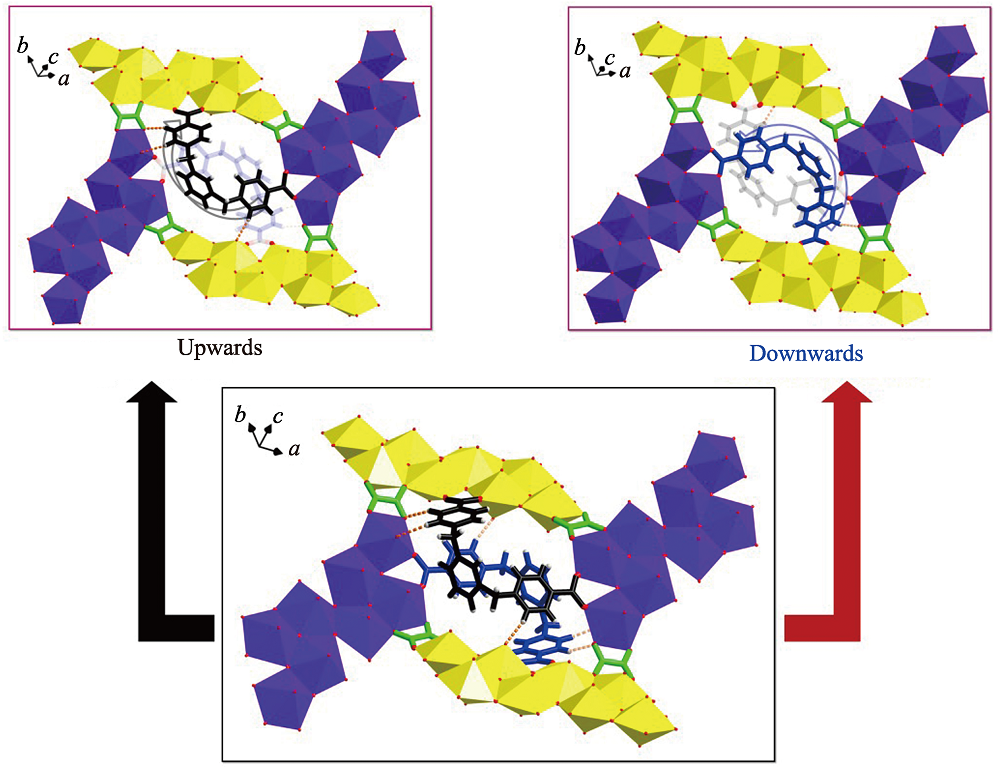
Fig. S9 Two ‘U’-shaped bidentate m-Xyl-BPy4CA ligands located in the cavity of rhombic loop crosslink all the four U8 motifs through coordination bonds and hydrogen bonds (bottom) where one m-Xyl-BPy4CA ligand points upwards (top left) and the other points downwards from the opposite direction (top right)
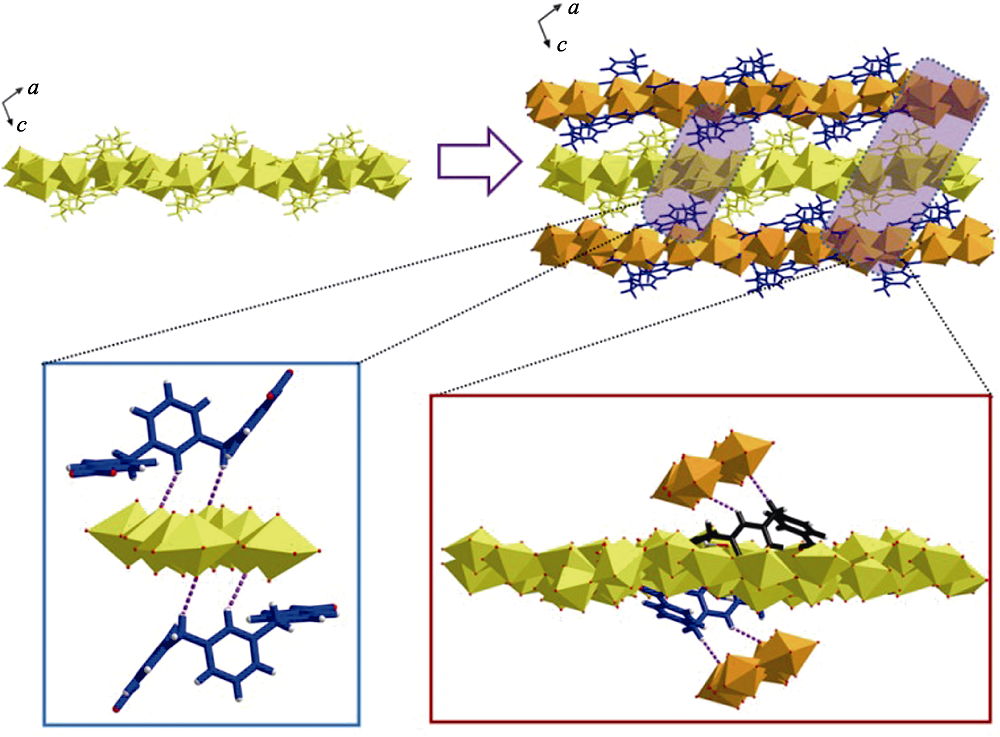
Fig. S10 Hydrogen bonds between adjacent layers of 2D sheets through U8 motifs that interact with neighboured m-Xyl-BPy4CA from another sheet or m-Xyl-BPy4CA interacting with neighboured uranyl group from another sheet
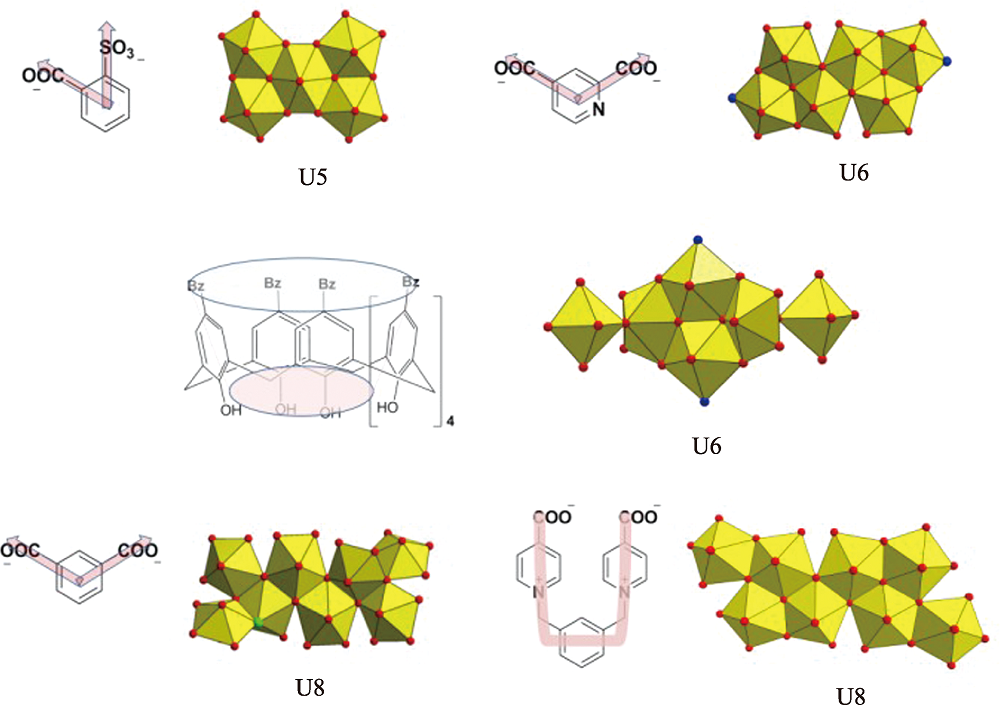
Fig. S11 Some examples of high-nuclear uranyl motif based on nonlinear multi-topic organic ligands, as suggested by the cases of pentanuclear (U5), hexanuclear (U6) and octanuclear (U8) uranyl motifs derived from sulfobenzoate precursors[2], ortho-position or meta-position aromatic/heteroaromatic dicarboxylate[3,4], calixarene ligand[3] and U-shaped linkers used in this work
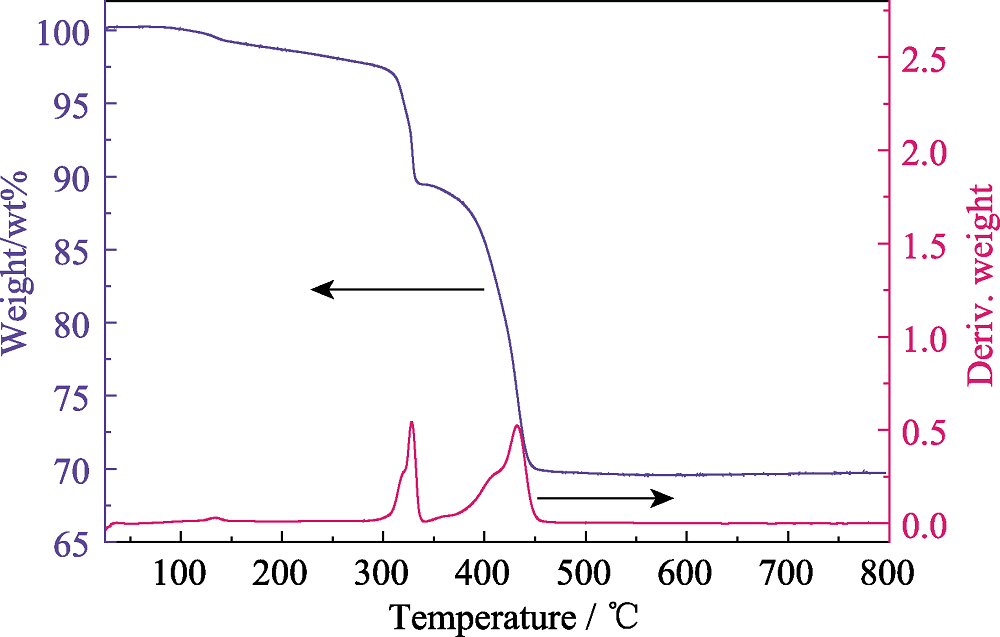
Fig. S13 Thermogravimetric analysis (TGA) of compounds 1, where 1 starts to decompose at ~295 ℃, and finally transforms to U3O8 with residual weight of 69.31% (theoretical value: 70.25%)
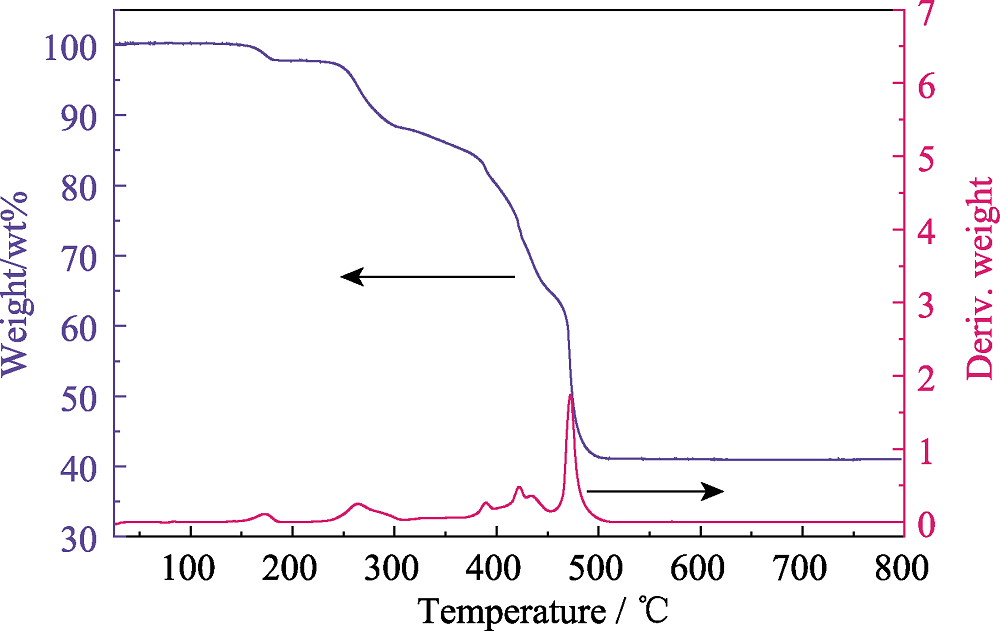
Fig. S14 Thermogravimetric analysis (TGA) of compounds 2, where 2 starts to decompose at ~233 ℃, and finally transforms to U3O8 with residual weight of 40.95% (theoretical value: 40.20%)
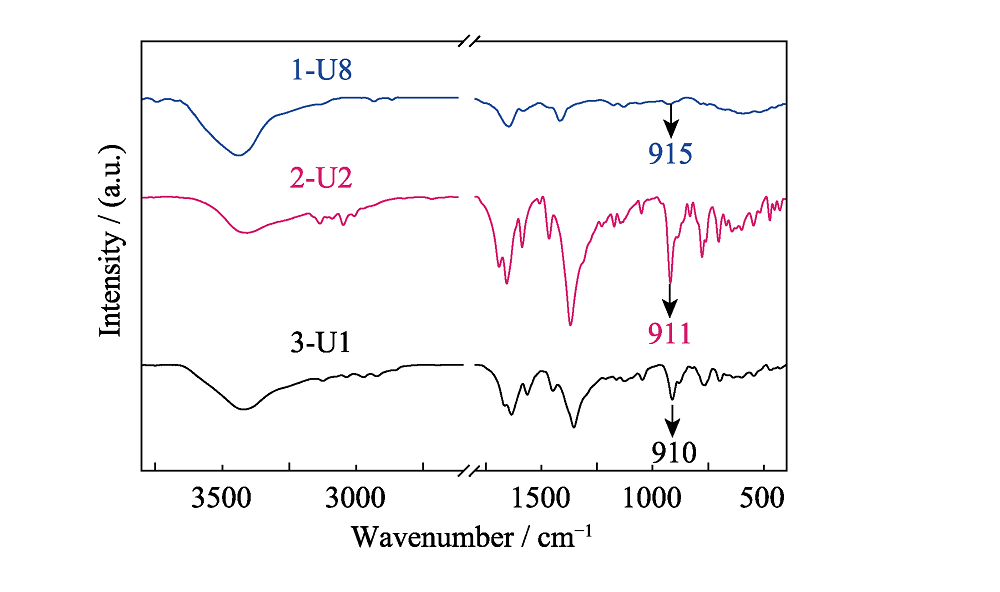
Fig. S15 Fourier transform infrared (IR) spectra of compounds 1 (U8 motif, blue line), 2 (U2 motif, red line) and 3 (U1 motif, black line) with characteristic symmetric ν1vibrations at 915, 911 and 910 nm, respectively
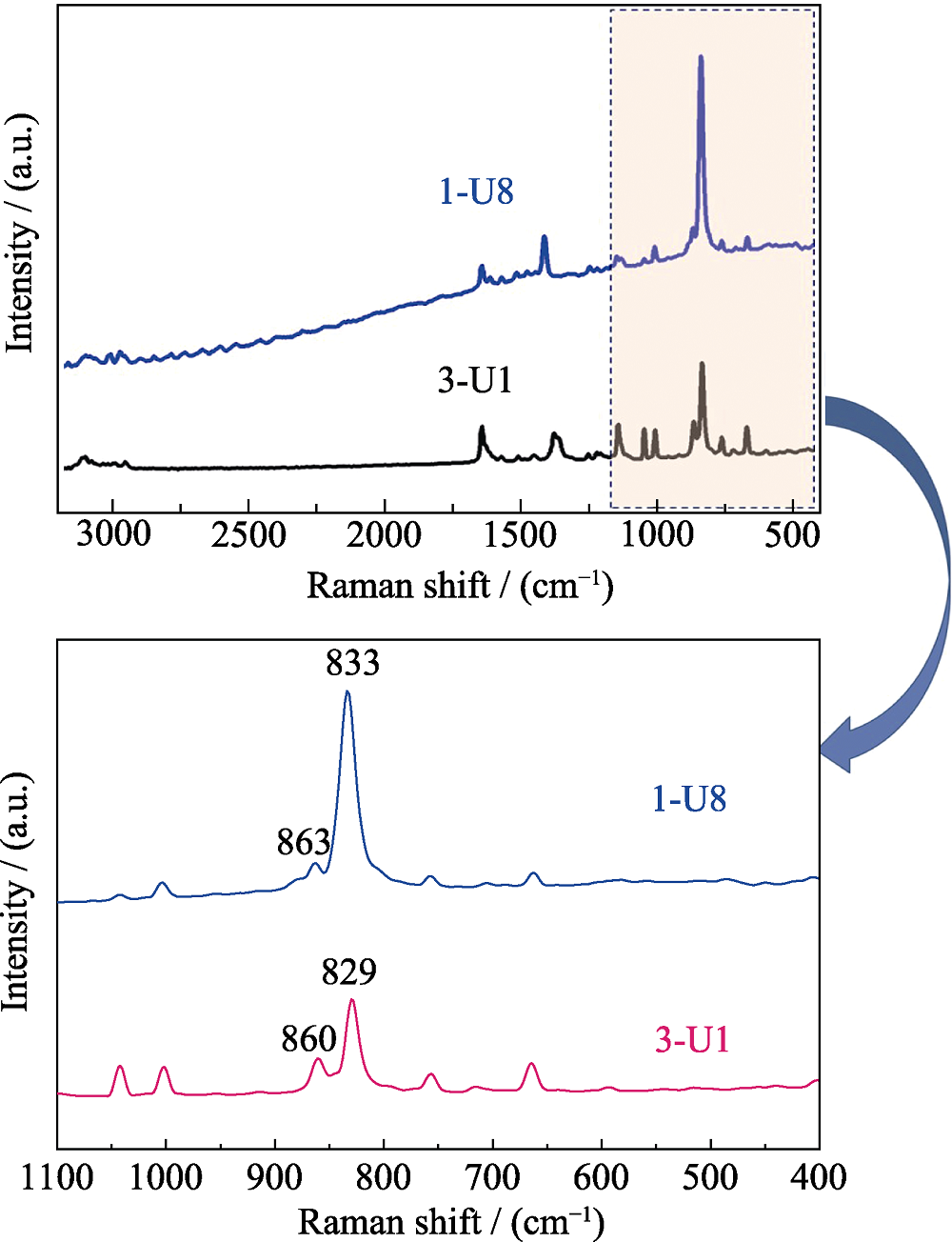
Fig. S16 The Raman spectra of compounds s 1 (U8 motif) and 3 (U1 motif) with characteristic asymmetric ν3 vibrations (1: 833 and 863 cm-1; 3: 829 and 860 cm-1)
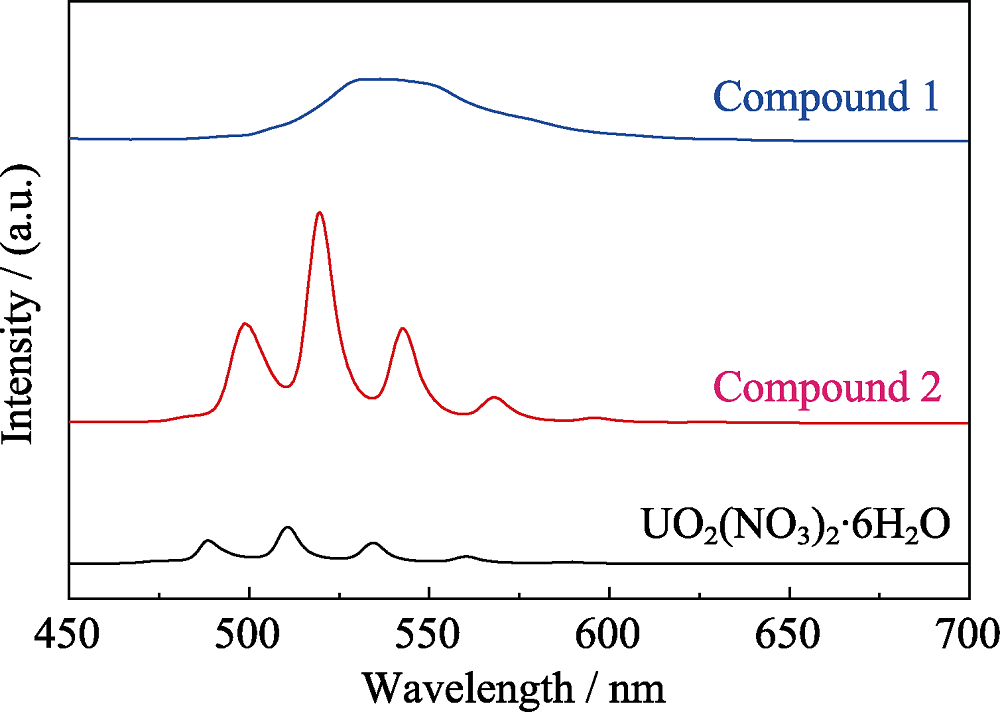
Fig. S17 Solid-state fluorescence spectra of compound 1 and 2 as compared to that of uranyl nitrate (UO2(NO3)2): 1, a broad peak ranging from 530 to 550 nm; 2, five main emission bands located at 499, 520, 543, 568 and 596 nm; UO2(NO3)2, 488, 511, 534, 561 and 589 nm
| Compound 1 | |||
|---|---|---|---|
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1748(17) | U(2)-O(3) | 0.1752(15) |
| U(1)-O(2) | 0.1770(2) | U(2)-O(4) | 0.1751(15) |
| U(1)-O(9) | 0.2208(13) | U(2)-O(9) | 0.2275(12) |
| U(1)-O(12) | 0.2327(15) | U(2)-O(10) | 0.2193(14) |
| U(1)-O(13) | 0.2506(14) | U(2)-O(15) | 0.2466(14) |
| U(1)-O(14) | 0.2440(18) | U(2)-O(16) | 0.2578(14) |
| U(1)-O(18) | 0.2426(16) | U(2)-O(17) | 0.2380(17) |
| U(3)-O(5) | 0.1746(17) | U(4)-O(7) | 0.165(3) |
| U(3)-O(6) | 0.178(2) | U(4)-O(8) | 0.171(2) |
| U(3)-O(9) | 0.2344(14) | U(4)-O(10) | 0.2200(14) |
| U(3)-O(10) | 0.2237(14) | U(4)-O(11) | 0.242(2) |
| U(3)-O(11c) | 0.248(2) | U(4)-O(11c) | 0.2461(14) |
| U(3)-O(12) | 0.2349(17) | U(4)-O(16) | 0.249(2) |
| U(3)-O(19a) | 0.2439(16) | U(4)-O(20d) | 0.2399(16) |
| Compound 2 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1776(2) | U(1)-O(4) | 0.2364(2) |
| U(1)-O(2) | 0.1784(2) | U(1)-O(5a) | 0.2358(2) |
| U(1)-O(7) | 0.2325(2) | U(1)-O(7a) | 0.2339(2) |
| U(1)-O(1W) | 0.2576(2) | ||
| Compound 3 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.182(3) | U(1)-O(4b) | 0.244(2) |
| U(1)-O(1a) | 0.182(3) | U(1)-O(5) | 0.237(2) |
| U(1)-O(2) | 0.240(2) | U(1)-O(6) | 0.2307(18) |
| U(1)-O(3) | 0.2397(19) | ||
| Compound 1 | |||
|---|---|---|---|
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1748(17) | U(2)-O(3) | 0.1752(15) |
| U(1)-O(2) | 0.1770(2) | U(2)-O(4) | 0.1751(15) |
| U(1)-O(9) | 0.2208(13) | U(2)-O(9) | 0.2275(12) |
| U(1)-O(12) | 0.2327(15) | U(2)-O(10) | 0.2193(14) |
| U(1)-O(13) | 0.2506(14) | U(2)-O(15) | 0.2466(14) |
| U(1)-O(14) | 0.2440(18) | U(2)-O(16) | 0.2578(14) |
| U(1)-O(18) | 0.2426(16) | U(2)-O(17) | 0.2380(17) |
| U(3)-O(5) | 0.1746(17) | U(4)-O(7) | 0.165(3) |
| U(3)-O(6) | 0.178(2) | U(4)-O(8) | 0.171(2) |
| U(3)-O(9) | 0.2344(14) | U(4)-O(10) | 0.2200(14) |
| U(3)-O(10) | 0.2237(14) | U(4)-O(11) | 0.242(2) |
| U(3)-O(11c) | 0.248(2) | U(4)-O(11c) | 0.2461(14) |
| U(3)-O(12) | 0.2349(17) | U(4)-O(16) | 0.249(2) |
| U(3)-O(19a) | 0.2439(16) | U(4)-O(20d) | 0.2399(16) |
| Compound 2 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.1776(2) | U(1)-O(4) | 0.2364(2) |
| U(1)-O(2) | 0.1784(2) | U(1)-O(5a) | 0.2358(2) |
| U(1)-O(7) | 0.2325(2) | U(1)-O(7a) | 0.2339(2) |
| U(1)-O(1W) | 0.2576(2) | ||
| Compound 3 | |||
| Bond | Distance/nm | Bond | Distance/nm |
| U(1)-O(1) | 0.182(3) | U(1)-O(4b) | 0.244(2) |
| U(1)-O(1a) | 0.182(3) | U(1)-O(5) | 0.237(2) |
| U(1)-O(2) | 0.240(2) | U(1)-O(6) | 0.2307(18) |
| U(1)-O(3) | 0.2397(19) | ||
| Compound 1 | ||||
|---|---|---|---|---|
| Hydrogen bond | D-H/nm | H··A/nm | D··A/nm | Angle/(°) |
| C6-H6···O6 | 0.093 | 0.215 | 0.305 | 165 |
| C17-H17···O1 | 0.093 | 0.243 | 0.316 | 135 |
| C18-H18···O13 | 0.093 | 0.242 | 0.330 | 159 |
| C15-H15···O5 | 0.093 | 0.245 | 0.322 | 141 |
| C16-H16A···O3 | 0.097 | 0.242 | 0.321 | 138 |
| Compound 2 | ||||
| Hydrogen bond | D-H/nm | H···A/nm | D···A/nm | Angle/(°) |
| O7-H7···O10 | 0.073 | 0.216 | 0.285 | 161 |
| C16-H16···O10 | 0.093 | 0.258 | 0.324 | 128 |
| C15-H16···O9 | 0.093 | 0.298 | 0.358 | 123 |
| Compound 1 | ||||
|---|---|---|---|---|
| Hydrogen bond | D-H/nm | H··A/nm | D··A/nm | Angle/(°) |
| C6-H6···O6 | 0.093 | 0.215 | 0.305 | 165 |
| C17-H17···O1 | 0.093 | 0.243 | 0.316 | 135 |
| C18-H18···O13 | 0.093 | 0.242 | 0.330 | 159 |
| C15-H15···O5 | 0.093 | 0.245 | 0.322 | 141 |
| C16-H16A···O3 | 0.097 | 0.242 | 0.321 | 138 |
| Compound 2 | ||||
| Hydrogen bond | D-H/nm | H···A/nm | D···A/nm | Angle/(°) |
| O7-H7···O10 | 0.073 | 0.216 | 0.285 | 161 |
| C16-H16···O10 | 0.093 | 0.258 | 0.324 | 128 |
| C15-H16···O9 | 0.093 | 0.298 | 0.358 | 123 |
| Compound 1 | Compound 2 | Compound 3 | |
|---|---|---|---|
| Formula | C22H16N2O20U4 | C40H38N6O22U2 | C8H5NO8U |
| Formula weight | 1580.49 | 1430.82 | 481.16 |
| Crystal system | monoclinic | triclinic | orthorhombic |
| Space group | P21/c | P-1 | Ibam |
| a/nm | 1.15944(14) | 0.98277(3) | 2.6039(4) |
| b/nm | 1.9854(3) | 1.05830(4) | 1.17462(13) |
| c/nm | 1.5002(2) | 1.15097(4) | 0.91646(17) |
| α/(º) | 90 | 82.951(2) | 90 |
| β/(º) | 105.390(3) | 88.168(2) | 90 |
| γ/(º) | 90 | 66.735(2) | 90 |
| V/nm3 | 3.3296(8) | 1.09126(7) | 2.8031(7) |
| Z | 4 | 1 | 8 |
| T/K | 296 | 297 | 293 |
| F(000) | 2760 | 680 | 1728 |
| Dc/(g·cm-3) | 3.153 | 2.177 | 2.280 |
| μ/mm-1 | a 19.480 | b 7.507 | c 32.914 |
| Rint | 0.073 | 0.028 | 0.088 |
| R1, wR2 (all data) | 0.0646, 0.1536 | 0.0227, 0.0491 | 0.0755, 0.2833 |
| Compound 1 | Compound 2 | Compound 3 | |
|---|---|---|---|
| Formula | C22H16N2O20U4 | C40H38N6O22U2 | C8H5NO8U |
| Formula weight | 1580.49 | 1430.82 | 481.16 |
| Crystal system | monoclinic | triclinic | orthorhombic |
| Space group | P21/c | P-1 | Ibam |
| a/nm | 1.15944(14) | 0.98277(3) | 2.6039(4) |
| b/nm | 1.9854(3) | 1.05830(4) | 1.17462(13) |
| c/nm | 1.5002(2) | 1.15097(4) | 0.91646(17) |
| α/(º) | 90 | 82.951(2) | 90 |
| β/(º) | 105.390(3) | 88.168(2) | 90 |
| γ/(º) | 90 | 66.735(2) | 90 |
| V/nm3 | 3.3296(8) | 1.09126(7) | 2.8031(7) |
| Z | 4 | 1 | 8 |
| T/K | 296 | 297 | 293 |
| F(000) | 2760 | 680 | 1728 |
| Dc/(g·cm-3) | 3.153 | 2.177 | 2.280 |
| μ/mm-1 | a 19.480 | b 7.507 | c 32.914 |
| Rint | 0.073 | 0.028 | 0.088 |
| R1, wR2 (all data) | 0.0646, 0.1536 | 0.0227, 0.0491 | 0.0755, 0.2833 |
| [1] | ALTMAIER M, GAONA X, FANGHANEL T , et al. Recent advances in aqueous actinide chemistry and thermodynamics. Chemical Reviews, 2013,113(2):901-943. |
| [2] | JONES M B, GAUNT A J . Recent developments in synthesis and structural chemistry of nonaqueous actinide complexes. Chemical Reviews, 2013,113(2):1137-1198. |
| [3] | WANG K X, CHEN J S . Extended structures and physicochemical properties of uranyl-organic compounds. Accounts of Chemical Research, 2011,44(7):531-540. |
| [4] | ANDREWS M B, CAHILL C L . Uranyl bearing hybrid materials: synthesis, speciation, and solid-state structures. Chemical Reviews, 2013,113(2):1121-1136. |
| [5] | YANG W T, PARKER T G, SUN Z M , et al. Structural chemistry of uranium phosphonates. Coordination Chemistry Reviews, 2015,303(1):86-109. |
| [6] | LOISEAU T, MIHALCEA I, HENRY N , et al. The crystal chemistry of uranium carboxylates. Coordination Chemistry Reviews, 2014,266(35):69-109. |
| [7] | RAI D, FELMY A R, RYAN J L , et al. Uranium (IV) hydrolysis constants and solubility product of UO2·xH2O(am). Inorganic Chemistry, 1990,29(2):260-264. |
| [8] | AHRLAND S . On the complex chemistry of the uranyl ion Ι. The hydrolysis of the 6-valent uranium in aqueous solutions. Acta Chemica Scandinavica, 1949,3(4):374-400. |
| [9] | ZANONATO P, DI BERNARDO P, BISMONDO A , et al. Hydrolysis of uranium (VI) at variable temperatures (10-85 ℃). Journal of the American Chemical Society, 2004,126(17):5515-5522. |
| [10] | SALMON L, THUERY P, EPHRITIKHINE M , et al. Crystal structure of the first octanuclear uranium (IV) complex with compartmental schiff base ligands. Polyhedron, 2004,23(4):623-627. |
| [11] | MIHALCEA I, HENRY N, CLAVIER N , et al. Occurence of an octanuclear motif of uranyl isophthalate with cation-cation interactions through edge-sharing connection mode. Inorganic Chemistry, 2011,50(13):6243-6249. |
| [12] | PASQUALE S, SATTIN S, ESCUDERO-ADAN E C , et al. Giant regular polyhedra from calixarene carboxylates and uranyl. Nature Communications, 2012,3(1):785. |
| [13] | THUERY P . A highly adjustable coordination system: nanotubular and molecular cage species in uranyl ion complexes with kemp's triacid. Crystal Growth & Design, 2014,14(3):901-904. |
| [14] | WANG L H, SHANG R, ZHENG Z , et al. Two systems of [DabcoH2]2+/[PipH2]2+-uranyl-oxalate showing reversible crystal-to- crystal transformations controlled by the diammonium/uranyl/oxalate ratios in aqueous solutions ([DabcoH2]2+=1,4-diazabicyclo- [2.2.2]-octaneH2 and [PipH2]2+ = PiperazineH2). Crystal Growth & Design, 2013,13(6):2597-2606. |
| [15] | CHAPELET-ARAB B, NOWOGROCKI G, ABRAHAM E , et al. Crystal structure of new uranyl oxalates (NH4)2[UO2(C2O4)·2H2O] and (NH4)2-x(N2H5)x[UO2(C2O4)3]·3H2O (x=0 and x=1). Comparison with other uranyl oxalates. Radiochimica Acta, 2005,93(5):279-285. |
| [16] | GIESTING P A, PORTER N J, BURNS P C , et al. A series of sheet-structured alkali metal uranyl oxalate hydrates: structures and IR spectra. Zeitschrift für Kristallographie, 2006,221(8):589-599. |
| [17] | GIESTING P A, PORTER N J, BURNS P C , et al. Uranyl oxalate hydrates: structures and IR spectra. Zeitschrift für Kristallographie, 2006,221(4):252-259. |
| [18] | DUVIEUBOURG L, NOWOGROCKI G, ABRAHAM F , et al. Hydrothermal synthesis and crystal structures of new uranyl oxalate hydroxides: α- and β-[(UO2)2 (C2O4)(OH)2(H2O)2] and [(UO2)2((C2O4)(OH)2(H2O)2]·H2O. Journal of Solid State Chemistry, 2005,178(11):3437-3444. |
| [19] | THUERY P . Reaction of uranyl nitrate with carboxylic diacids under hydrothermal conditions. Crystal structure of complexes with L(+)-tartaric and oxalic acids. Polyhedron, 2007,26(1):101-106. |
| [20] | VOLOGZHANINA A V, SEREZHKINA L B, NEKLYUDOVA N A , et al. Synthesis and characterisation of a trinuclear uranyl complex: crystal structure of (CN3H6)5[(UO2)3O(OH)2(CH3COO)(C2O4)3]. Inorganica Chimica Acta, 2009,362(14):4921-4925. |
| [21] | CHUGH C A, SHARMA A, SHARMA A , et al. Kinetics and mechanism of thermal decomposition of uranyl oxalate. Asian Journal of Chemistry, 2011,23(4):1865-1866. |
| [22] | BARTLETT J R, COONEY R P , et al. On the determination of uranium oxygen bond lengths in dioxouranium (VI) compounds by raman-spectroscopy. Journal of Molecular Structure, 1989,193(1):295-300. |
| [23] | BRACHMANN A, GEIPEL G, BERNHARD G , et al. Study of uranyl (VI) malonate complexation by time resolved laser-induced fluorescence spectroscopy (TRLFS). Radiochimica Acta, 2002,90(3):147-153. |
| [24] | MEI L, WANG C Z, ZHU L Z , et al. Exploring new assembly modes of uranyl terephthalate: templated syntheses and structural regulation of a series of rare 2d→3d polycatenated frameworks. Inorganic Chemistry, 2017,56(14):7694-7706. |
| [25] | NATRAJAN L S . Developments in the photophysics and photochemistry of actinide ions and their coordination compounds. Coordination Chemistry Reviews, 2012,256(15/16):1583-1603. |
| [26] | THUERY P, HARROWFIELD J . Solvent effects in solvo-hydrothermal synthesis of uranyl ion complexes with 1,3-adamantanediacetate. CrystEngComm, 2015,17(21):4006-4018. |
| [27] | THUERY P, HARROWFIELD J . Structural variations in the uranyl/4,4'-biphenyldicarboxylate system. rare examples of 2d→3d polycatenated uranyl-organic networks. Inorganic Chemistry, 2015,54(16):8093-8102. |
| [28] | THUERY P, RIVIERE E, HARROWFIELD J , et al. Uranyl and uranyl-3d block cation complexes with 1,3-adamantanedicarboxylate: crystal structures, luminescence, and magnetic properties. Inorganic Chemistry, 2015,54(6):2838-2850. |
| [1] | WANG Xiaobo, ZHU Yuliang, XUE Wenchao, SHI Ruchuan, LUO Bofeng, LUO Chengtao. Effect of PbTiO3 Content Variation on High-power Performance of PMN-PT Single Crystal [J]. Journal of Inorganic Materials, 2025, 40(7): 840-846. |
| [2] | TANG Xinli, DING Ziyou, CHEN Junrui, ZHAO Gang, HAN Yingchao. In vivo Distribution and Metabolism of Calcium Phosphate Nanomaterials Based on Fluorescent Labeling with Rare Earth Europium Ions [J]. Journal of Inorganic Materials, 2025, 40(7): 754-764. |
| [3] | CHAI Runyu, ZHANG Zhen, WANG Menglong, XIA Changrong. Preparation of Ceria Based Metal-supported Solid Oxide Fuel Cells by Direct Assembly Method [J]. Journal of Inorganic Materials, 2025, 40(7): 765-771. |
| [4] | WANG Lujie, ZHANG Yuxin, LI Tongyang, YU Yuan, REN Pengwei, WANG Jianzhang, TANG Huaguo, YAO Xiumin, HUANG Yihua, LIU Xuejian, QIAO Zhuhui. Corrosion and Wear Behavior of Silicon Carbide Ceramic in Deep-sea Service Environment [J]. Journal of Inorganic Materials, 2025, 40(7): 799-807. |
| [5] | YU Leyangyang, ZHAO Fangxia, ZHANG Shuxin, XU Yixiang, NIU Yaran, ZHANG Zhenzhong, ZHENG Xuebin. Preparation of High-entropy Boride Powders for Plasma Spraying by Inductive Plasma Spheroidization [J]. Journal of Inorganic Materials, 2025, 40(7): 808-816. |
| [6] | YANG Guang, ZHANG Nan, CHEN Shujin, WANG Yi, XIE An, YAN Yujie. WO3 Films Based on Porous ITO Electrodes: Preparation and Electrochromic Property [J]. Journal of Inorganic Materials, 2025, 40(7): 781-789. |
| [7] | SUN Jing, LI Xiang, MAO Xiaojian, ZHANG Jian, WANG Shiwei. Effect of Lauric Acid Modifier on the Hydrolysis Resistance of Aluminum Nitride Powders [J]. Journal of Inorganic Materials, 2025, 40(7): 826-832. |
| [8] | HU Zhichao, YANG Hongyu, YANG Hongcheng, SUN Chengli, YANG Jun, LI Enzhu. Usage of the P-V-L Bond Theory in Regulating Properties of Microwave Dielectric Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 609-626. |
| [9] | LI Wenyuan, XU Jianan, DENG Han'ao, CHANG Aimin, ZHANG Bo. Effect of V5+ Substitution on Microstructure and Microwave Dielectric Properties of LaTaO4 Ceramics [J]. Journal of Inorganic Materials, 2025, 40(6): 697-703. |
| [10] | DONG Chenyu, ZHENG Weijie, MA Yifan, ZHENG Chunyan, WEN Zheng. Characterizations by Piezoresponse Force Microscopy on Relaxor Properties of Pb(Mg,Nb)O3-PbTiO3 Ultra-thin Films [J]. Journal of Inorganic Materials, 2025, 40(6): 675-682. |
| [11] | HE Guoqiang, ZHANG Kaiheng, WANG Zhentao, BAO Jian, XI Zhaochen, FANG Zhen, WANG Changhao, WANG Wei, WANG Xin, JIANG Jiapei, LI Xiangkun, ZHOU Di. Ba(Nd1/2Nb1/2)O3: Au Underrated K40 Microwave Dielectric Ceramic [J]. Journal of Inorganic Materials, 2025, 40(6): 639-646. |
| [12] | ZHANG Jiawei, CHEN Ning, CHENG Yuan, WANG Bo, ZHU Jianguo, JIN Cheng. Electrical Properties of Bismuth Layered Piezoelectric Bi4Ti3O12 Ceramics with A/B-site Doping [J]. Journal of Inorganic Materials, 2025, 40(6): 690-696. |
| [13] | XIONG Siyu, MO Chen, ZHU Xiaowei, ZHU Guobin, CHEN Deqin, LIU Laijun, SHI Xiaodong, LI Chunchun. Low-temperature Sintering of LiBxAl1-xSi2O6 Microwave Dielectric Ceramics with Ultra-low Permittivity [J]. Journal of Inorganic Materials, 2025, 40(5): 536-544. |
| [14] | CUI Ning, ZHANG Yuxin, WANG Lujie, LI Tongyang, YU Yuan, TANG Huaguo, QIAO Zhuhui. Single-phase Formation Process and Carbon Vacancy Regulation of (TiVNbMoW)Cx High-entropy Ceramics [J]. Journal of Inorganic Materials, 2025, 40(5): 511-520. |
| [15] | AN Ran, LIN Si, GUO Shigang, ZHANG Chong, ZHU Shun, HAN Yingchao. Iron-doped Nano-hydroxyapatite: Preparation and Ultraviolet Absorption Performance [J]. Journal of Inorganic Materials, 2025, 40(5): 457-465. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||